Abstract
Pan-drug resistant Gram-negative bacteria, being resistant to most available antibiotics, represent a huge threat to the medical community. Colistin is considered the last therapeutic option for patients in hospital settings. Thus, we were concerned in this study to demonstrate the membrane permeabilizing activity of colistin focusing on investigating its efficiency toward those pan-drug resistant isolates which represent a critical situation. We determined the killing dynamics of colistin against pan-drug resistant isolates. The permeability alteration was confirmed by different techniques as: leakage, electron microscopy and construction of an artificial membrane model; liposomes. Moreover, selectivity of colistin against microbial cells was also elucidated. Colistin was proved to be rapid bactericidal against pan-drug resistant isolates. It interacts with the outer bacterial membrane leading to deformation of its outline, pore formation, leakage of internal contents, cell lysis and finally death. Furthermore, variations in membrane composition of eukaryotic and microbial cells provide a key for colistin selectivity toward bacterial cells. Colistin selectively alters membrane permeability of pan-drug resistant isolates which leads to cell lysis. Colistin was proved to be an efficient last line treatment for pan-drug resistant infections which are hard to treat.
Keywords
Pan-drug resistant; Leakage; Permeability alteration; Colistin; Ultrastructure
Introduction
Recently, it has been witnessed worldwide that Gram-negative bacteria resistant to many classes of antibiotics represents a fearful situation toward the emergence of a future medical disaster.11 Sharma R, Sharma CL, Kapoor B. Antibacterial resistance: current problems and possible solutions. Indian J Med Sci. 2005;59:120-129. There are 2 terms commonly describing those superbugs; which are multi-drug resistant (MDR) and pan-drug resistant (PDR). An isolate is considered MDR if it exhibited resistance toward 5 out of the 7 anti-pseudomonal classes of antimicrobial agents, i.e. anti-pseudomonal penicillins, cephalosporins, carbapenems, monobactams, quinolones, aminoglycosides, and colistin, while it is a PDR if it showed resistance toward all 7 anti-pseudomonal antimicrobial agents, including colistin.22 Falagas ME, Bliziotis IA, Kasiakou SK, et al. Outcome of infections due to pandrug-resistant (PDR) Gram-negative bacteria. BMC Infect Dis. 2005;5:24 There was another view considering that PDR isolates were those resistant to all antibiotics but only susceptible to polymyxins.33 Wang CY, Jerng JS, Chen KY, et al. Pandrug-resistant Pseudomonas aeruginosa among hospitalised patients: clinical features, risk-factors and outcomes. Clin Microbiol Infect. 2006;12:63-68.,44 Marais E, de Jong G, Ferraz V, Maloba B, Duse AG. Interhospital transfer of pan-resistant Acinetobacter strains in Johannesburg, South Africa. Am J Infect Control. 2004;32:278-281. Although there is no apparent definition for the term PDR throughout literature, it generally denotes resistance against a variety of antibiotics excluding polymyxins.55 Falagas ME, Koletsi PK, Bliziotis IA. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J Med Microbiol. 2006;55(Pt 12):1619-1629. Such view is adopted in the present work. In the past few decades there have been a tremendous increase in resistance to currently available antibiotics and a significant decline in development of new ones.66 Li J, Nation RL, Turnidge JD, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6:589-601. This leads to the revival of older agents as polymyxins, for the treatment of such PDR infections.22 Falagas ME, Bliziotis IA, Kasiakou SK, et al. Outcome of infections due to pandrug-resistant (PDR) Gram-negative bacteria. BMC Infect Dis. 2005;5:24
Polymyxins are a group of polypeptide cationic antibiotics that were isolated from Bacillus polymyxa in the 1940s.77 Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis. 2005;40:1333-1341. Since then, polymyxin E (colistin) and polymyxin B were extensively used in clinical practice for Gram-negative organisms.88 Nord NM, Hoeprich PD. Polymyxin B and colistin: a critical comparison. N Engl J Med. 1964;270:1030-1035.,99 Yow EM, Tan E, Shane L, Schonfeld S, Abu-Nassar H. Colistin (coly-mycin) in resistant bacterial infections. A clinical appraisal. Arch Intern Med. 1961;108:664-670. However, they were gradually withdrawn from the market and abandoned during the last two decades due to claimed reports of toxicity. Therefore, during that time, there have been limited studies on the clinical use, pharmacokinetics and pharmacodynamics of colistin.1010 Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int J Antimicrob Agents. 2005;25:11-25. Emergence of the PDR pathogens, necessitated the re-evaluation of polymyxin therapies.1111 Stein A, Raoult D. Colistin: an antimicrobial for the 21st century?. Clin Infect Dis. 2002;35:901-902.
