Abstracts
The objective of the present study was to estimate the rates of parasitism of Lysiphlebus testaceipes (Cresson) on Schizaphis graminum (Rond.) and Aphis gossypii Glover under choice and no-choice condition tests. The experiments were carried out in a controlled environmental chamber at 25±1ºC temperature, 70±10% RH, and 12h photophase. Second and third instar aphid nymphs were used as hosts and for each colony one less than a day old L. testaceipes female was used. The previously mated and oviposition inexperienced female remained in contact with the hosts for 24 h. In the choice test 10 colonies of S. graminum and 10 colonies of A. gossypii having sections of sorghum leaves as substrate were used. For the no-choice test 10 colonies of S. graminum maintained on sections of sorghum leaves and 10 colonies of A. gossypii maintained on pepper leaves were used. In the choice test the parasitism was 67% and 46% for S. graminum and A. gossypii, respectively whereas in the no-choice test the parasitism was 76% for S. graminum and 56% for A. gossypii. Superparasitism was not observed for S. graminum. The aphid S. graminum was the most suitable for multiplication of the parasitoid L. testaceipes under laboratory conditions.
Insecta; aphid; biological control; parasitoid
Este trabalho teve como objetivo estimar a taxa de parasitismo de Lysiphlebus testaceipes (Cresson) em Schizaphis graminum (Rond.) e Aphis gossypii Glover, em testes com e sem escolha. Os ensaios foram conduzidos em câmara climática com 25±1ºC, 70±10% UR e 12h de fotofase. Foram utilizadas, como hospedeiros, ninfas de pulgões de 2º e 3º ínstares e, para cada colônia, utilizou-se uma fêmea de L. testaceipes com menos de um dia de vida. A fêmea era previamente acasalada e sem experiência de oviposição e ficou em contato com os hospedeiros por 24h. No teste com escolha, foram utilizadas 10 colônias de S. graminum e 10 de A. gossypii, tendo como substrato seções de folhas de sorgo. Para o teste sem escolha, foram utilizadas 10 colônias de S. graminum, mantidas em seções de folhas de sorgo e 10 de A. gossypii, em folhas de pimentão. No teste com chance de escolha, o parasitismo foi de 67% e 46% para S. graminum e A. gossypii, respectivamente, enquanto que no teste sem chance de escolha, foram obtidos 76% de parasitismo para S. graminum e 56% para A. gossypii. Não foi observado superparasitismo em S. graminum. O pulgão S. graminum mostrou-se mais adequado para a multiplicação do parasitóide L. testaceipes em condições de laboratório.
Insecta; pulgões; controle biológico; parasitóide
a17v30n4
BIOLOGICAL CONTROL
Parasitism Rates of Lysiphlebus testaceipes (Cresson) (Hym.: Aphidiidae) on Schizaphis graminum (Rond.) and Aphis gossypii Glover (Hem.: Aphididae)
SANDRA M.M. RODRIGUES AND VANDA H.P. BUENO
Depto. Entomologia, Universidade Federal de Lavras, C. postal 37, 372000-000, Lavras, MG
Taxas de Parasitismo de Lysiphlebus testaceipes (Cresson) (Hym.: Aphidiidae) em Schizaphis graminum (Rond.) e Aphis gossypii Glover (Hem.: Aphididae)
RESUMO Este trabalho teve como objetivo estimar a taxa de parasitismo de Lysiphlebus testaceipes (Cresson) em Schizaphis graminum (Rond.) e Aphis gossypii Glover, em testes com e sem escolha. Os ensaios foram conduzidos em câmara climática com 25±1oC, 70±10% UR e 12h de fotofase. Foram utilizadas, como hospedeiros, ninfas de pulgões de 2o e 3o ínstares e, para cada colônia, utilizou-se uma fêmea de L. testaceipes com menos de um dia de vida. A fêmea era previamente acasalada e sem experiência de oviposição e ficou em contato com os hospedeiros por 24h. No teste com escolha, foram utilizadas 10 colônias de S. graminum e 10 de A. gossypii, tendo como substrato seções de folhas de sorgo. Para o teste sem escolha, foram utilizadas 10 colônias de S. graminum, mantidas em seções de folhas de sorgo e 10 de A. gossypii, em folhas de pimentão. No teste com chance de escolha, o parasitismo foi de 67% e 46% para S. graminum e A. gossypii, respectivamente, enquanto que no teste sem chance de escolha, foram obtidos 76% de parasitismo para S. graminum e 56% para A. gossypii. Não foi observado superparasitismo em S. graminum. O pulgão S. graminum mostrou-se mais adequado para a multiplicação do parasitóide L. testaceipes em condições de laboratório.
