Abstract
Large quantities of kitchen waste are produced in modern society and its disposal poses serious environmental and social problems. The aim of this study was to isolate degradative strains from kitchen waste and to develop a novel and effective microbial agent. One hundred and four strains were isolated from kitchen waste and the 84 dominant strains were used to inoculate protein-, starch-, fat- and cellulose-containing media for detecting their degradability. Twelve dominant strains of various species with high degradability (eight bacteria, one actinomycetes and three fungi) were selected to develop a compound microbial agent "YH" and five strains of these species including H7 (Brevibacterium epidermidis), A3 (Paenibacillus polymyxa), E3 (Aspergillus japonicus), F9 (Aspergillus versicolor) and A5 (Penicillium digitatum), were new for kitchen waste degradation. YH was compared with three commercial microbial agents-"Tiangeng" (TG), "Yilezai" (YLZ) and Effective Microorganisms (EM), by their effects on reduction, maturity and deodorization. The results showed that YH exerted the greatest efficacy on mass loss which decreased about 65.87% after 14 days. The agent inhibited NH3 and H2S emissions significantly during composting process. The concentration of NH3 decreased from 7.1 to 3.2 ppm and that of H2S reduced from 0.7 to 0.2 ppm. Moreover, E4/E6 (Extinction value460nm/Extinction value665nm) of YH decreased from 2.51 to 1.31, which meant YH had an obvious maturity effect. These results highlighted the potential application of YH in composting kitchen waste.
Keywords:
Kitchen waste; Dominant strains; Degradative activity; Deodorization
Introduction
Kitchen waste is the most common type of anthropogenic organic waste and includes many types of discarded food residue. The major chemical components of kitchen waste are starch, protein, fat, cellulose, and others. Due to continuous urbanization and population growth in many countries, the production of kitchen waste has increased annually, and in many districts, its leachate is discharged directly into the sewer system. This represents a waste of environmental resources.11 Jurado M, López MJ, Suárez-Estrella F, Vargas-García MC, López-González JA, Moreno J. Exploiting composting biodiversity: study of the persistent and biotechnologically relevant microorganisms from lignocellulose-based composting. Bioresour Technol. 2014;162:283-293. The deterioration of kitchen waste produces large amounts of toxins and foul odors, such as NH3 and H2S. Ammonia (NH3) has a strong, pungent odor and can cause serious burns to the skin, eyes and respiratory tract.22 Chowdhury MA, de Neergaard A, Jensen LS. Potential of aeration flow rate and bio-char addition to reduce greenhouse gas and ammonia emissions during manure composting. Chemosphere. 2014;97:16-25. Reducing NH3 emission during kitchen waste composting is important for environmental protection and safety. Hydrothion (H2S) is an acidic flammable gas that has an odor reminiscent of rotten eggs and is highly toxic to humans.33 Yuan J, Yang Q, Zhang Z, Li G, Luo W, Zhang D. Use of additive and pretreatment to control odors in municipal kitchen waste during aerobic composting. J Environ Sci. 2015;37:83-90. These gases cause serious water and air pollution. Therefore, the efficient and environmentally responsible disposal of kitchen waste is important.
Because of its high organic matter content, comprehensive nutrient profile and abundant microorganisms, compost enables kitchen waste to be degraded effectively. Using biological composting technology, organic fertilizer can facilitate the safe and non-polluting recycling of nutrient resources. During composting, the strain selection is key for the preparation of effective, complex microbial agents.44 Agyeman FO, Tao W. Anaerobic co-digestion of food waste and dairy manure: effects of food waste particle size and organic loading rate. J Environ Manage. 2014;133:268-274. Optimal efficacy requires the fermenting and culturing of various microorganisms in appropriate proportions to prevent antagonism. During the past forty years, many microbial agents such as EM (University of the Ryukyus in Japan) and ABS-GC (AquaticBioScience Company),55 Yang F, Li GX, Yang QY, Luo WH. Effect of bulking agents on maturity and gaseous emissions during kitchen waste composting. Chemosphere. 2013;93:1393-1399. have been studied and developed for purification of domestic sewage, treatment of industrial wastewater, and degradation of organics and garden waste.66 Alvarezmartin P, Florez A, Hernandezbarranco A, et al. Interaction between dairy yeasts and lactic acid bacteria strains during milk fermentation. Food Control. 2008;19:62-70.,77 Dawkar VV, Jadhav UU, Tamboli DP, et al. Efficient industrial dye decolorization by Bacillus sp. VUS with its enzyme system. Ecotox Environ Safe. 2010;73:1696-1703. EM has been widely used in more than ninety countries in applications including agricultural production, garden waste treatment and soil fertility improvement.
Several studies on substance conversion in kitchen waste compost have been conducted, including those discussing the effect of inoculation on composting88 Kato K, Miura N. Effect of matured compost as a bulking and inoculating agent on the microbial community and maturity of cattle manure compost. Bioresour Technol. 2008;99:3372-3380. and improvement of the agent application method.99 Gaggia F, Baffoni L, Gioia DD, et al. Inoculation with microorganisms of Lolium perenne L.: evaluation of plant growth parameters and endophytic colonization of roots. New Biotechnol. 2013;30:695-704. However, reports on the selection of effective degradative strains from kitchen waste and development of novel microbial agents are rare. For kitchen waste disposal, reduction is the most important aim and the optimal indicator of efficacy. Maturity is a useful indicator of environmental protection.1010 Awasthi MK, Pandey AK, Khan J, Bundela PS, Wong JW, Selvam A. Evaluation of thermophilic fungal consortium for organic municipal solid waste composting. Bioresour Technol. 2014;168:214-221. Deodorization is also significant for surroundings,1111 Jiang T, Schuchardt F, Li GX, Guo R, Luo YM. Gaseous emission during the composting of pig feces from Chinese Ganqinfen system. Chemosphere. 2013;90:1545-1551. due to the emission of byproducts discharged by kitchen waste during storage and composting. Therefore, the objectives of this study were (i) to identify microbial strains that degrade kitchen waste effectively, (ii) to develop a new microbial agent, and (iii) to compare this new agent's efficacy with that of other commercially available agents in treating kitchen waste, in terms of reduction, maturity and deodorization (NH3 and H2S emission mitigation).