Colistin has been recently considered as last option treatment for patients with nosocomial PDR infections, which have become an important public health issue, owing to its favorable properties of rapid bacterial killing, a narrow spectrum of activity, and slow development of resistance.1212 Falagas ME, Kasiakou SK, Tsiodras S, Michalopoulos A. The use of intravenous and aerosolized polymyxins for the treatment of infections in critically ill patients: a review of the recent literature. Clin Med Res. 2006;4:138-146.,1313 Landman D, Georgescu C, Martin DA, Quale J. Polymyxins revisited. Clin Microbiol Rev. 2008;21:449-465. Colistin interacts electrostatically with the outer membrane of Gram-negative bacteria and competitively displaces divalent cations which stabilize the lipopolysaccharide layer thus disrupting the membrane integrity. It is then subsequently taken up via the self-promoted uptake pathway.1313 Landman D, Georgescu C, Martin DA, Quale J. Polymyxins revisited. Clin Microbiol Rev. 2008;21:449-465. It is believed that colistin forms cracks in the outer membrane which promotes its uptake inside the cell and permits the passage of different molecules.1414 Hancock RE. Peptide antibiotics. Lancet. 1997;349:418-422. Thus, polymyxins produce a disruptive detergent effect, leading to increased permeability in the outer membrane, leakage of the absorbing cytoplasmic contents, cell lysis and finally death.1515 Hermsen ED, Sullivan CJ, Rotschafer JC. Polymyxins: pharmacology, pharmacokinetics, pharmacodynamics, and clinical applications. Infect Dis Clin North Am. 2003;17:545-562. The chemical composition of bacterial membranes being rich in phosphatidylethanolamine and negatively charged lipids allows such electrostatic attraction with cationic peptides in contrast to eukaryotic cells in which cholesterol is the predominant component providing a clue for the selectivity of action toward microbial versus host cells.1616 Glukhov E, Stark M, Burrows LL, Deber CM. Basis for selectivity of cationic antimicrobial peptides for bacterial versus mammalian membranes. J Biol Chem. 2005;280:33960-33967. Such a situation prompted the present microbiological study to investigate the membrane permeability alteration of colistin and it bactericidal effect on PDR Gram-negative clinical isolates including Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae and Escherichia coli.
Materials and methods
Microorganisms and antibiotics
Four clinical bacterial isolates, identified by classical microscopic and biochemical procedures,1717 Finegold SM, Baron EJ. Bailey and Scott’s Diagnostic Microbiology. St. Louis: The C. V. Mosby Company;1986:70–125, 173–201, 355–387, 397–437, 859–914.,1818 Collee JG, Miles RS. Tests for identification of bacteria. In: Collee JG, Duguid JP, Fraser AG, Marmion BP, eds. Mackie and McCartney, Practical Medical Microbiology. New York: ChurchillLivingstone; 1989:141–160. were used in this study. These are: A. baumannii (A182), P. aeruginosa (P103), E. coli (E9) and K. pneumoniae (K103). The identified isolates were maintained by freezing in 15% glycerol broth 1919 Howard DH. The preservation of bacteria by freezing in glycerol broth. J Bacteriol. 1956;71:625 (Oxoid Ltd.; Basingostok; Hampshire, England). Colistin sulphate was obtained as powder from Pharco pharmaceutical Co., Egypt. It was dissolved in water to prepare stock solutions. The susceptibility of the tested isolates, to the different classes of antibiotics was determined by the standard disc agar diffusion technique according to Bauer et al.2020 Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493-496. with some modifications.2121 Andrews JM. BSAC standardized disc susceptibility testing method (version 5). J Antimicrob Chemother. 2006;58:511-529. MIC of colistin was determined by the standard broth dilution technique.2222 Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48(suppl 1):5-16.