PALAVRAS-CHAVE: Insecta, pulgões, controle biológico, parasitóide.
ABSTRACT The objective of the present study was to estimate the rates of parasitism of Lysiphlebus testaceipes (Cresson) on Schizaphis graminum (Rond.) and Aphis gossypii Glover under choice and no-choice condition tests. The experiments were carried out in a controlled environmental chamber at 25±1ºC temperature, 70±10% RH, and 12h photophase. Second and third instar aphid nymphs were used as hosts and for each colony one less than a day old L. testaceipes female was used. The previously mated and oviposition inexperienced female remained in contact with the hosts for 24 h. In the choice test 10 colonies of S. graminum and 10 colonies of A. gossypii having sections of sorghum leaves as substrate were used. For the no-choice test 10 colonies of S. graminum maintained on sections of sorghum leaves and 10 colonies of A. gossypii maintained on pepper leaves were used. In the choice test the parasitism was 67% and 46% for S. graminum and A. gossypii, respectively whereas in the no-choice test the parasitism was 76% for S. graminum and 56% for A. gossypii. Superparasitism was not observed for S. graminum. The aphid S. graminum was the most suitable for multiplication of the parasitoid L. testaceipes under laboratory conditions.
KEY WORDS: Insecta, aphid, biological control, parasitoid.
Species from the family Aphidiidae parasitize exclusively aphids and are used in biological control programs on protected crops in several countries. The Aphidiidae females seem to randomly search on a leaf sometimes along the leaf veins or leaf margins generally detecting the aphids through antennae contact (Hagvar & Hofsvang 1991). After finding a potential host the female evaluates its suitability and quality for offspring development by antenna and ovipositor probing. Examples of stimuli that may induce a host examination are: shape, texture, size, chemical compounds and host movements. A potential host has to be adequate to the wasp profile of response and has to satisfy the minimal physiological and dietary needs for the development and growth of the immature insects (Mackauer et al. 1996).
The parasitoid Lysiphlebus testaceipes (Cresson) has as its hosts the aphids Aphis craccivora Koch, Aphis fabae Scopoli, Aphis gossypii Glover, Aphis nerii Boyer de Fonscolombe, Rhopalosiphum maidis (Fitch), Rhopalosiphum padi (Linnaeus), Schizaphis graminum (Rondani), and Toxoptera aurantii (Boyer de Fonscolombe) (Stary et al. 1988, Kring & Kring 1988).
The selection of host species, that will ensure a reasonable production of offspring, is of vital importance for the parasitoid. When several host species are present, the quality (in terms of fitness gain of the parasitoid) as well as the abundance and distribution pattern of the host will affect the choice by the parasitoid (Hagvar & Hofsvang 1991).
Preferences for some host species were demonstrated in laboratory studies. It was found that the parasitoid oviposited with a higher frequency on some host species than on others, when the host species are simultaneously as well as separately offered (Powell & Wright 1988).
The objective of this study was to estimate the rate of parasitism of L. testaceipes on S. graminum and A. gossypii under choice and no-choice conditions tests.
Materials and Methods
This research was carried out in an incubator at 25±1ºC, 70±10% RH, and 12h photophase. The aphids were obtained from a laboratory rearing on sorghum and cotton plants, maintained under greenhouse conditions, at the Department of Entomology, Federal University of Lavras, Lavras County, State of Minas Gerais, Brazil. The parasitoid L. testaceipes was collected from mummies of A. gossypii on cotton plants. Multiplication of S. graminum was achieved using sections of sorghum (Sorghum bicolor L., cv. BR300) leaves maintained in plastic cups. A . gossypii was multiplied on pepper (Capsicum annum L.) plants and on cotton (Gossypium hirsutum L.) seedlings. L. testaceipes were reared on colonies of S. graminum.
