Abstract
Surveillances and interventions on antibiotics use have been suggested to improve serious drug-resistance worldwide. Since 2007, our hospital have proposed many measures for regulating surgical prophylactic antibiotics (carbapenems, third gen. cephalosporins, vancomycin, etc.) prescribing practices, like formulary restriction or replacement for surgical prophylactic antibiotics and timely feedback. To assess the impacts on drug-resistance after interventions, we enrolled infected patients in 2006 (pre-intervention period) and 2014 (post-intervention period) in a tertiary hospital in Shanghai. Proportions of targeted pathogens were analyzed: methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus spp. (VRE), imipenem-resistant Escherichia coli (IREC), imipenem-resistant Klebsiella pneumoniae (IRKP), imipenem-resistant Acinetobacter baumannii (IRAB) and imipenem-resistant Pseudomonas aeruginosa (IRPA) isolates. Rates of them were estimated and compared between Surgical Department, ICU and Internal Department during two periods. The total proportions of targeted isolates in Surgical Department (62.44%, 2006; 64.09%, 2014) were more than those in ICU (46.13%, 2006; 50.99%, 2014) and in Internal Department (44.54%, 2006; 51.20%, 2014). Only MRSA has decreased significantly (80.48%, 2006; 55.97%, 2014) (p < 0.0001). The percentages of VRE and IREC in 3 departments were all <15%, and the slightest change were also both observed in Surgical Department (VRE: 0.76%, 2006; 2.03%, 2014) (IREC: 2.69%, 2006; 2.63%, 2014). The interventions on surgical prophylactic antibiotics can be effective for improving resistance; antimicrobial stewardship must be combined with infection control practices.
Keywords:
Surgical prophylactic antibiotics; Intervention; Resistance; Nosocomial infection
Introduction
Since turn of the 21st century, the emergence of organisms with increased resistance to available antibiotics has been continuously concerned by the public.11 Livermore DM. Fourteen years in resistance. Int J Antimicrob Agents. 2012;39(4):283-294.
2 Resistance EA. Healthcare-Associated Infections P. Antibiotic resistance in Europe: the challenges ahead. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2009;14(45):.
3 Hu FP, Guo Y, Zhu DM, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005-2014. Clin Microbiol Infect. 2016;22(suppl 1):S9-S14.-44 Jean SS, Hsueh PR. High burden of antimicrobial resistance in Asia. Int J Antimicrob Agents. 2011;37(4):291-295. Simultaneously, it has become a global threat to human health, provoking adverse outcomes, high care costs and prolonged hospital stays.55 Huh K, Chung DR, Park HJ, et al. Impact of monitoring surgical prophylactic antibiotics and a computerized decision support system on antimicrobial use and antimicrobial resistance. Am J Infect Control. 2016. The unrestrained use of antibiotics has been implicated as a significant reservoir of the greatly changed resistance.11 Livermore DM. Fourteen years in resistance. Int J Antimicrob Agents. 2012;39(4):283-294.,66 Polk R. Optimal use of modern antibiotics: emerging trends. Clin Infect Dis. 1999;29(2):264-274. Thus optimizing use of antibiotics has become one of the most essential parts to contain resistance.66 Polk R. Optimal use of modern antibiotics: emerging trends. Clin Infect Dis. 1999;29(2):264-274.,77 Cheng VC, Wong SC, Ho PL, Yuen KY. Strategic measures for the control of surging antimicrobial resistance in Hong Kong and mainland of China. Emerg Microbes Infect. 2015;4(2):e8. A study in France has showed successful control on fluoroquinolone-resistant Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus (MRSA) by fluoroquinolone prescription restriction.88 Lafaurie M, Porcher R, Donay JL, Touratier S, Molina JM. Reduction of fluoroquinolone use is associated with a decrease in methicillin-resistant Staphylococcus aureus and fluoroquinolone-resistant Pseudomonas aeruginosa isolation rates: a 10 year study. J Antimicrob Chemother. 2012;67(4):1010-1015. Similarly, another multifaceted proactive intervention program including antimicrobial stewardship has also minimized the nosocomial transmission and outbreaks of vancomycin-resistant Enterococcus spp. (VRE) in HongKong.99 Cheng VC, Tai JW, Chen JH, et al. Proactive infection control measures to prevent nosocomial transmission of vancomycin-resistant enterococci in Hong Kong. J Formos Med Assoc = Taiwan yi zhi. 2014;113(10):734-741. What's better, reports from the US and Korea have demonstrated that the monitoring on antibiotics (surgical prophylactic antibiotics (SPAs)) could reduce the antimicrobial use and length of hospital stay, improve the clinical and financial outcomes and even slow down the previously increasing antimicrobial resistance rates of some pathogens.55 Huh K, Chung DR, Park HJ, et al. Impact of monitoring surgical prophylactic antibiotics and a computerized decision support system on antimicrobial use and antimicrobial resistance. Am J Infect Control. 2016.,1010 Kim ES, Park SW, Lee CS, Gyung Kwak Y, Moon C, Kim BN. Impact of a national hospital evaluation program using clinical performance indicators on the use of surgical antibiotic prophylaxis in Korea. Int J Infect Dis. 2012;16(3):e187-e192.,1111 Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect. 2014;69(3):216-225.