Materials and methods
Kitchen waste materials
Kitchen waste was obtained from the dining rooms of Beijing Forestry University (Beijing, China). The initial water content of this waste was 62.6%, due to the waste's high contents of fresh vegetables and meat. The initial pH value was 5.28. Kitchen waste was stored for 3 days prior to the experiment, during which period microorganisms were fermenting the nutrients, producing some organic acids, such as acetic acid and butyric acid. Therefore, the pH value of the kitchen waste was mostly lower than 7.
The proportions (wet mass) of the main components were as follows: staple food, 27.9%; fruits and vegetables, 31.5%; bone and eggshell, 9.1%; meat, 0.8%; peels, 28.2%; and shells and pits, 2.5%. The bone and eggshell composition of the Kitchen waste materials was relatively high. CaCO3 the major component of bone and eggshells, can react with acidic materials to adjust the pH value. When the concentration of H+ in the system reached a certain level, the carbonate adsorbed the H+, causing the pH value to trend increasingly toward neutral, which was beneficial for microbial growth.1212 von Willert FJ, Stehouwer RC. Compost and calcium surface treatment effects on subsoil chemistry in acidic minespoil columns. J Environ Qual. 2003;32:781-788.
Strain isolation and identification
Ten gram samples of fresh kitchen waste were weighed and placed into flasks filled with 90 mL sterile water, maintained at 30 °C and shaken at 160 r/min for 30 min. The supernatant obtained was diluted with sterile water and a 10-fold series of dilutions (10-1-10-6) was carried out. Then, microbial strains were isolated by spreading the dilutions onto agar plates. The agar plates used were LB medium (tryptone 1%, yeast power 0.5%, NaCl 0.5%, agar 15%, H2O 1000 mL) and PDA medium (potato 20%, sucrose 2%, H2O 1000 mL, pH 6.0-7.0) (w/v).
Microbial strains were identified using Bergey's Manual of Systematic Bacteriology (Ninth Edition) and the Fungal Identification Manual by Jingchao Wei (1979.9).
Molecular identification of microbes was achieved by means of DNA sequencing. DNA was extracted from bacteria using the TIANamp DNA Kit (Tiangen) and from fungi using a fungal DNA kit (Omega). Then, PCR amplification of the 16S rRNA gene (bacteria and actinomycetes) and 5.8S-ITS region (fungi) sequences was performed and the products were subjected to purification and sequencing.11 Jurado M, López MJ, Suárez-Estrella F, Vargas-García MC, López-González JA, Moreno J. Exploiting composting biodiversity: study of the persistent and biotechnologically relevant microorganisms from lignocellulose-based composting. Bioresour Technol. 2014;162:283-293. Partial or nearly full-length nucleotide sequences were compared by BLAST search (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Closely related strains were retrieved from NCBI for further analysis.
Screening of strains with high degradative activity
The dominant strains were inoculated into starch-, fat-, protein- and cellulose-containing media. The starch medium (1000 mL) comprised soluble starch 2%, NaCl 0.5%, peptone 0.5%, and agar 2%; the fat medium (1000 mL) comprised peptone 1%, NaCl 1%, CaCl2·7H2O 0.01%, Tween-80 1%, and agar 2%, pH 7.4-7.8; the protein medium (1000 mL) comprised nonfat dried milk 5% and agar 1.8%; and the cellulose medium (1000 mL) comprised K2HPO4 0.05%, microcrystalline cellulose 0.188%, MgSO4 0.025%, gelatin 0.2%, Congo red 0.02%, and agar 1.4% (w/v).1313 Chen TS. Manufacture and Application of Microbial Mediums. Beijing, China: China Agriculture Press; 1999. The presence of "clearance zones" surrounding the colonies was taken to indicate degradative activity. Moreover, iodine was added to the starch medium and neutral red dye to the fat medium to increase the color contrast. In triplicate, we measured the degradative activity and subsequently calculated the mean. Strains with the greatest degradative activity were selected to comprise the new agent named YH (abbreviation from its Chinese name "Yuanhui").
Optimization of fermentation conditions
The selected strains (1%, v/v) were each inoculated in mixed-fermenting medium (potato 20%, sucrose 1%, glucose 1%, tryptone 0.3%, yeast power 0.3%, H2O 1000 mL, pH 6.0-7.0) (w/v). An orthogonal experiment of three factors and three levels was designed, and the response surface methodology was applied to optimize the reaction parameters for the fermenting strains with the kitchen waste. The optimum conditions for microbial strain preparation were as follows: 35 °C, intermittent oscillation, and culture for 108 h. According to the optimum conditions for strain fermentation, the mixed germ solution was compounded with sterile wheat bran (1 mL/10 g) and kept for 24 h at 35 °C to make up the microbial agent, YH. The optimum conditions for kitchen waste disposal were 45 °C temperature, 6% microbial agent (magent × 100%/mwaste, where "m" is "mass", as below) and 30% sawdust (msawdust × 100%/mwaste, diameter: 0.30-0.42 mm) (e.g., 100 g waste was mixed with 6 g agent and 30 g sawdust). These results have been published previously.1414 Tang H, Xu R, Wang XL, Cao AX, Zhao GZ, Zhou CB. Optimization of the new technology of microbial agents using response surface methodology. Chin J Environ Eng. 2015;9:102-107. YH has been produced and marketed as a dry granular agent under the same conditions used in the present study, facilitating production and transportation.