The rates of parasitism of L. testaceipes on S. graminum and A. gossypii were determined by choice and no-choice tests. Sections of sorghum leaves and leaves of pepper, with the bottom tips and the petioles, respectively, wrapped with cotton pads moistened in a nutritive solutions of N-Benzoiladein at 0.1 ppm were used. The leaves were maintained in glass tubes (4.5 cm in diameter and 24.5 cm long) sealed in one of the ends with cheese cloth and a rubber band and in the other end with PVC film.
Second and third instars S. graminum and A. gossypii aphids were than transferred to these substrates 4h before the female parasitoids were released. One less than 24h old female, previously mated and without oviposition experience, was released in each tube, remaining in contact with the aphids for a 24h period. Little drops of honey and water were fed to L. testaceipes on the walls of the tubes. The vegetal substrate was renewed every three days or when needed.
After a 24h contact period of the female parasitoid with the host aphids, the A. gossypii nymphs were transferred, with the aid of a fine brush, to petri dishes (5 cm in diameter) containing discs of pepper leaves arranged on top of filter paper moistened with nutritive solution. This procedure was needed because sorghum in not a suitable host plant for A. gossypii. The petri dishes were than sealed with PVC film and pierced with an entomological pin to allow ventilation. The S. graminum nymphs remained on the sections of sorghum leaves in the glass tubes after the contact with the parasitoid. The colonies of S. graminum and A. gossypii were monitored until no mummy could be found. The nymphs that died before becoming mummies were dissected to check for the presence the parasitoid larvae.
In the choice test, 10 L. testaceipes females to 10 colonies of aphids (one female/colony) were tested, keeping 20 specimens of S. graminum and 20 specimens of A. gossypii in the same tube. In the no-choice test 10 colonies of S. graminum were evaluated on sections of sorghum leaves and 10 colonies of A. gossypii were evaluated on pepper leaves. One colony containing 10 specimens of S. graminum and 10 specimens of A. gossypii, and one female of the parasitoid were maintained in each container.
Incomplete parasitism was observed when larvae of the parasitoid were found after dissection of host nymphs that had died before mummification; complete parasitism was recorded when mummies were found. The total parasitism data were obtained by the addition of the incomplete parasitism figures to the complete parasitism figures. Superparasitism was observed when more than one larva of the parasitoid was found on the host aphid.
The proportion of parasitized aphids was calculated dividing the number of parasitized aphids by the total number of aphids provided. Data were analyzed using the Exact Confidence Interval (E.C.I), according to Leemis and Trivedi (1996).
Results and Discussion
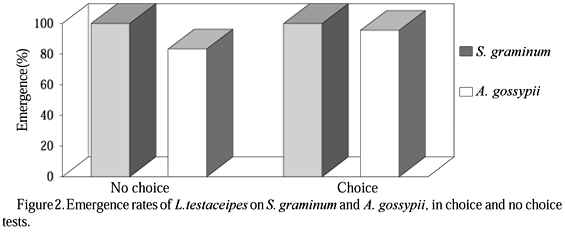
In the choice test, the rates of parasitism on S. graminum and on A. gossypii were 67% and 46%, respectively, with an E.C.I of [ 17.67 <P> 13.92], at 95% probability. The rates of incomplete parasitism were 20% for S. graminum and 35% for A. gossypii, and the rates of complete parasitism were 47% and 11% for S. graminum and A. gossypii, respectively (Fig. 1). Superparasitism was not found on S. graminum in this test but for the species A. gossypii a 9% rate was found. The emergence rates of L. testaceipes on S. graminum and on A. gossypii were, respectively, 100% and 95% in the same test (Fig. 2).