A study once performed in China collecting 230,800 prescriptions between 2007 and 2009 has revealed excessive overprescribing, including twice as many prescriptions as recommended by the WHO.1212 Li Y, Xu J, Wang F, et al. Overprescribing in China, driven by financial incentives, results in very high use of antibiotics, injections, and corticosteroids. Health Affairs (Project Hope). 2012;31(5):1075-1082. Herein, a hospital-wide intervention project was launched in our hospital to improve the quality of SPAs in 2007, and the backend alerts of Hospital Information System (HIS) and a restriction system were introduced one year later.1313 Chen-rong M, Yu-xing N, Wan-hua Y, et al. Effectiveness analysis of continuous improvement in antibacterial drugs prophylactic using during perioperative period. Chin Hosp Manag. 2013;33(4):41-42.,1414 Chen-rong M, Yu-xing N, Mu S, et al. Computer network application of managing antibiotic agents utilization in hospitals. Chin Hosp Manag. 2011;31(4):61-62. All measures intended to minimize the unnecessary usage of antibiotics by timely feedback and prospective audit. Although they have been confirmed to enhance the appropriateness of antibiotic use and improve the quality of treatment,1313 Chen-rong M, Yu-xing N, Wan-hua Y, et al. Effectiveness analysis of continuous improvement in antibacterial drugs prophylactic using during perioperative period. Chin Hosp Manag. 2013;33(4):41-42. the effects on changes in antimicrobial resistance warranted further researches. Thus we conducted the study to compare the antimicrobial resistant rates of six major nosocomial pathogens between the pre- and post-intervention periods. To our knowledge, most studies to date focused more on the rapid growth of drug-resistant bacteria due to the antibiotics abuse in China or the decreases of drug consumption due to antimicrobial stewardship.1515 Tang Q, Song P, Li J, Kong F, Sun L, Xu L. Control of antibiotic resistance in China must not be delayed: the current state of resistance and policy suggestions for the government, medical facilities, and patients. Biosci Trends. 2016;10(1):1-6.
16 Xu J, Sun Z, Li Y, Zhou Q. Surveillance and correlation of antibiotic consumption and resistance of Acinetobacter baumannii complex in a tertiary care hospital in northeast China, 2003-2011. Int J Environ Res Public Health. 2013;10(4):1462-1473.
17 Shen J, Sun Q, Zhou X, et al. Pharmacist interventions on antibiotic use in inpatients with respiratory tract infections in a Chinese hospital. Int J Clin Pharm. 2011;33(6):929-933.-1818 Hvistendahl M. Public health. China takes aim at rampant antibiotic resistance. Science (New York, N.Y.). 2012;336(6083):795. Whether the interventions on SPAs were effective to antimicrobial resistance, however, has not been documented well in Shanghai, even in China.
Materials and methods
Setting
Ruijin Hospital is a tertiary university-affiliated hospital, located in Shanghai, a large metropolitan region in China of over 24 million inhabitants. It is a general 1800-bed hospital integrated with emergency, intensive care unit (ICU), surgery and other departments, serving approximately 115,000 patient visits per year. In addition to native residents, patients from other provinces in China also come for better medical treatment.
The study was approved by Ruijin Hospital Ethics Committee (Shanghai Jiao Tong University School of Medicine), and the Review Board exempted request for informed consent because no patient-level data were obtained.
Interventions
The intervention measures were described before.1414 Chen-rong M, Yu-xing N, Mu S, et al. Computer network application of managing antibiotic agents utilization in hospitals. Chin Hosp Manag. 2011;31(4):61-62. Briefly, medical records of the patients prescribed SPAs (with emphasis on carbapenems, third gen. cephalosporins and vancomycin) were selective examined, for example, timing of antibiotic administration, appropriateness of the regimen and duration of SPAs. Moreover, the backend alerts of HIS and a restriction system were designed to monitor and improve the use of SPAs in line with the inclusion of assessing the use of SPAs. Ratios of adherence to the recommendations were measured regularly and timely feedback was given to surgical staffs. The surgeons were provided with systematical lectures on the rational use of SPAs, which may facilitate the propagation.