Efficacy of YH agent
The new compound microbial agent (YH) was compared with three known commercially available agents in terms of reduction, maturity and deodorization. The three commercial agents were Taiwan "Tiangeng" (TG) (Fugeng Ecological Co., Ltd.), Hangzhou "Yilezai" (YLZ) (Lvjian Agricultural Leisure Co., Ltd.) and the Japanese EM Inoculant (Beijing Kangyuanlvzhou Biological Technology Co., Ltd.).
Kitchen waste was homogenized in a blender, and 150 g portions were transferred to conical flasks.1515 Wei L, Shutao W, Jin Z, Tong X. Biochar influences the microbial community structure during tomato stalk composting with chicken manure. Bioresour Technol. 2014;154:148-154. Each microbial agent (5 g) was placed into 200 mL PDA liquid medium and cultured for 48 h under optimal conditions. The microbial suspension (10 mL) was inoculated into conical flasks containing 150 g kitchen waste for the experimental groups; 10 mL PDA liquid medium were used as the negative control. Each sample was placed in an incubator at 45 °C with 140 r/min shaking. The mean values of three replicates per group were analyzed.
-
Reduction: the samples were weighed every 24 h for 14 days to determine loss of mass. The mass at day "n" was presented as "m n" (i.e., the mass at day 3 was "m 3") and the rate of mass reduction from 0 to 3 days was ((m0 - m3) × 100%/m0).
-
Maturity: E4/E6 is the ratio of the composting humic acid absorbance at wavelength 460 nm to that at 665 nm. The change in humification E4/E6 can be monitored to evaluate the maturity of the compost. The control group and experimental groups were prepared using the above method. Samples (5 g) were removed every 48 h, and deionized water was added to the samples at a 9:1 (v/m) ratio. Supernatant was removed after 1 h of incubation in ambient temperature with 200 r/min horizontal oscillation. The E4 and E6 values were measured using a 751 ultraviolet spectrophotometer (Shanghai Shunyuhengping Technology Co., Ltd.) for 14 days.
-
Deodorization: The mitigation of emission of NH3 and H2S was determined in the kitchen waste treatment. The control and experimental groups were prepared using the above method. Samples (10 g) were removed from conical flasks and diluted two-fold with sterile water. An NH3 and H2S detector (GC 310 gas detector) (Shenzhen Yuanzhiheng Technology Ltd.) was used to continuously test samples for 14 days.1616 Zhang H, Schuchardt F, Li G, Yang J, Yang Q. Emission of volatile sulfur compounds during composting of municipal solid waste (MSW). Waste Manage. 2013;33:957-963.
Results and discussion
Strain identification and screening
One hundred and four microbial strains (63 bacteria, 14 actinomycetes and 27 fungi) were screened from kitchen waste (Appendix-Supplementary material, Table A Appendix A Supplementary data Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bjm.2016.12.011. ). Morphological and molecular identification indicated the presence of 5 genera and 12 species of bacteria, 2 genera and 3 species of actinomycetes, and 2 genera and 5 species of fungi. Among them, 84 strains of 14 species were dominant and accounted for 81% of the total strains isolated.
Bacteria were the predominant community because of their small volume and large specific surface area, which enables rapid absorption of soluble substrates. The genus Bacillus exhibited the greatest number of species and strains. Members of this genus form endospores tolerant to high temperatures, corrosion and detrimental environmental parameters.1717 Bosma EF, van de Weijer AH, Daas MJ, van der Oost J, de Vos WM, van Kranenburg R. Isolation and screening of thermophilic Bacilli from compost for electrotransformation and fermentation: characterization of Bacillus smithii ET 138 as a new biocatalyst. Appl Environ Microbiol. 2015;81:1874-1883. They can grow and reproduce even at 80 °C and are important microorganisms in the composting process. The actinomycetes solely comprised the genera Brevibacterium and Streptomyces, which are commonly found in compost. Brevibacterium epidermidis (six strains) was the dominant species, likely because of its tolerance to high temperatures and ability to metabolize proteins into amino acids.1818 Liu C, Sheng J, Chen L, et al. Biocontrol Activity of Bacillus subtilis isolated from Agaricus bisporus mushroom compost against pathogenic fungi. J Agric Food Chem. 2015;63:6009-6018. Abundant fungi were detected, including the genera Aspergillus and Phlebia. Extracellular enzymes produced by fungi mediate the biodegradation and transformation of organic compounds.1919 Urbanová M, Šnajdr J, Baldrian P. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol Biochem. 2015;84:53-64. Moreover, fungi such as Aspergillus spp. and Phlebia spp. can produce spores, which are resistant to high temperatures.
However, Aspergillus flavus produces aflatoxin, a compound potentially harmful to the environment and human health2020 Naseer R, Sultana B, Khan M, Naseer D, Nigam P. Utilization of waste fruit-peels to inhibit aflatoxins synthesis by Aspergillus flavus: a biotreatment of rice for safer storage. Bioresour Technol. 2014;172:423-428. and Streptomyces somaliensis produces several secondary metabolites (i.e., streptomycin), which inhibit the growth of other microorganisms.2121 Li P, Li J, Guo Z, et al. An efficient blue-white screening based gene inactivation system for Streptomyces. Appl Microbiol Biotechnol. 2015;99:1923-1933. These two species were excluded from further study.
Examination of degradative activity
The majority of species were able to degrade protein, starch, fat, or cellulose after 48 h of culture. The diameters (d) of the clearance zones around colonies were measured to assess the degradative activity of the strains.