Bactericidal activity of colistin using the viable count technique
For each tested isolate, two concentrations of colistin (1/2MIC and MIC) were prepared in sterile nutrient broth. Each concentration was inoculated with overnight culture to give a final inoculum of 106 cfu/mL. A control without antibiotic was prepared for each of the tested isolates. The systems were mixed well and incubated at 37 °C with shaking. Samples were aseptically withdrawn from each test tube at 0, 1, 3, 6 and 24 h and serially diluted with sterile saline. Then, 10 µL portions were dropped onto the surface of overdried nutrient agar plates. The plates were left to dry and incubated inverted at 37 °C for 24 h, the resulting colonies were counted and the original viable count was determined.2323 Barry AL, Craig WA, Nadler H, et al. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline. In: NCCLS document M26-A. PA, USA: NCCLS; 1999
Effect of colistin on the cytoplasmic membrane by leakage technique
Bacterial suspensions of the selected isolates were prepared by streaking an overnight broth culture onto nutrient agar slants (Oxoid Ltd.; Basingostok; Hampshire, England). The slants were incubated at 37 °C for 16–18 h. The resulting growth of 3 slants was resuspended in 5 mL sterile 0.9% saline to produce heavy inoculum (O.D600 adjusted to 2) and transferred into sterile test tubes. The obtained bacterial suspensions were centrifuged at 12,000 × g for 5 min. The formed pellets were washed twice with sterile saline and then were resuspended in 5 mL sterile saline. Aliquots of the prepared bacterial suspension of each isolate were treated with 50 mg/L of colistin. A control was included in each test containing untreated bacterial suspension. Both the treated and untreated bacterial suspensions were incubated at 37 °C for 24 h. After incubation, the bacterial suspensions were centrifuged at 12,000 × g for 5 min. The absorbance of the clear supernatant was estimated at 260 and 280 nm against saline solution using the spectrophotometer (Thermospectronic, Helios alpha, NC 9423UV A 1002E, England).2424 Carson CF, Mee BJ, Riley TV. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob Agents Chemother. 2002;46:1914-1920.
Effect of colistin on the leakage of red blood cells
One milliliter of fresh human blood was centrifuged at 2000 × g for 5 min and the cells were washed 4× with sterile 0.9% saline discarding the supernatant every time. The sedimented red blood cells (RBCs) were resuspended in 1 mL buffer (5 mM sodium phosphate and 150 mM sodium chloride [pH 7.4]). The RBCs suspension in buffer was distributed in sterile eppendorf tubes in 25 µL aliquots and 1 mL of colistin solution dissolved in the same buffer was added to each eppendorf in concentrations ranging from 0.78 mg/L to 100 mg/L. The resulting suspensions were incubated at room temperature for 2 h. The systems were then centrifuged at 2000 × g for 10 min. The release of hemoglobin was monitored by measuring the absorbance of the supernatant at 540 nm by spekol (Carl Zeiss, Jena, Germany). Absorbance of control untreated cells in saline was measured and used as a blank. Total haemolysis was also measured by lysing colistin-untreated RBCs with distilled water.2525 Cirioni O, Ghiselli R, Silvestri C, et al. Efficacy of tachyplesin III, colistin, and imipenem against a multiresistant Pseudomonas aeruginosa strain. Antimicrob Agents Chemother. 2007;51:2005-2010.