Isolates
We performed an interrupted time series study of patients with infections in 2006 (pre-intervention period) and 2014 (post-intervention period). Cases were identified from the laboratory database of the Department of Clinical Microbiology. Only the first isolate from the same species was reviewed and recorded per patient among the two periods. To evaluate the effect of interventions in SPAs on antimicrobial resistance, pathogens belonging to six major species or genus were enrolled in current study referring to the primer studies as following11 Livermore DM. Fourteen years in resistance. Int J Antimicrob Agents. 2012;39(4):283-294.,33 Hu FP, Guo Y, Zhu DM, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005-2014. Clin Microbiol Infect. 2016;22(suppl 1):S9-S14.: S. aureus, Enterococcus spp., Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii and P. aeruginosa. Antimicrobial susceptibility data, including MRSA, VRE, imipenem-resistant E. coli (IREC), imipenem-resistant K. pneumoniae (IRKP), imipenem-resistant A. baumannii (IRAB) and imipenem-resistant P. aeruginosa (IRPA) were collected. Species were identified by standard biochemical methods or the VITEK 2 compact system (bioMérieux, Marcyl'Étoile, France). The antimicrobial susceptibilities of clinical isolates were determined by the disk diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) criteria or the VITEK 2 compact system (bioMérieux, Marcyl'Étoile, France) following the specifications, and results were interpreted using the CLSI criteria.1919 Institute CaLS. Performance standards for antimicrobial susceptibility testing. In: 24th informational supplement, M100-S24. 2014. Variations in the susceptibility phenotype above were estimated from pre- and post-intervention periods among different departments (ICU, Internal Department and Surgical Department).
Statistical analysis
Values were presented as a percentage of the group. Pearson's chi square test was used for testing the differences of proportions and resistant rates between two groups with Fisher's exact test as appropriate. A two-tailed p value of <0.05 was regarded as statistically significant. All statistical analysis was performed by SAS 8.2 (SAS Institute Inc., Cary, NC, USA).
Results
Total characteristic
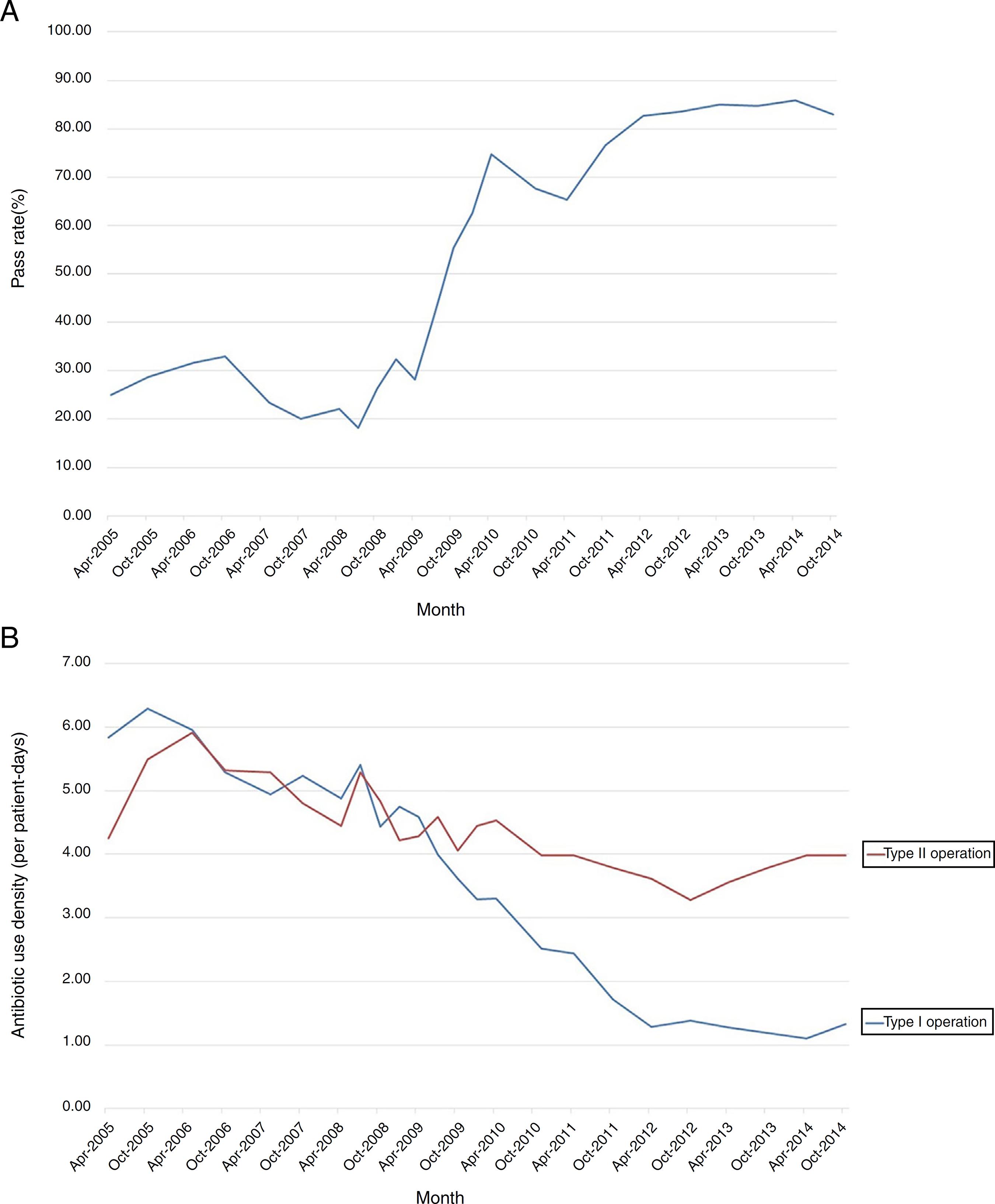
The secular trend of the qualified SPAs use in total after interventions in 2007 was on a sharp increase and the rate became stable at 85% since 2013 (Fig. 1A). The antibiotic use density in type I and II operation both showed downward trends, and the slope change in type I operation was more positive (Fig. 1B). Besides, the descending curves have been gradual since 2014, this was also why 2014 as a post-intervention period in our study.