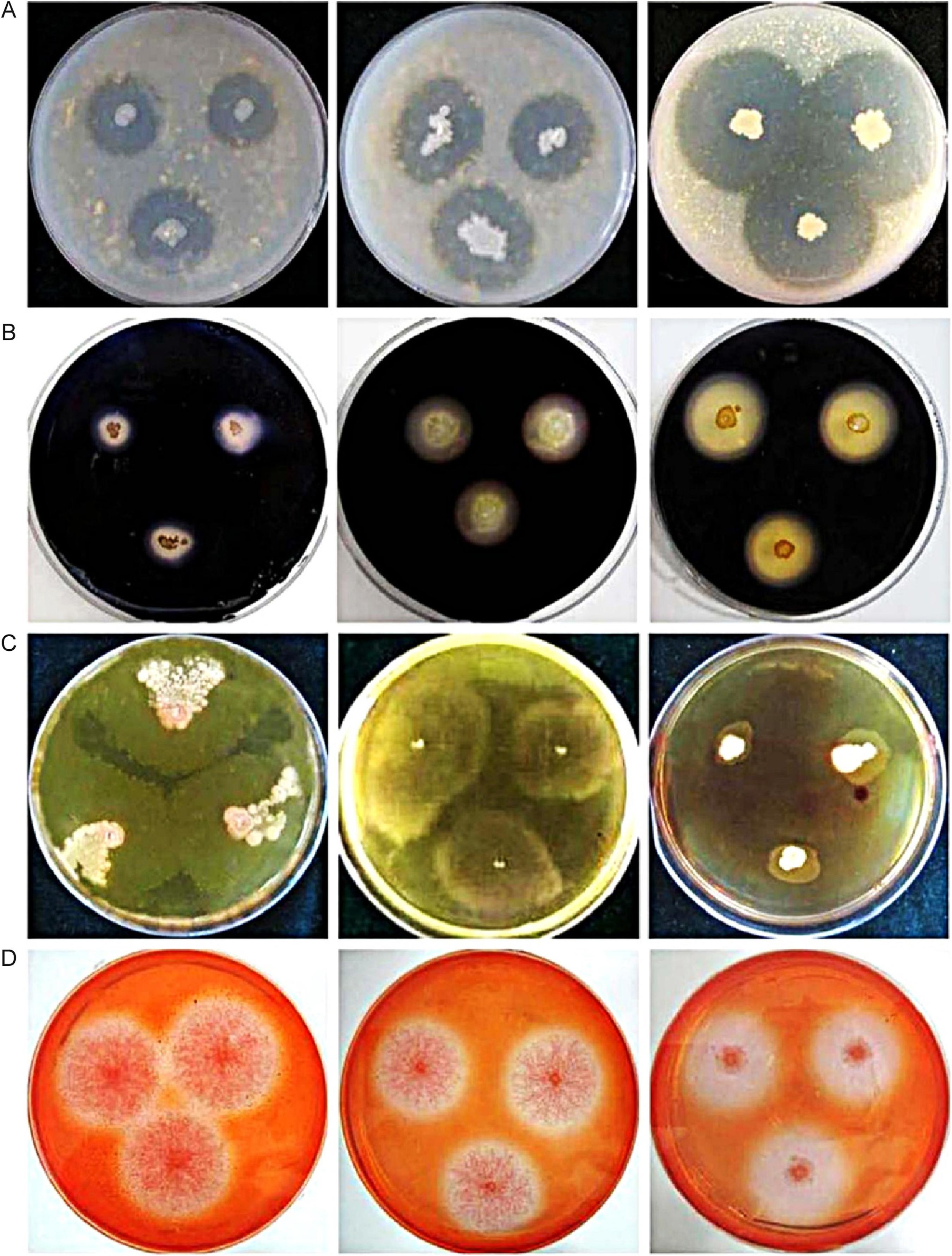
Our results indicated that all bacteria were capable of degrading protein. The minimum clearance zone diameter was 9 mm (Bacillus amyloliquefaciens, D6) and the maximum was 34 mm (Lysinibacillus xylanilyticus, I3). Strains D2 (Bacillus licheniformis, d = 22 mm), I4 (Bacillus pseudomycoides, d = 26 mm) and I3 (L. xylanilyticus, d = 34 mm) were selected for inclusion in YH (Fig. 1A).
Degradation characteristics of 12 microbial strains. (A) Proteolysis, left to right: D2 (Bacillus licheniformis, d = 22 mm), I4 (Bacillus pseudomycoides, d = 26 mm), and I3 (Lysinibacillus xylanilyticus, d = 34 mm). (B) Amylolysis, left to right: A3 (Paenibacillus polymyxa, d = 12 mm), J2 (Bacillus cereus, d = 16 mm), and D6 (Bacillus amyloliquefaciens, d = 23 mm). (C) Lipolysis, left to right: H7 (Brevibacterium epidermidis), J2 (Bacillus cereus), and F1 (Paenibacillus jamilae). (D) Firinolysis, left to right: A5 (Phlebia acanthocystis, d = 23 mm), E3 (Aspergillus japonicus, d = 25 mm), and F9 (Aspergillus versicolor, d = 28 mm).
These three strains grow and reproduce rapidly while degrading protein. Some study showed that efficient protein degradation mainly contributed to nitrogen circulation in compost systems and promoted compost fermentation.2222 Lim JW, Chiam JA, Wang JY. Microbial community structure reveals how microaeration improves fermentation during anaerobic co-digestion of brown water and food waste. Bioresour Technol. 2014;171:132-138.
Iodine was added to starch medium to identify amylolytic strains. Carbohydrates are required for cell growth and metabolism. Microorganisms were inoculated into starch media and transformed starch into carbohydrates of low molecular mass. Five bacterial strains had high starch degradative activity and top three were A3 (Paenibacillus polymyxa, d = 12 mm), J2 (Bacillus cereus, d = 16 mm) and D6 (B. amyloliquefaciens, d = 23 mm) (Fig. 1B).
Only four bacterial strains exhibited clearance zones on fat-containing medium. Because of the presence of Tween-80 (sorbitan monooleate polyoxyethylene) and the low agar concentration (9 g/L), the fat-containing medium was soft and the colony shapes were irregular, making measurement difficult. Addition of neutral red dye to the fat medium resulted in dark orange clearance zones, which were easily measured. The strains with the highest fat-degradative activity were H7 (B. epidermidis), J2 (B. cereus) and F1 (Paenibacillus jamilae) (Fig. 1C). The other strain was F2 (Bacillus subtilis).