Effect of colistin on the ultrastructure of bacterial cells using the transmission electron microscope
Two clinical isolates were used in this study; a sensitive and an induced resistant population for each isolate. The resistance was induced by serial passaging in increasing colistin concentrations. 0.5 mL of overnight broth culture was inoculated in flasks containing 50 mL sterile nutrient broth. Flasks were incubated in the orbital shaking incubator (100 rpm) at 37 °C till reaching acceptable turbidity for about 4–5 h. The selected clinical isolates were treated with 100 mg/L of colistin for 1 h. Proper controls lacking colistin were included for each isolate population. The obtained bacterial suspensions were centrifuged at 6000 × g for 5 min. The supernatants were discarded. The obtained colistin-treated and control bacterial pellets were then resuspended in 3 mL Trump's fixative solution and further processed as previously described.2626 Kim Y, Farrah S, Baney RH. Membrane damage of bacteria by silanols treatment. Electron J Biotechnol. 2007;10:252-259.,2727 Suganuma A. Further studies on the plasma membrane of Staphylococcus aureus. J Cell Biol. 1966;30:208-210. The samples were examined and photographed at an accelerating voltage of 80 kV using Joel CX 100 transmission electron microscope at the Electron Microscope Unit, Faculty of Science, Alexandria University.
Effect of colistin on the cytoplasmic membrane using artificial cytoplasmic membrane model
Negatively-charged unilamellar cholesterol free liposomes were prepared by reverse-phase evaporation method.2828 Szoka FJR, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978;75:4194-4198. The liposomes were then treated by colistin (100 mg/L) for 24 h and were morphologically examined by the phase contrast microscope (Olympus, CX 41 RF) using oil-immersion objective lens (100×) for any damage in their shape following colistin treatment.
Effect of colistin on the formation of spheroplasts
One hundred µL of exponential cell culture, adjusted to have O.D600 of 0.05 were inoculated into 3 mL Müller-Hinton broth containing 0.3 M of sucrose and were mixed well. Then, aliquots of 100 µL were distributed in sterile eppendorf tubes. Ten µL of colistin were added to 3 eppendorfs in concentration of 1/2 MIC. Ten µL of ceftazidime were added to 3 other eppendorfs in concentration of 1/2 MIC. A control eppendorf was included containing 0.9% saline instead of antibiotic solution. The eppendorfs were incubated in the orbital shaking incubator at 37 °C, 100 × g for 90 min. Samples were then taken and examined by the phase contrast microscope using oil-immersion objective lens (100×).
Results and discussion
Globally, there is a growing threat from the emergence of MDR and PDR organisms especially Gram-negative bacteria, such as P. aeruginosa, A. baumannii, Klebsiella and Enterobacter species in hospital settings.2929 Gupta S, Govil D, Kakar PN, et al. Colistin and polymyxin B: a re-emergence. Indian J Crit Care Med. 2009;13:49-53. PDR pathogens represent a fearful clinical situation with tremendous implications. So, this study aimed at investigating the deleterious effects of colistin on those PDR pathogens. The four bacterial isolates used in this study were confirmed to be pan-drug resistant by the antibiotic susceptibility testing being resistant to all antibiotic classes except colistin (data not shown). The MIC of colistin against the tested isolates ranged from 0.625 to 1.25 mg/L as determined by broth dilution method (Table 1). All the tested isolates were sensitive to colistin according to the CLSI resistance breakpoint which is 4 mg/L (Table 1).
The dynamics of killing of colistin was determined by the viable count technique against the tested isolates using ½ MIC and MIC of colistin. Colistin was shown to be a rapid bactericidal agent in a concentration dependent manner in all the tested PDR isolates. Rapid and significant declines (>2 log) in bacterial survival were observed after 3 h in all the tested isolates at 1/2 MIC level reaching almost 6 logs at the MIC level. The rapid bactericidal activity of colistin is related to its permeabilizing action on the cell membrane following self-promoted uptake.3030 Li J, Turnidge J, Milne R, Nation RL, Coulthard K. In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob Agents Chemother. 2001;45:781-785. However, re-growth was observed as early as 6 h and substantial re-growth occurred at 24 h in all the tested PDR clinical isolates at 1/2MIC while only 2 isolates showed re-growth at the MIC level (Fig. 1). This might be caused by the heteroresistant subpopulations which grow probably at a slower rate than the sensitive subpopulation and hence, temporary inhibition could be mistaken. This phenomenon is of potential risk as it can lead to therapy failure if colistin monotherapy is used.3131 Li J, Rayner CR, Nation RL, et al. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2006;50:2946-2950.