Changing trends of SPAs use among Surgical Department from 2005 to 2014. (A) The rates of qualified SPAs prescriptions in total, (B) the antibiotic use density in type I and II operation.
The total numbers of clinical isolates were 3774 in 2006 and 5127 in 2014. The proportions of targeted isolates among all reported isolates were shown in Table 1. The isolates enrolled accounted for 51.27% (2006) and 55.14% (2014) of all reported isolates. The proportion of targeted isolates in Surgical Department (62.36% in 2006; 64.09% in 2014) was more than that in ICU (46.13% in 2006; 50.99% in 2014) and Internal Department (44.54% in 2006; 51.20% in 2014) (Table 1). A sharp decrease was only found in the proportion of S. aureus among all 3 departments (Total: 11.02% in 2006; 6.20% in 2014) (p ≤ 0.0001).
Proportions of targeted pathogens belonging to 6 species or genus among different departments.
Changes of resistant rates in surgical department
Only the percentage of MRSA isolates has dropped significantly after the interventions (80.48% in 2006; 55.97% in 2014) (p < 0.0001). In contrast, VRE (0.76% in 2006; 2.03% in 2014), IRKP (2.91% in 2006; 13.11% in 2014), IRAB (28.21% in 2006; 69.64% in 2014) and IRPA (46.53% in 2006; 54.73% in 2014) rose within post-intervention period. Notably, the slight changes of VRE, IREC and IRPA have not been regarded as significant (p > 0.05). These findings were presented in Table 2.
Changes of resistant rates in different departments
The percentages of MRSA in Surgical Department (80.48% in 2006; 55.97% in 2014) stayed between those in ICU (90.74% in 2006; 78.85% in 2014) and Internal Department (58.56% in 2006; 44.23% in 2014) but with the greatest decreasing tendency. The percentages of VRE and IREC in 3 departments were all below 15%, and the narrowest margins were observed both in Surgical Department (Fig. 2). A substantial climb in the incidence of IRAB has been observed with similar ranges in all 3 departments. Separately, the minimum increased ranges of IRKP (0.82% in 2006; 6.15% in 2014) and IRPA (46.88% in 2006; 51.22% in 2014) were in Internal Department and ICU respectively (Fig. 2).
Rates of MRSA, VRE, IREC, IRKP, IRAB and IRPA among 3 departments in 2006 and 2014. ‘Internal' means Internal Department; ‘Surgical' means Surgical Department; ‘Total' means the total rates of MRSA, VRE, IREC, IRKP, IRAB and IRPA in 3 departments.
Discussion
It is notorious that extensive use of antibiotics can potentiate antibiotic resistance, which will be difficult and costly to control.44 Jean SS, Hsueh PR. High burden of antimicrobial resistance in Asia. Int J Antimicrob Agents. 2011;37(4):291-295. And evidently, the control of antibiotic use deserves to be undertaken. Reports form several countries have claimed that a web-based audit and feedback intervention can increase adherence to empirical antibiotic prescribing guidelines.2020 Hogli JU, Garcia BH, Skjold F, Skogen V, Smabrekke L. An audit and feedback intervention study increased adherence to antibiotic prescribing guidelines at a Norwegian hospital. BMC Infect Dis. 2016;16:96.
21 Yardley L, Douglas E, Anthierens S, et al. Evaluation of a web-based intervention to reduce antibiotic prescribing for LRTI in six European countries: quantitative process analysis of the GRACE/INTRO randomised controlled trial. Implement Sci. 2013;8:134.