Degradation of cellulose-containing medium (microcrystalline cellulose as the only carbon source) was easily detected. The medium contained indicator-Congo red dye, which was decomposed by microorganisms forming transparent clearance zones around the colonies. Three fungal and five bacterial strains had considerable clearance zones. The three fungi exhibited significantly superior cellulose degradation compared with the five bacteria. They were A5 (Phlebia acanthocystis, d = 23 mm), E3 (Aspergillus japonicus, d = 25 mm) and F9 (Aspergillus versicolor, d = 28 mm) (Fig. 1D). Some studies also proved that many saprophytic fungi, such as Phlebia spp. and Neurospora spp., had high cellulose-degradative activity.2323 Hildén K, Martinez AT, Hatakka A, Lundell T. The two manganese peroxidases Pr-MnP2 and Pr-MnP3 of Phlebia radiata, a lignin-degradative basidiomycete, are phylogenetically and structurally divergent. Fungal Genet Biol. 2005;42:403-419.,2424 Morgenstern I, Powlowski J, Tsang A. Fungal cellulose degradation by oxidative enzymes: from dysfunctional GH61 family to powerful lytic polysaccharide monooxygenase family. Brief Funct Genomics. 2014;13:471-481. Otherwise, F2 (B. subtilis) had greatest degradability in 5 bacterial strains and this species has been previously reported to use in kitchen waste degradation by its high temperature tolerance.2525 Nair J, Okamitsu K. Microbial inoculants for small scale composting of putrescible kitchen wastes. Waste Manage. 2010;30:977-982. In this study, due to its distinguished fat- and cellulose-degradabilities, F2 was added in YH agent.
Twelve dominant strains with high degradative activity (8 bacteria, 1 actinomycetes and 3 fungi) were chosen to be included in YH agent (Tables 1 and 2). Among them, D2 (B. licheniformis), F2 (B. subtilis), J2 (B. cereus) and I4 (B. pseudomycoides) have been reported to have high waste-degradative activities.2626 Gopinath KP, Sahib HAM, Muthukumar K, Velan M. Improved biodegradation of Congo Red by using Bacillus sp.. Bioresour Technol. 2009;100:670-675.
Bacillus species can interact with each other and form a stable micro-ecology system to improve the degradation rate of organic matters, increasing the maturity of kitchen waste and reducing toxin products of the reaction.2727 Tuo B, Yan J, Fan B, Yang Z, Liu J. Biodegradation characteristics and bioaugmentation potential of a novel quinoline-degrading strain of Bacillus sp. isolated from petroleum-contaminated soil. Bioresour Technol. 2012;107:55-60. Strains I3 (L. xylanilyticus), F1 (P. jamilae) and D6 (B. amyloliquefaciens) were specific to waste degradation. The morphology and function of Lysinibacillus and Paenibacillus were similar to those of Bacillus. They can produce abundant spores, which was very significant for compost fermentation.2525 Nair J, Okamitsu K. Microbial inoculants for small scale composting of putrescible kitchen wastes. Waste Manage. 2010;30:977-982.B. amyloliquefaciens can produce amylase and specifically degrades starch to small molecule carbohydrate. It has been shown to be a benign, ambient plant root-colonizing bacteria. B. amyloliquefaciens has been developed as a biofertilizer and biocontrol agents via genomics and genetic engineering techniques.2828 Chowdhury SP, Hartmann A, Gao XW, Borriss R. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42-a review. Front Microbiol. 2015;6:780. To the best of our knowledge, this was the first report to use strains H7 (B. epidermidis), A3 (P. polymyxa), E3 (A. japonicus), A5 (P. acanthocystis) and F9 (A. versicolor) in a kitchen waste degradation agent.2929 Jin M, Wang XW, Gong TS, et al. A novel membrane bioreactor enhanced by effective microorganisms for the treatment of domestic wastewater. Appl Microbiol Biotechnol. 2005;69:229-235.
B. epidermidis (H7) has been reported to have considerable tolerance to salt and high temperature.3030 Siddikee MA, Chauhan PS, Anandham R, Han GH, Sa T. Isolation, characterization, and use for plant growth promotion under salt stress, of ACC deaminase-producing halotolerant bacteria derived from coastal soil. J Microbiol Biotechnol. 2010;20:1577-1584. It is a powerful decomposer of organics containing benzene under conditions of weak alkalinity, and the optimum pH value of this species' living environment is about 7.5.3131 Forquin-Gomez MP, Weimer BC. Brevibacterium. In: Carl A, Batt, eds. Encyclopedia of Food Microbiology. 2nd ed. Ithaca, NY, USA: Cornell University; 2014:324–330.32 Zhang et al. showed that P. polymyxa (A3) had been widely used for plant disease control to inhibit pathogenic bacteria and reduce the toxicity of compost.3232 Zhang B, Zhang L, Zhang S, Shi H, Cai W. The influence of pH on hydrolysis and acidogenesis of kitchen wastes in two-phase anaerobic digestion. Environ Technol. 2005;26:329-340. Three strains of fungi (E3, F9 and A5) were commonly used in cellulose degradation to promote the carbon cycle.3333 van Kuijk S, Sonnenberg A, Baars J, Hendriks W, Cone J. Fungal treated lignocellulosic biomass as ruminant feed ingredient: a review. Biotechnol Adv. 2015;33:191-202.A. japonicus (E3) and A. versicolor (F9) were widely used in garden waste composting and their hyphae could penetrate lignocellulose to decompose woody structures. P. acanthocystis (A5), a kind of white rot fungi, was of significant importance in degrading organics. These strains did not antagonize each other in mixed cultures and the basic characteristics of the 12 strains are shown in Table 1. The degradative activities of the strains are shown in Table 2.