Bactericidal activity of colistin against PDR isolates. (A) A. baumannii (A182), (B) P. aeruginosa (P103), (C) K. pneumonie (K103) and (D) E. coli (E9). Filled circles represent the control untreated cells, filled squares represent 1/2 MIC of colistin, filled triangles represent MIC of colistin.
A leakage study was conducted to confirm the membrane permeability alteration effect of colistin. The effect of 50 mg/L of colistin on the cytoplasmic membrane against 4 representative PDR clinical isolates was determined spectrophotometrically. The difference between the values of absorbance at 260 and 280 nm of the supernatant of both the treated cells and the untreated cells corresponding to the net leakage due to treatment is illustrated in Fig. 2.
Leakage of absorbing materials from PDR clinical isolates in the presence of colistin. White bars represent 260 nm absorbing materials and black bars represent 280 nm absorbing materials.
Colistin resulted in a net loss of 260 and 280 nm-absorbing materials. The extent of leakage differed from one organism to another. The maximum leakage was observed for the P103 isolate.
This leakage effect is due to the destabilization of membrane integrity arising after the electrostatic interaction of colistin with the outer membrane displacing the divalent cations from the LPS layer.3232 Dixon RA, Chopra I. Leakage of periplasmic proteins from Escherichia coli mediated by polymyxin B nonapeptide. Antimicrob Agents Chemother. 1986;29:781-788. This results in an increase in the permeability of cell membrane, leakage of cell contents and finally cell death.
Furthermore, to demonstrate the selectivity of colistin toward microbial membranes, the effect of colistin on the haemolysis of human RBCs was studied. The release of hemoglobin was monitored by measuring the absorbance of the supernatants of the different reaction mixtures at 540 nm by the spekol. Then, the per cent of haemolysis of RBCs was calculated. It is shown in Fig. 3 that the per cent of RBCs haemolysis increased by increasing colistin concentration. It produced about 1.3% haemolysis at a concentration of 12.5 mg/L which is nearly more than ten times the average MIC of the tested PDR clinical isolates. At low concentrations, it produced insignificant haemolysis less than 1%. However, there was a sharp increase in the per cent of haemolysis at concentrations above 50 mg/L which are unachievable in vivo. This confirms the selectivity of colistin toward the cytoplasmic membrane of the bacterial cells and denotes its in vivo safety at the normal administered doses. The composition of bacterial cells being rich in anionic phosphatidylglycerol and cardiolipin likely provides an important determinant for antimicrobial cationic peptides to target microbial membranes.3333 Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389-395.
Another evidence for the damaging effect of colistin on the cell envelope is obtained by electron microscope examination. The effect of colistin on the ultrastructure of 2 representative isolates (A182 and P103) was studied. The cells (sensitive and induced resistant) were treated by 100 mg/L of colistin for 30 min, centrifuged, fixed and prepared by encapsulating and cutting ultra thin sections from treated and untreated bacterial cell sediments followed by examination of the prepared section under the transmission electron microscope. A smooth continuous cell wall and cytoplasmic membrane structures were observed in the untreated sensitive bacterial strains. However, a slightly wavy cytoplasmic outline was observed for the induced resistant one (Fig. 4). Colistin-treated cells showed numerous projections and pores on the cell wall which was almost dissolved whereas the cytoplasmic membrane was partially damaged. The contents of some treated cells appeared depleted as part of the cytoplasmic material was released through cracks. Such phenomenon was more apparent with the sensitive isolates than with the corresponding isolates with induced colistin resistance (Fig. 5).
Ultrastructure of colistin treated A. baumannii cells. (A1,2,3): Sensitive A. baumannii A182 cells, (B1,2,3): Resistant (induced) A. baumannii A182 cells, A1 and B1 represent the control untreated cells for each category. Magnification: 40,000×: A2, A3, B3 and 50,000×: A1, B1, B2.