22 Prior M, Elouafkaoui P, Elders A, et al. Evaluating an audit and feedback intervention for reducing antibiotic prescribing behaviour in general dental practice (the RAPiD trial): a partial factorial cluster randomised trial protocol. Implement Sci. 2014;9:50.-2323 Popovski Z, Mercuri M, Main C, et al. Multifaceted intervention to optimize antibiotic use for intra-abdominal infections. J Antimicrob Chemother. 2015;70(4):1226-1229. Better than that, since 2000 when a US study found that antibiotic-utilization interventions may be particularly important for control of multidrug-resistant (MDR) K. pneumonia,2424 Patterson JE, Hardin TC, Kelly CA, Garcia RC, Jorgensen JH. Association of antibiotic utilization measures and control of multiple-drug resistance in Klebsiella pneumoniae. Infect Control Hosp Epidemiol. 2000;21(7):455-458. there have been worldwide reports on the reduction of resistance rates in major pathogens due to antibiotic interventions.55 Huh K, Chung DR, Park HJ, et al. Impact of monitoring surgical prophylactic antibiotics and a computerized decision support system on antimicrobial use and antimicrobial resistance. Am J Infect Control. 2016.,1111 Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect. 2014;69(3):216-225.,2525 Cheng VC, Chan JF, Wong SC, et al. Proactive infection control measures to prevent nosocomial transmission of carbapenem-resistant Enterobacteriaceae in a non-endemic area. Chin Med J. 2013;126(23):4504-4509.,2626 Bantar C, Sartori B, Vesco E, et al. A hospitalwide intervention program to optimize the quality of antibiotic use: impact on prescribing practice, antibiotic consumption, cost savings, and bacterial resistance. Clin Infect Dis. 2003;37(2):180-186. China manufactured and consumed the largest quantity of antibiotics by country according to a survey in 2007.1818 Hvistendahl M. Public health. China takes aim at rampant antibiotic resistance. Science (New York, N.Y.). 2012;336(6083):795. Since the same year, many measures for regulating SPAs prescribing practices have been proposed in our hospital, such as continuing educational sessions for SPAs prescribing, signature of liability forms, formulary restriction or replacement and timely feedback.1313 Chen-rong M, Yu-xing N, Wan-hua Y, et al. Effectiveness analysis of continuous improvement in antibacterial drugs prophylactic using during perioperative period. Chin Hosp Manag. 2013;33(4):41-42.,1414 Chen-rong M, Yu-xing N, Mu S, et al. Computer network application of managing antibiotic agents utilization in hospitals. Chin Hosp Manag. 2011;31(4):61-62. The preliminary study have demonstrated that the interventions had resulted in the increased rates of qualified SPAs prescriptions and the decreased duration of SPAs.1313 Chen-rong M, Yu-xing N, Wan-hua Y, et al. Effectiveness analysis of continuous improvement in antibacterial drugs prophylactic using during perioperative period. Chin Hosp Manag. 2013;33(4):41-42. Of interest, these findings were in accordance with those of Bantar et al.,2626 Bantar C, Sartori B, Vesco E, et al. A hospitalwide intervention program to optimize the quality of antibiotic use: impact on prescribing practice, antibiotic consumption, cost savings, and bacterial resistance. Clin Infect Dis. 2003;37(2):180-186. but opposite to those of Gyssens et al.,2727 Gyssens IC, Blok WL, van den Broek PJ, Hekster YA, van der Meer JW. Implementation of an educational program and an antibiotic order form to optimize quality of antimicrobial drug use in a department of internal medicine. Eur J Clin Microbiol Infect Dis. 1997;16(12):904-912. who reported a 25% growth in antibiotic use after similar intervention. Considering we also noted unstable utilization levels of SPAs on the baseline phase (Fig. 1), this is not surprising. Therefore, the current study aimed to assess the impacts of interventions on antimicrobial susceptibility between pre- and post-intervention periods.
In this study, the highest proportion of targeted isolates belonging to 6 species/genus was found in Surgical Department (Table 1), which supported the necessity of interventions on SPAs in our hospital. Monitoring of SPAs and timely feedback were widely applied strategies to improve professional prescription practice. A multicenter project to prevent surgical site infections also presented improvement in antibiotic use in the US.2828 Dellinger EP, Hausmann SM, Bratzler DW, et al. Hospitals collaborate to decrease surgical site infections. Am J Surg. 2005;190(1):9-15. In addition, the interventions on prescription have been proved to account for a diminishing tendency of antibiotics use,55 Huh K, Chung DR, Park HJ, et al. Impact of monitoring surgical prophylactic antibiotics and a computerized decision support system on antimicrobial use and antimicrobial resistance. Am J Infect Control. 2016.,2626 Bantar C, Sartori B, Vesco E, et al. A hospitalwide intervention program to optimize the quality of antibiotic use: impact on prescribing practice, antibiotic consumption, cost savings, and bacterial resistance. Clin Infect Dis. 2003;37(2):180-186. then for the reduction of antimicrobial resistance.88 Lafaurie M, Porcher R, Donay JL, Touratier S, Molina JM. Reduction of fluoroquinolone use is associated with a decrease in methicillin-resistant Staphylococcus aureus and fluoroquinolone-resistant Pseudomonas aeruginosa isolation rates: a 10 year study. J Antimicrob Chemother. 2012;67(4):1010-1015.,2929 Kaier K, Frank U, Hagist C, Conrad A, Meyer E. The impact of antimicrobial drug consumption and alcohol-based hand rub use on the emergence and spread of extended-spectrum beta-lactamase-producing strains: a time-series analysis. J Antimicrob Chemother. 2009;63(3):609-614. So improving the appropriateness of SPAs use may become a good start for prevention and management of infections.