In the no-choice test, the rates of parasitism on S. graminum and on A. gossypii were 76% and 56%, respectively. These figures were found with an E.C.I. of [867.85 <P> 80.42], at 95% probability. The rates of incomplete parasitism observed were 9% for S. graminum and 41% for A. gossypii. The rates of complete parasitism were 76% for S. graminum and 15 % for A. gossypii (Fig. 3). In this test, superparasitism was also not found for S. graminum, whereas for A. gossypii a 32% figure was observed (Fig. 3). The rates of emergence of the parasitoid L. testaceipes in this test were 100% on S. graminum and 83% on A. gossypii (Fig. 2).
The occurrence of incomplete parasitism in these tests may have been a consequence of the incapacity of the host nymph in metabolizing nutrients in sufficient quantities to guarantee the development of the nymph itself as well as of the parasitoid larva. In that case, the parasitoid has to compete with the host tissue for the minimum supply of nutrients of the hemolymph (Vinson & Iwansch 1980). In the parasitized host it is necessary an increment of the metabolic rate in order to compensate the additional consumption by the larva of the parasitoid; when that is not possible, the parasitized host dies.
The fact of not finding superparasitism on S. graminum in both tests does not mean that the parasitoid did not lay more than one egg on the host once the conditions were identical for both species. In this study it was observed a lower mortality of parasitized nymphs (incomplete parasitism) on S. graminum than on A. gossypii (Figs. 1 and 3). Nevertheless the probability of finding more than one larva was higher for A. gossypii.
The lack of superparasitism on S. graminum can also be related to the defense behavior of the aphids against the attack of the parasitoid. The aphids many times defend themselves from the attack by kicking, shaking, walking and dropping from the plant, and this may affect the rate of parasitism observed among the hosts (Gardner et al. 1984). According to Sampaio (1999) A. gossypii was almost always passive with Aphidius colemani Viereck, not avoiding new oviposition whereas Myzus persicae (Sulzer) was more defensive. S. graminum is considered not quite passive, according to Brown (1974) who observed that specimens of this species avoided the attack of coccinelids by moving rapidly their bodies. The defensive behavior of Acyrtosiphon pisum (Harris) and A. kondoi Shinji limited the number of eggs laid by the parasitoid A. ervi Haliday despite not having avoided the parasitism (Bueno et al. 1993). Gardner et al. (1984) observed that the aphids Metopolophium dirhodum (Walker) dodged from the attack of A. rhopalosiphi (De-Stefani-Perez).
Comparing the rates of superparasitism on A. gossypii in both tests (Figs. 1 and 3) it becomes clear that it was higher (32%) in the no-choice test than in the choice test (9%). The number of host insects provided in each test may have caused the difference. In the no-choice test, L. testaceipes had access to a density of 20 A. gossypii aphids, whereas in the choice test 40 nymphs were offered, where 20 were S. graminum and the other 20 were A. gossypii. In the higher density (choice test) the parasitoid L. testaceipes had lower chance of laying eggs on a host already parasitized. Similarly, Hofsvang & Hagvar (1983) found that superparasitism of Ephedrus cerasicola Stary on M. persicae was 44% on a density of 20 aphids and 15% on a density of 40 aphids. Cloutier (1984) reported lower superparasitism of Aphidius nigripes (Ashmead) when the density of the host Macrosiphon euphorbiae (Thomas) increased.
In both tests, L. testaceipes oviposited on S. graminum as well as on A. gossypii, however the rate of parasitism was higher on S. graminum. This may indicate a preference of the parasitoid for this species. Although further studies such as rates of encounters and rejections among parasitoids and hosts with higher density of hosts and shorter time of contact between parasitoid and host, are needed to assure this premise. It is also possible to infer that as L. testaceipes was raised for many generations on S. graminum, an unexpected condition may have occurred, i.e., the parasitoid preferred to lay its eggs on the host on which it developed. After emergence, the female parasitoid can contact its meconium or the remains of the host that may provide some signals about the host on which it successfully developed (Vinson 1998). For example, when the parasitoid A. ervi was raised on A. pisum more mummies were produced than on Microlophium carnosum (Buckton) (Powell & Wright 1998).
Therefore, it may be concluded that S. graminum and A. gossypii were adequate for the development of the parasitoid L. testaceipes. However S. graminum proved to be more suitable for possible mass raisings under laboratory conditions.