Ultrastructure of colistin treated P. aeruginosa cells. (A1,2,3): Sensitive P. aeruginosa P103 cells, (B1,2,3): Resistant (induced) P. aeruginosa P103 cells, A1 and B1 represent the control untreated cells for each category. Magnification: 30,000×: A3, B1, B2, 40,000×: A1, B3 and 50,000×: A2.
Moreover, the effect of colistin on an artificial cytoplasmic membrane model was determined by examination under the phase contrast microscope. The effect of colistin (100 mg/L) on the negatively-charged unilamellar cholesterol free liposomes compared to the control untreated ones is illustrated in Fig. 6. Colistin resulted in an overall deformation in the structure of the artificial cytoplasmic membrane model. Various effects were observed on the phospholipid membrane ranging from roughness and distortion of the outline, pore formation and complete rupture of the membrane and liberation of the internal contents.
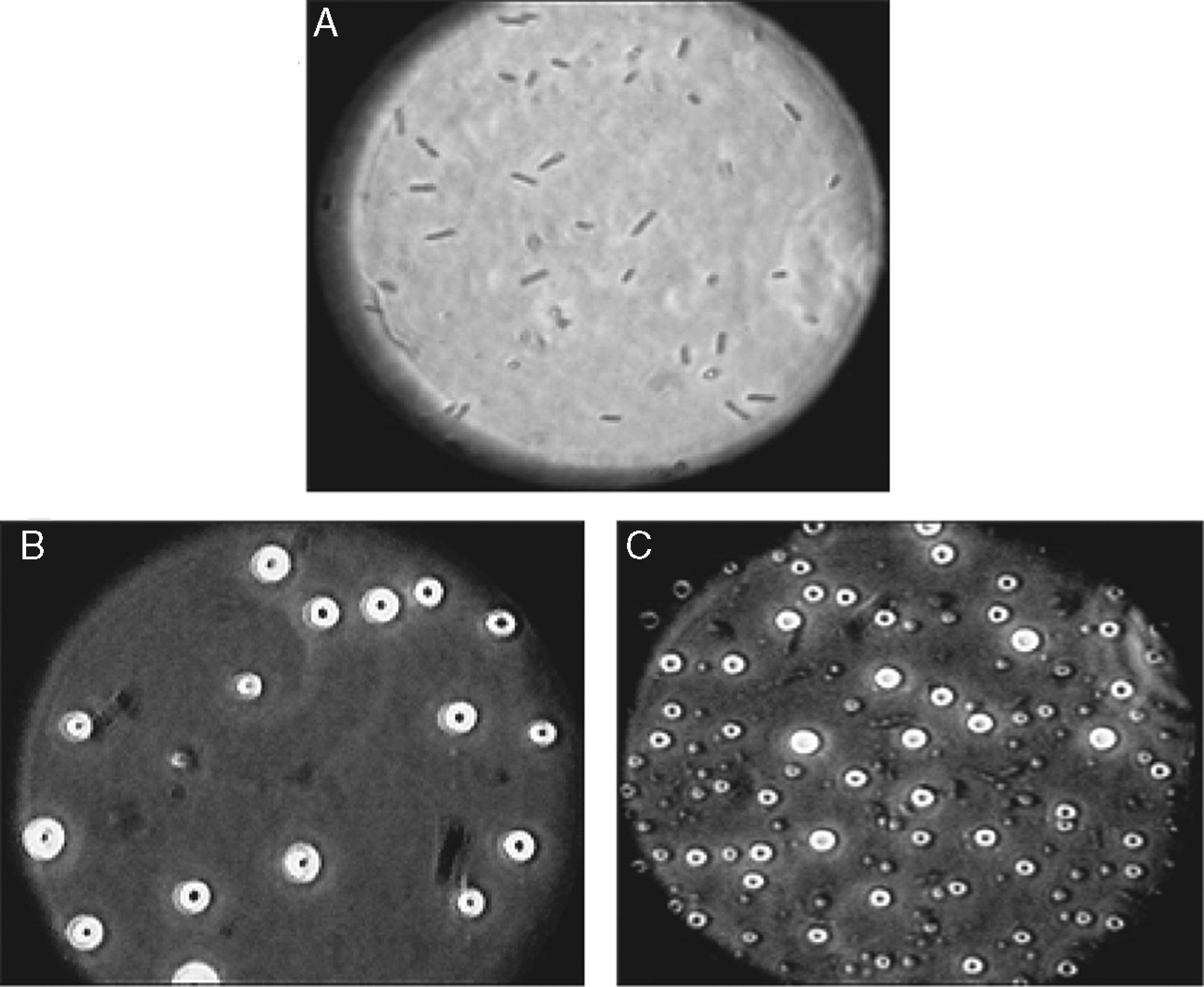
Morphology of liposomes examined under oil-immersion objective lens (total magnification 1000×). (A) Untreated control, (B) Colistin-treated [additional optical zoom: A1 and B1 (2×), A2 and B2 (3×)].