Effect of microbial agent "YH"
Reduction
Reduction of kitchen waste mass is the most important indicator of efficacy.3434 Selvam A, Xu SY, Gu XY, Wong JW. Food waste decomposition in leached reactor: role of neutralizing solutions on the leachate quality. Bioresour Technol. 2010;101:1707-1714. In this study, the initial masses of CK and the 4 other experimental groups ranged from 127.6 to 140.5 g. The trends in mass reduction of CK, YH, TG, YLZ, and EM groups had an initial decline (0-4 d) followed by a gradual stabilization during days 4-14 (Fig. 2A). In the initial stage of fermentation, abundant nutrients and vigorous microbial metabolism resulted in a rapid mass loss. With the composting process, the readily decomposable organic matter was consumed by microorganisms, resulting in exhaustion of the available carbon and nitrogen sources. The remaining mass was more difficult to decompose.3535 Demirer GN, Chen S. Anaerobic biogasification of undiluted dairy manure in leaching bed reactors. Waste Manage. 2008;28:112-119. Meanwhile, due to the constant temperature (45 °C) of the fermentation system, microbial physiology and proliferation were relatively stable. This led to the loss of mass rate of the whole system slowing gradually. After 6 days, the O2 concentration decreased due to its conversion into CO2 by microbial respiration. Exhaustion of readily decomposable organic material resulted in microbial use of less-readily degradable material, particularly organic acids,3636 Khalid A, Arshad M, Anjum M, Mahmood T, Dawson L. The anaerobic digestion of solid organic waste. Waste Manage. 2011;31:1737-1744. which decreased further the rate of degradation by suppressing microbial reproduction and metabolism. As a result, the masses of the CK, YH, TG, YLZ and EM groups at day 14 were 75.87, 45.72, 49.2, 53.91 and 55.1 g, representing mass losses of 41.95%, 65.87%, 61.44%, 59.65% and 60.77%, respectively (Fig. 2A).
Effects of the microbial agents. (A) Composting mass. (B) E 4/E 6 values. (C) NH3 emission. (D) H2S emission. The experimental period was 14 days.
Microbial metabolic activity was the primary reason for kitchen waste mass loss. Microbes decomposed organic components into micro-molecular organics such as glucose and produced and emitted large amounts of CO2.3737 Zhang C, Xiao G, Peng L, Su H, Tan T. The anaerobic co-digestion of food waste and cattle manure. Bioresour Technol. 2013;129:170-176. With fermentation reactions, water evaporation also caused mass reduction. The gases emitted were important components of the natural atmosphere and were harmless to the environment. Reductions in mass in all experimental agent groups were significantly greater than that in CK (Fig. 2A). This result strongly supports the suggestion that the addition of agents with abundant microorganisms to compost effectively promotes the composting process, especially for mass loss. Addition of YH resulted in the greatest reduction in mass and was 23.92% greater than CK, followed by TG, YLZ and EM groups. This indicates that YH had a considerable effect on reduction for kitchen waste degradation.
Maturity
E4/E6 is a simple, direct and commonly used index of maturity. During composting, the accumulation of high molecular weight humic acid reduced the E 4/E 6 values. Measurement of water absorption by fertilizers can be used to assess humification.2525 Nair J, Okamitsu K. Microbial inoculants for small scale composting of putrescible kitchen wastes. Waste Manage. 2010;30:977-982.E 4 and E 6 values were determined every 48 h in this study. The initial E 4/E 6 value of kitchen waste without addition of agent was 2.4. In all five groups, the E 4/E 6 values first increased and then decreased (Fig. 2B). With waste composting, high-molecular-weight humus was accumulated continuously, leading to fully mature compost. During the initial stage, composting organic matter such as starch, protein, and oil were decomposed into small molecules by microorganisms, resulting in a gradual increase in the E 4/E 6 value. The E 4/E 6 values peaked in CK, YH, TG, YLZ, and EM at day 4 and were 2.78, 2.51, 2.67, 2.49, 2.71, respectively, then the values declined. Through the physiological metabolism of microorganisms, the composited macromolecular substances gradually formed as the more complex matters (e.g., humic acid) and considerable quantities of ammonia gas (NH3) emission resulted in the formation of compact and solid compost. This type of compost was fully degraded, resulting in a lower E 4/E 6 value.3838 Zhai N, Zhang T, Yin D, et al. Effect of initial pH on anaerobic co-digestion of kitchen waste and cow manure. Waste Manage. 2015;38:126-131.
The E 4/E 6 values of those groups were detected at different time points in this study. The values of YH, TG and YLZ were 1.31, 1.23 and 1.15 at day 14, respectively, and they were significantly lower (higher maturity) than the group of CK (1.76) and EM (1.7) (Fig. 2B). Though E 4/E 6 values of YH were a little higher (lower maturity) than TG and YLZ, It was effective on maturity and reached a requirement for composting kitchen waste. Then, more work on screening strains might to further improve maturity of YH.
Deodorization
(1) Ammonia emission: At days 0-4, NH3 emission increased rapidly and peaked at 10.2, 7.1, 4.5, 9.1 and 8.1 ppm for CK, YH, TG, YLZ and EM, respectively (Fig. 2C). Microbial consumption of protein and other substances in kitchen waste, which involved hydrolysis of ammonium to NH3, resulted in an increase in the NH3 concentration.2222 Lim JW, Chiam JA, Wang JY. Microbial community structure reveals how microaeration improves fermentation during anaerobic co-digestion of brown water and food waste. Bioresour Technol. 2014;171:132-138.,3939 Jiang T, Li G, Tang Q, Ma X, Wang G, Schuchardt F. Effects of aeration method and aeration rate on greenhouse gas emissions during composting of pig feces in pilot scale. J Environ Sci. 2015;31:124-132. Following the peak, the NH3 emission decreased and then gradually stabilized in all groups (Fig. 2C), which was in accordance with previously published studies.33 Yuan J, Yang Q, Zhang Z, Li G, Luo W, Zhang D. Use of additive and pretreatment to control odors in municipal kitchen waste during aerobic composting. J Environ Sci. 2015;37:83-90. This effect was likely mediated by promotion of the nitrogen cycle, which inhibited the production of NH3. The metabolic capacity of ammonia-producing strains subsequently decreased gradually, and available nitrogen was exhausted. Much of the nitrogen organic matter was solidified. Thus, the structure of the kitchen waste compost became denser. Microbial metabolism results in production of water, which reduces the free air space and NH3 emission by 30-70%.4040 El Kader NA, Robin P, Paillat JM, Leterme P. Turning, compacting and the addition of water as factors affecting gaseous emissions in farm manure composting. Bioresour Technol. 2007;98:2619-2628.