Acknowledgments
The authors would like to thank Marcelo Teixeira Tavares (Department of Exact and Natural Sciences, University Center of Araraquara, Araraquara County, State of São Paulo, Brazil) for the identification of the parasitoid; to CAPES and CNPq for the scholarship granted; and to the Research Support Foundation of Minas Gerais State (FAPEMIG) for the financial support.
Literature Cited
Received 26/11/99. Accepted 06/06/01.
- Brown, H.D. 1974. Defensive behaviour of the wheat aphid Schizaphis graminum (Rondani) (Hemiptera: Aphididae), against Coccinelidae. J. Entomol. 48: 157-165.
- Bueno, V.H.P., A.P. Gutierrez & P. Ruggle.1993. Parasitism by Aphidius ervi (Hym.: Aphidiidae): Preference for pea aphid and blue alfafa aphid (Hom.: Aphididae) and competition with A. smithi Entomophaga 38: 273-284.
- Cloutier, C. 1984 The effect of host density on egg distribution by the solitary parasitoid Aphidius nigripes (Hymenoptera: Aphidiidae). Can. Entomol. 116: 805-811.
- Gardner, S.M., S.A. Ward & A.F.G. Dixon. 1984. Limitation of superparasitism by Aphidius rhopalosiphi: a consequence of aphid defensive behaviour. Ecol. Entomol. 9: 149-155.
- Hĺgvar, E.B. & T. Hofsvang. 1991. Aphid parasitoids (Hymenoptera, Aphidiidae): biology, host selection and use in biological control. Biocontrol News Inf. 12: 13-41.
- Hofsvang, T. & E.B. Hĺgvar. 1983 Superparasitism and host discrimination by Ephedrus cerasicola (Hym.: Aphidiidae), an aphidiid parasitoid of Myzus persicae (Hom.: Aphididae). Entomophaga 28: 379-386.
- Kring, T.J. & J.B. Kring. 1988 Aphid fecundity, reproductive longevity, and parasite development in the Schizaphis graminum (Rondani) (Homoptera: Aphididae)-Lysiphlebus testaceipes (Cresson) (Hymenoptera: Braconidae) system. Can. Entomol. 120: 1079-1083.
- Leemis, L.M. & K.S. Trivedi. 1996 A comparison of approximate interval estimators for the Bernoulli parameter. Am. Stat. 50: 63-68.
- Mackauer, M., J.P. Michaud & W. Völkl. 1996 Host choice by aphidiid parasitoids (Hymenoptera: Aphidiidae): host recognition, host quality, and host value. Can. Entomol. 128: 959-980.
- Powell, W. & A.F. Wright. 1988. The abilities of the aphid parasitoids Aphidius ervi Haliday and A. rhopalosiphi de Stefani Perez (Hymenoptera: Braconidae) to transfer between different known host species and implications for the use of alternative host in pest strategies. Bul. Entomol. Res. 78: 683-693.
- Sampaio, M.V. 1999. Parasitismo de Aphidius colemani Viereck, 1912 (Hymenoptera: Aphidiidae) em diferentes densidades de Myzus persicae (Sulzer, 1776) e preferęncia por M. persicae e Aphis gossypii Glover, 1877 (Hemiptera: Aphididae). Dissertaçăo de mestrado, UFLA, Lavras, 73p.
- Starý, P., J.P. Lyon & F. Leclant. 1988. Post-colonisation host range of Lysiphlebus testaceipes in the Mediterranean area (Hymenoptera, Aphidiidae). Act. Entomol. Boh. 85: 1-11.
- Vinson, S.B. 1998. The general host selection behaviour of parasitoid hymenoptera and a comparison of initial strategies utilized by larvaphagous and oophagous species. Biol. Control 11: 79-96.
- Vinson, S.B. & G.F. Iwantsch. 1980. Host suitability for insect parasitoids. Annu. Rev. Entomol. 25: 397-419.
Publication Dates
-
Publication in this collection
17 June 2002 -
Date of issue
Dec 2001
History
-
Accepted
06 June 2001 -
Received
26 Nov 1999