It was a thought of interest whether colistin affects only the outer membrane or it also affects the cell wall. So, the effect of colistin on the cell wall was compared to that of ceftazidime which is a β-lactam antibiotic known to act on the cell wall by using the isolate P103. Ceftazidime causes spheroplast formation prior to cell lysis in Gram-negative bacteria due to inhibition of transpeptidases (Penicillin-Binding Proteins) which catalyze the cross linking of peptidoglycan chains. Thus, it disrupts the synthesis of peptidoglycan resulting in the release of autolysins which enzymatically degrade the cell walls forming spheroplast, which is osmotically-sensitive cell lacking rigidity of the cell wall.3434 Kitano K, Tomasz A. Triggering of autolytic cell wall degradation in Escherichia coli by beta-lactam antibiotics. Antimicrob Agents Chemother. 1979;16:838-848. Sucrose 0.3 M acts as a stabilizer for the formed spheroplasts. By using sub-inhibitory concentrations of ceftazidime in the present study, spheroplasts were formed and viewed by the phase contrast microscope (Fig. 7). When comparing that to colistin, similar structures were obtained at 1/2 MIC level. Formation of such osmotically unstable structures indicates that colistin not only acts on the cytoplasmic membrane but also on the cell wall. Therefore, it could be assumed that treatment of sensitive Gram-negative cells with colistin in the presence of hypertonic solution might have resulted in the release of autolysins which degraded most of the cell wall leaving spheroplasts supported by the surrounding high osmotic pressure.3535 Hocquet D, Berthelot P, Roussel-Delvallez M, et al. Pseudomonas aeruginosa may accumulate drug resistance mechanisms without losing its ability to cause bloodstream infections. Antimicrob Agents Chemother. 2007;51:3531-3536. Hence, it can be elucidated that colistin acts on multiple layers of Gram-negative cells, initially on the outer membrane as demonstrated by the electron microscopy study and then on the inner membrane as shown by the spheroplasts data.
Spheroplasts of P103 isolate formed at ½ MIC of ceftazidime (B) and colistin (C) in hypertonic solution compared to shape of control cells (A).
In conclusion, it can be demonstrated that colistin is still a promising drug for the treatment of infections due to PDR clinical isolates. It induces alterations in the permeability of the bacterial cytoplasmic membrane, leakage of the intracellular contents and ultimately cell death. However, colistin should not be misused to avoid the global problem of development of resistance. Further in vivo investigations are still required concerning the pharmacokinetics, pharmacodynamics and toxicodynamics of colistin.
-
Associate Editor: Roxane Maria Fontes Piazza
-
☆
The work was conducted in Pharmaceutical Microbiology Department, Faculty of Pharmacy, Alexandria University, Egypt.
References
-
1Sharma R, Sharma CL, Kapoor B. Antibacterial resistance: current problems and possible solutions. Indian J Med Sci 2005;59:120-129.