The most important results of the study were the effects of the interventions on antimicrobial resistance. Fig. 2 revealed the same trend but different volatility of antimicrobial resistant rates to the targeted pathogens in different departments between the 2 periods. Only the great decreasing ratio of MRSA was observed in Surgical Department after the interventions performed (Table 2, Fig. 2). Successful controls of MRSA by prescription restriction through HIS have also been reported previously.11 Livermore DM. Fourteen years in resistance. Int J Antimicrob Agents. 2012;39(4):283-294.,88 Lafaurie M, Porcher R, Donay JL, Touratier S, Molina JM. Reduction of fluoroquinolone use is associated with a decrease in methicillin-resistant Staphylococcus aureus and fluoroquinolone-resistant Pseudomonas aeruginosa isolation rates: a 10 year study. J Antimicrob Chemother. 2012;67(4):1010-1015. Some other infection control policies might have contributed to the inverse, such as promoting alcohol-based hand hygiene to all conscious patients and healthcare workers in our hospital,3030 Chen-rong M, Gui-ting X, Li-jun Z, et al. Practice of improving compliance of hand hygiene of healthcare workers. Chin Nurs Manag. 2009;9(6):16-18. which appeared to be useful in preventing nosocomial outbreaks due to epidemiologically important MDR organisms or viruses in clinical settings.99 Cheng VC, Tai JW, Chen JH, et al. Proactive infection control measures to prevent nosocomial transmission of vancomycin-resistant enterococci in Hong Kong. J Formos Med Assoc = Taiwan yi zhi. 2014;113(10):734-741.,2525 Cheng VC, Chan JF, Wong SC, et al. Proactive infection control measures to prevent nosocomial transmission of carbapenem-resistant Enterobacteriaceae in a non-endemic area. Chin Med J. 2013;126(23):4504-4509.,3131 Stone SP, Fuller C, Savage J, et al. Evaluation of the national Cleanyourhands campaign to reduce Staphylococcus aureus bacteraemia and Clostridium difficile infection in hospitals in England and Wales by improved hand hygiene: four year, prospective, ecological, interrupted time series study. BMJ (Clin Res Ed). 2012;344:e3005.,3232 Cheng VC, Tai JW, Lee WM, et al. Infection control preparedness for human infection with influenza A H7N9 in Hong Kong. Infect Control Hosp Epidemiol. 2015;36(1):87-92. In contrast to these positive shifts for MRSA, the resistance changes amongst Enterococcus spp. and Gram-negative bacilli seemed to be for the worse (Table 2, Fig. 2). Compared with those in Internal Department and ICU, the proportions of VRE and IREC in Surgical Department fluctuated minimally and insignificantly, which can demonstrate the effectiveness of interventions on SPAs (Fig. 2, Table 2). What's more, the different volatilities between different departments' added evidence to the importance of interventions on antibiotics for controlling antimicrobial resistance.
The increased prevalence of carbapenem-resistant Gram-negative bacilli isolates (i.e. K. pneumoniae, A. baumanii and P. aeruginosa) have been widely documented due to the reliance on carbapenems to treat these pathogens.3333 Nordmann P, Naas T, Poirel L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17(10):1791-1798.
34 Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007;20(3):440-458.-3535 Hackel MA, Badal RE, Bouchillon SK, Biedenbach DJ, Hoban DJ. Resistance rates of intra-abdominal isolates from intensive care units and non-intensive care units in the United States: the study for monitoring antimicrobial resistance trends 2010-2012. Surg Infect (Larchmt). 2015;16(3):298-304. As to IRAB, the marked rise has been observed with similar ranges in 3 departments (Fig. 2). Unexpectedly, the minimum increased ranges of IRKP and IRPA were in Internal Department and ICU respectively, and the proportions of IRPA and IRAB in Surgical Department in 2014 were far higher than that in CHINET surveillance system.33 Hu FP, Guo Y, Zhu DM, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005-2014. Clin Microbiol Infect. 2016;22(suppl 1):S9-S14. These seemed we could not assert that the reduced use of SPAs could bring about the positive volatilities in antimicrobial resistance especially of non-fermentative Gram-negative bacilli. Actually, the dramatically growing emergence of carbapenem-resistant K. pneumonia isolates has been deemed a big concern over the past decade, mostly due to the diversity of emerging carbapenemase types.11 Livermore DM. Fourteen years in resistance. Int J Antimicrob Agents. 2012;39(4):283-294.,3434 Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007;20(3):440-458.P. aeruginosa and A. baumannii, the two most frequent non-fermenters causing nosocomial infections and outbreaks, were notorious for the multiple mechanisms of antimicrobial resistance and could survive under a wide range of environmental conditions and persists for prolonged periods on surfaces, which made the clonal dissemination of clinical MDR strains.44 Jean SS, Hsueh PR. High burden of antimicrobial resistance in Asia. Int J Antimicrob Agents. 2011;37(4):291-295.,3636 Sanchez A, Gattarello S, Rello J. New treatment options for infections caused by multiresistant strains of Pseudomonas aeruginosa and other nonfermenting gram-negative bacilli. Semin Respir Crit Care Med. 2011;32(2):151-158. Therefore, prudent use of antibiotics is not the only strategy for fighting antibiotic resistance. It still requires a combination of good infection control practices, like hand hygiene, environmental disinfection, and the screening of especially non-fermenters infected patients in order to achieve a comprehensive control of resistance. Friedrich even found a correlation between use of single antibiotics and changes in the resistance profiles of some Gram-negative aerobes by using data analysis (i.e. simple linear regression).3737 Friedrich LV, White RL, Bosso JA. Impact of use of multiple antimicrobials on changes in susceptibility of gram-negative aerobes. Clin Infect Dis. 1999;28(5):1017-1024. One last thing, clinical and economical costs were still questionable and warranted further follow-up.