The NH3 concentrations of the YH and TG groups were significantly lower than those of the CK, YLZ and EM groups. Therefore, application of YH and TG resulted in marked inhibition of NH3 emission.
(2) Hydrothion emission: Throughout the composting process, H2S emission first increased rapidly and then decreased, similar to NH3 emission (Fig. 2D). During the first 4 days, H2S emission increased rapidly because of the active metabolism of the microorganisms, specifically decomposing protein and fat. At day 4, CK, TG, YLZ and EM had maximum H2S emission (1.1, 0.6, 0.9 and 0.9 ppm, respectively), and at day 5, YH reached an emission peak (0.7 ppm). H2S is a highly reactive gas that can be both reduced and oxidized. The H2S produced in the system combined with small inorganic molecules, accelerating the reduction in mass and enhancing the maturity of kitchen waste, which closely corresponded to the data in Fig. 2A. At days 4-10, H2S emission showed a decreasing trend in all groups. On the one hand, microbial metabolism weakened gradually during the composting process. On the other hand, organic macromolecules in kitchen waste were gradually broken down to the less readily decomposable humic acid, resulting in reduced H2S emission.1515 Wei L, Shutao W, Jin Z, Tong X. Biochar influences the microbial community structure during tomato stalk composting with chicken manure. Bioresour Technol. 2014;154:148-154. After 10 days, microbial metabolism almost stopped and H2S emission decreased to negligible levels.
Therefore, addition of microbial agents reduced H2S emission, of which YH and TG exerted the greatest effects. YH is comprised of strains that can degrade H2S and produce more stable sulfur-containing compounds during composting, leading to reduced H2S emission.4040 El Kader NA, Robin P, Paillat JM, Leterme P. Turning, compacting and the addition of water as factors affecting gaseous emissions in farm manure composting. Bioresour Technol. 2007;98:2619-2628.
Conclusions
The microbial consortium containing 12 selected dominant strains were used in degradation of kitchen waste, which processed the resource waste efficiently and environmentally. The novel agent YH included five strains used for the first time in the degradation of kitchen waste and seven strains with known waste-degradative activity. YH had the great effects in terms of reduction and E 4/E 6 values, and significantly decreased NH3 and H2S emissions, which provided the benefit of converting waste residues into organic fertilizer.
Acknowledgements
We thank at least two professional editors from TEXTCHECK (http://www.textcheck.com/certificate/2×3rxv), for their English improvement to the paper. This work was financially supported by the Fundamental Research Funds for the Central Universities at Beijing Forestry University (TD2012-03), and the National Natural Science Foundation of China (Nos. 31570019, 41601513).
Appendix A Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bjm.2016.12.011.
References
-
1Jurado M, López MJ, Suárez-Estrella F, Vargas-García MC, López-González JA, Moreno J. Exploiting composting biodiversity: study of the persistent and biotechnologically relevant microorganisms from lignocellulose-based composting. Bioresour Technol 2014;162:283-293.
-
2Chowdhury MA, de Neergaard A, Jensen LS. Potential of aeration flow rate and bio-char addition to reduce greenhouse gas and ammonia emissions during manure composting. Chemosphere 2014;97:16-25.
-
3Yuan J, Yang Q, Zhang Z, Li G, Luo W, Zhang D. Use of additive and pretreatment to control odors in municipal kitchen waste during aerobic composting. J Environ Sci 2015;37:83-90.
-
4Agyeman FO, Tao W. Anaerobic co-digestion of food waste and dairy manure: effects of food waste particle size and organic loading rate. J Environ Manage. 2014;133:268-274.
-
5Yang F, Li GX, Yang QY, Luo WH. Effect of bulking agents on maturity and gaseous emissions during kitchen waste composting. Chemosphere 2013;93:1393-1399.
-
6Alvarezmartin P, Florez A, Hernandezbarranco A, et al. Interaction between dairy yeasts and lactic acid bacteria strains during milk fermentation. Food Control. 2008;19:62-70.
-
7Dawkar VV, Jadhav UU, Tamboli DP, et al. Efficient industrial dye decolorization by Bacillus sp. VUS with its enzyme system. Ecotox Environ Safe 2010;73:1696-1703.
-
8Kato K, Miura N. Effect of matured compost as a bulking and inoculating agent on the microbial community and maturity of cattle manure compost. Bioresour Technol. 2008;99:3372-3380.
-
9Gaggia F, Baffoni L, Gioia DD, et al. Inoculation with microorganisms of Lolium perenne L.: evaluation of plant growth parameters and endophytic colonization of roots. New Biotechnol. 2013;30:695-704.
-
10Awasthi MK, Pandey AK, Khan J, Bundela PS, Wong JW, Selvam A. Evaluation of thermophilic fungal consortium for organic municipal solid waste composting. Bioresour Technol. 2014;168:214-221.
-
11Jiang T, Schuchardt F, Li GX, Guo R, Luo YM. Gaseous emission during the composting of pig feces from Chinese Ganqinfen system. Chemosphere 2013;90:1545-1551.
-
12von Willert FJ, Stehouwer RC. Compost and calcium surface treatment effects on subsoil chemistry in acidic minespoil columns. J Environ Qual. 2003;32:781-788.
-
13Chen TS. Manufacture and Application of Microbial Mediums Beijing, China: China Agriculture Press; 1999.
-
14Tang H, Xu R, Wang XL, Cao AX, Zhao GZ, Zhou CB. Optimization of the new technology of microbial agents using response surface methodology. Chin J Environ Eng. 2015;9:102-107.
-
15Wei L, Shutao W, Jin Z, Tong X. Biochar influences the microbial community structure during tomato stalk composting with chicken manure. Bioresour Technol. 2014;154:148-154.
-
16Zhang H, Schuchardt F, Li G, Yang J, Yang Q. Emission of volatile sulfur compounds during composting of municipal solid waste (MSW). Waste Manage. 2013;33:957-963.
-
17Bosma EF, van de Weijer AH, Daas MJ, van der Oost J, de Vos WM, van Kranenburg R. Isolation and screening of thermophilic Bacilli from compost for electrotransformation and fermentation: characterization of Bacillus smithii ET 138 as a new biocatalyst. Appl Environ Microbiol. 2015;81:1874-1883.
-
18Liu C, Sheng J, Chen L, et al. Biocontrol Activity of Bacillus subtilis isolated from Agaricus bisporus mushroom compost against pathogenic fungi. J Agric Food Chem. 2015;63:6009-6018.
-
19Urbanová M, Šnajdr J, Baldrian P. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol Biochem. 2015;84:53-64.
-
20Naseer R, Sultana B, Khan M, Naseer D, Nigam P. Utilization of waste fruit-peels to inhibit aflatoxins synthesis by Aspergillus flavus: a biotreatment of rice for safer storage. Bioresour Technol. 2014;172:423-428.
-
21Li P, Li J, Guo Z, et al. An efficient blue-white screening based gene inactivation system for Streptomyces. Appl Microbiol Biotechnol. 2015;99:1923-1933.
-
22Lim JW, Chiam JA, Wang JY. Microbial community structure reveals how microaeration improves fermentation during anaerobic co-digestion of brown water and food waste. Bioresour Technol. 2014;171:132-138.
-
23Hildén K, Martinez AT, Hatakka A, Lundell T. The two manganese peroxidases Pr-MnP2 and Pr-MnP3 of Phlebia radiata, a lignin-degradative basidiomycete, are phylogenetically and structurally divergent. Fungal Genet Biol. 2005;42:403-419.
-
24Morgenstern I, Powlowski J, Tsang A. Fungal cellulose degradation by oxidative enzymes: from dysfunctional GH61 family to powerful lytic polysaccharide monooxygenase family. Brief Funct Genomics. 2014;13:471-481.
-
25Nair J, Okamitsu K. Microbial inoculants for small scale composting of putrescible kitchen wastes. Waste Manage 2010;30:977-982.
-
26Gopinath KP, Sahib HAM, Muthukumar K, Velan M. Improved biodegradation of Congo Red by using Bacillus sp.. Bioresour Technol. 2009;100:670-675.
-
27Tuo B, Yan J, Fan B, Yang Z, Liu J. Biodegradation characteristics and bioaugmentation potential of a novel quinoline-degrading strain of Bacillus sp. isolated from petroleum-contaminated soil. Bioresour Technol. 2012;107:55-60.
-
28Chowdhury SP, Hartmann A, Gao XW, Borriss R. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42-a review. Front Microbiol 2015;6:780.
-
29Jin M, Wang XW, Gong TS, et al. A novel membrane bioreactor enhanced by effective microorganisms for the treatment of domestic wastewater. Appl Microbiol Biotechnol 2005;69:229-235.
-
30Siddikee MA, Chauhan PS, Anandham R, Han GH, Sa T. Isolation, characterization, and use for plant growth promotion under salt stress, of ACC deaminase-producing halotolerant bacteria derived from coastal soil. J Microbiol Biotechnol. 2010;20:1577-1584.
-
31Forquin-Gomez MP, Weimer BC. Brevibacterium. In: Carl A, Batt, eds. Encyclopedia of Food Microbiology 2nd ed. Ithaca, NY, USA: Cornell University; 2014:324–330.32
-
32Zhang B, Zhang L, Zhang S, Shi H, Cai W. The influence of pH on hydrolysis and acidogenesis of kitchen wastes in two-phase anaerobic digestion. Environ Technol. 2005;26:329-340.
-
33van Kuijk S, Sonnenberg A, Baars J, Hendriks W, Cone J. Fungal treated lignocellulosic biomass as ruminant feed ingredient: a review. Biotechnol Adv. 2015;33:191-202.
-
34Selvam A, Xu SY, Gu XY, Wong JW. Food waste decomposition in leached reactor: role of neutralizing solutions on the leachate quality. Bioresour Technol. 2010;101:1707-1714.
-
35Demirer GN, Chen S. Anaerobic biogasification of undiluted dairy manure in leaching bed reactors. Waste Manage 2008;28:112-119.
-
36Khalid A, Arshad M, Anjum M, Mahmood T, Dawson L. The anaerobic digestion of solid organic waste. Waste Manage 2011;31:1737-1744.
-
37Zhang C, Xiao G, Peng L, Su H, Tan T. The anaerobic co-digestion of food waste and cattle manure. Bioresour Technol 2013;129:170-176.
-
38Zhai N, Zhang T, Yin D, et al. Effect of initial pH on anaerobic co-digestion of kitchen waste and cow manure. Waste Manage. 2015;38:126-131.
-
39Jiang T, Li G, Tang Q, Ma X, Wang G, Schuchardt F. Effects of aeration method and aeration rate on greenhouse gas emissions during composting of pig feces in pilot scale. J Environ Sci. 2015;31:124-132.
-
40El Kader NA, Robin P, Paillat JM, Leterme P. Turning, compacting and the addition of water as factors affecting gaseous emissions in farm manure composting. Bioresour Technol 2007;98:2619-2628.
Publication Dates
-
Publication in this collection
Jul-Sep 2017
History
-
Received
5 Apr 2016 -
Accepted
26 Dec 2016