There are still some limitations in our study: first, the control of confounders like patient characteristics and effects of other infection control strategies and had been precluded. Second, we did not compare the patient-level outcomes between the 2 periods. However, it provides the step stone for the future expanded research associated with multicenter and further antibiotics prescribing surveillance to control possible resistance.
Conclusions
Our study indicated that the interventions on SPAs and timely feedback could lead to prompt and positive effects on the amount of antibiotic prescribed, and could be useful options to improve antimicrobial resistance of some pathogens (MRSA, VRE, IREC, etc.). In addition to administration of appropriate antimicrobial agents, strict infection control efforts are also essential, like hand hygiene, environmental disinfection, and the screening of especially non-fermenters infected patients. The periodic surveillance of drug resistance is crucial not just for epidemiological purposes but also for establishing and improving guidelines for the antimicrobial stewardship and infection control.
-
FundingThis work was supported by the National Natural Science Foundation of China (grant no. 81472010) and the Shanghai Three-Year Plan of the Key Subjects Construction in Public Health-Infectious Diseases and Pathogenic Microorganism (grant no. 15GWZK0102).
Acknowledgments
We would like to thank Shu-Zhen Xiao and Fei-Fei Gu for the preparation of the susceptibility data, and the Department of Clinical Microbiology at Ruijin Hospital for excellent laboratory provision and technical assistance.
References
-
1Livermore DM. Fourteen years in resistance. Int J Antimicrob Agents 2012;39(4):283-294.
-
2Resistance EA. Healthcare-Associated Infections P. Antibiotic resistance in Europe: the challenges ahead. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin 2009;14(45):.
-
3Hu FP, Guo Y, Zhu DM, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005-2014. Clin Microbiol Infect 2016;22(suppl 1):S9-S14.
-
4Jean SS, Hsueh PR. High burden of antimicrobial resistance in Asia. Int J Antimicrob Agents 2011;37(4):291-295.
-
5Huh K, Chung DR, Park HJ, et al. Impact of monitoring surgical prophylactic antibiotics and a computerized decision support system on antimicrobial use and antimicrobial resistance. Am J Infect Control 2016.
-
6Polk R. Optimal use of modern antibiotics: emerging trends. Clin Infect Dis 1999;29(2):264-274.
-
7Cheng VC, Wong SC, Ho PL, Yuen KY. Strategic measures for the control of surging antimicrobial resistance in Hong Kong and mainland of China. Emerg Microbes Infect 2015;4(2):e8.
-
8Lafaurie M, Porcher R, Donay JL, Touratier S, Molina JM. Reduction of fluoroquinolone use is associated with a decrease in methicillin-resistant Staphylococcus aureus and fluoroquinolone-resistant Pseudomonas aeruginosa isolation rates: a 10 year study. J Antimicrob Chemother 2012;67(4):1010-1015.
-
9Cheng VC, Tai JW, Chen JH, et al. Proactive infection control measures to prevent nosocomial transmission of vancomycin-resistant enterococci in Hong Kong. J Formos Med Assoc = Taiwan yi zhi 2014;113(10):734-741.
-
10Kim ES, Park SW, Lee CS, Gyung Kwak Y, Moon C, Kim BN. Impact of a national hospital evaluation program using clinical performance indicators on the use of surgical antibiotic prophylaxis in Korea. Int J Infect Dis 2012;16(3):e187-e192.
-
11Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect 2014;69(3):216-225.
-
12Li Y, Xu J, Wang F, et al. Overprescribing in China, driven by financial incentives, results in very high use of antibiotics, injections, and corticosteroids. Health Affairs (Project Hope) 2012;31(5):1075-1082.
-
13Chen-rong M, Yu-xing N, Wan-hua Y, et al. Effectiveness analysis of continuous improvement in antibacterial drugs prophylactic using during perioperative period. Chin Hosp Manag. 2013;33(4):41-42.
-
14Chen-rong M, Yu-xing N, Mu S, et al. Computer network application of managing antibiotic agents utilization in hospitals. Chin Hosp Manag 2011;31(4):61-62.
-
15Tang Q, Song P, Li J, Kong F, Sun L, Xu L. Control of antibiotic resistance in China must not be delayed: the current state of resistance and policy suggestions for the government, medical facilities, and patients. Biosci Trends 2016;10(1):1-6.
-
16Xu J, Sun Z, Li Y, Zhou Q. Surveillance and correlation of antibiotic consumption and resistance of Acinetobacter baumannii complex in a tertiary care hospital in northeast China, 2003-2011. Int J Environ Res Public Health 2013;10(4):1462-1473.
-
17Shen J, Sun Q, Zhou X, et al. Pharmacist interventions on antibiotic use in inpatients with respiratory tract infections in a Chinese hospital. Int J Clin Pharm 2011;33(6):929-933.
-
18Hvistendahl M. Public health. China takes aim at rampant antibiotic resistance. Science (New York, N.Y.). 2012;336(6083):795.
-
19Institute CaLS. Performance standards for antimicrobial susceptibility testing. In: 24th informational supplement, M100-S24 2014.
-
20Hogli JU, Garcia BH, Skjold F, Skogen V, Smabrekke L. An audit and feedback intervention study increased adherence to antibiotic prescribing guidelines at a Norwegian hospital. BMC Infect Dis 2016;16:96.
-
21Yardley L, Douglas E, Anthierens S, et al. Evaluation of a web-based intervention to reduce antibiotic prescribing for LRTI in six European countries: quantitative process analysis of the GRACE/INTRO randomised controlled trial. Implement Sci 2013;8:134.
-
22Prior M, Elouafkaoui P, Elders A, et al. Evaluating an audit and feedback intervention for reducing antibiotic prescribing behaviour in general dental practice (the RAPiD trial): a partial factorial cluster randomised trial protocol. Implement Sci. 2014;9:50.
-
23Popovski Z, Mercuri M, Main C, et al. Multifaceted intervention to optimize antibiotic use for intra-abdominal infections. J Antimicrob Chemother 2015;70(4):1226-1229.
-
24Patterson JE, Hardin TC, Kelly CA, Garcia RC, Jorgensen JH. Association of antibiotic utilization measures and control of multiple-drug resistance in Klebsiella pneumoniae. Infect Control Hosp Epidemiol 2000;21(7):455-458.
-
25Cheng VC, Chan JF, Wong SC, et al. Proactive infection control measures to prevent nosocomial transmission of carbapenem-resistant Enterobacteriaceae in a non-endemic area. Chin Med J 2013;126(23):4504-4509.
-
26Bantar C, Sartori B, Vesco E, et al. A hospitalwide intervention program to optimize the quality of antibiotic use: impact on prescribing practice, antibiotic consumption, cost savings, and bacterial resistance. Clin Infect Dis. 2003;37(2):180-186.
-
27Gyssens IC, Blok WL, van den Broek PJ, Hekster YA, van der Meer JW. Implementation of an educational program and an antibiotic order form to optimize quality of antimicrobial drug use in a department of internal medicine. Eur J Clin Microbiol Infect Dis 1997;16(12):904-912.
-
28Dellinger EP, Hausmann SM, Bratzler DW, et al. Hospitals collaborate to decrease surgical site infections. Am J Surg 2005;190(1):9-15.
-
29Kaier K, Frank U, Hagist C, Conrad A, Meyer E. The impact of antimicrobial drug consumption and alcohol-based hand rub use on the emergence and spread of extended-spectrum beta-lactamase-producing strains: a time-series analysis. J Antimicrob Chemother 2009;63(3):609-614.
-
30Chen-rong M, Gui-ting X, Li-jun Z, et al. Practice of improving compliance of hand hygiene of healthcare workers. Chin Nurs Manag 2009;9(6):16-18.
-
31Stone SP, Fuller C, Savage J, et al. Evaluation of the national Cleanyourhands campaign to reduce Staphylococcus aureus bacteraemia and Clostridium difficile infection in hospitals in England and Wales by improved hand hygiene: four year, prospective, ecological, interrupted time series study. BMJ (Clin Res Ed) 2012;344:e3005.
-
32Cheng VC, Tai JW, Lee WM, et al. Infection control preparedness for human infection with influenza A H7N9 in Hong Kong. Infect Control Hosp Epidemiol 2015;36(1):87-92.
-
33Nordmann P, Naas T, Poirel L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 2011;17(10):1791-1798.
-
34Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev 2007;20(3):440-458.
-
35Hackel MA, Badal RE, Bouchillon SK, Biedenbach DJ, Hoban DJ. Resistance rates of intra-abdominal isolates from intensive care units and non-intensive care units in the United States: the study for monitoring antimicrobial resistance trends 2010-2012. Surg Infect (Larchmt) 2015;16(3):298-304.
-
36Sanchez A, Gattarello S, Rello J. New treatment options for infections caused by multiresistant strains of Pseudomonas aeruginosa and other nonfermenting gram-negative bacilli. Semin Respir Crit Care Med 2011;32(2):151-158.
-
37Friedrich LV, White RL, Bosso JA. Impact of use of multiple antimicrobials on changes in susceptibility of gram-negative aerobes. Clin Infect Dis 1999;28(5):1017-1024.
Edited by
Publication Dates
-
Publication in this collection
Jul-Sep 2018
History
-
Received
11 May 2017 -
Accepted
1 Dec 2017