-
2Falagas ME, Bliziotis IA, Kasiakou SK, et al. Outcome of infections due to pandrug-resistant (PDR) Gram-negative bacteria. BMC Infect Dis 2005;5:24
-
3Wang CY, Jerng JS, Chen KY, et al. Pandrug-resistant Pseudomonas aeruginosa among hospitalised patients: clinical features, risk-factors and outcomes. Clin Microbiol Infect 2006;12:63-68.
-
4Marais E, de Jong G, Ferraz V, Maloba B, Duse AG. Interhospital transfer of pan-resistant Acinetobacter strains in Johannesburg, South Africa. Am J Infect Control 2004;32:278-281.
-
5Falagas ME, Koletsi PK, Bliziotis IA. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa J Med Microbiol. 2006;55(Pt 12):1619-1629.
-
6Li J, Nation RL, Turnidge JD, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 2006;6:589-601.
-
7Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis 2005;40:1333-1341.
-
8Nord NM, Hoeprich PD. Polymyxin B and colistin: a critical comparison. N Engl J Med 1964;270:1030-1035.
-
9Yow EM, Tan E, Shane L, Schonfeld S, Abu-Nassar H. Colistin (coly-mycin) in resistant bacterial infections. A clinical appraisal. Arch Intern Med 1961;108:664-670.
-
10Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int J Antimicrob Agents 2005;25:11-25.
-
11Stein A, Raoult D. Colistin: an antimicrobial for the 21st century?. Clin Infect Dis 2002;35:901-902.
-
12Falagas ME, Kasiakou SK, Tsiodras S, Michalopoulos A. The use of intravenous and aerosolized polymyxins for the treatment of infections in critically ill patients: a review of the recent literature. Clin Med Res 2006;4:138-146.
-
13Landman D, Georgescu C, Martin DA, Quale J. Polymyxins revisited. Clin Microbiol Rev 2008;21:449-465.
-
14Hancock RE. Peptide antibiotics. Lancet 1997;349:418-422.
-
15Hermsen ED, Sullivan CJ, Rotschafer JC. Polymyxins: pharmacology, pharmacokinetics, pharmacodynamics, and clinical applications. Infect Dis Clin North Am 2003;17:545-562.
-
16Glukhov E, Stark M, Burrows LL, Deber CM. Basis for selectivity of cationic antimicrobial peptides for bacterial versus mammalian membranes. J Biol Chem 2005;280:33960-33967.
-
17Finegold SM, Baron EJ. Bailey and Scott’s Diagnostic Microbiology St. Louis: The C. V. Mosby Company;1986:70–125, 173–201, 355–387, 397–437, 859–914.
-
18Collee JG, Miles RS. Tests for identification of bacteria. In: Collee JG, Duguid JP, Fraser AG, Marmion BP, eds. Mackie and McCartney, Practical Medical Microbiology New York: ChurchillLivingstone; 1989:141–160.
-
19Howard DH. The preservation of bacteria by freezing in glycerol broth. J Bacteriol 1956;71:625
-
20Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 1966;45:493-496.
-
21Andrews JM. BSAC standardized disc susceptibility testing method (version 5). J Antimicrob Chemother 2006;58:511-529.
-
22Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother 2001;48(suppl 1):5-16.
-
23Barry AL, Craig WA, Nadler H, et al. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline. In: NCCLS document M26-A PA, USA: NCCLS; 1999
-
24Carson CF, Mee BJ, Riley TV. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob Agents Chemother 2002;46:1914-1920.
-
25Cirioni O, Ghiselli R, Silvestri C, et al. Efficacy of tachyplesin III, colistin, and imipenem against a multiresistant Pseudomonas aeruginosa strain. Antimicrob Agents Chemother 2007;51:2005-2010.
-
26Kim Y, Farrah S, Baney RH. Membrane damage of bacteria by silanols treatment. Electron J Biotechnol 2007;10:252-259.
-
27Suganuma A. Further studies on the plasma membrane of Staphylococcus aureus J Cell Biol 1966;30:208-210.
-
28Szoka FJR, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A 1978;75:4194-4198.
-
29Gupta S, Govil D, Kakar PN, et al. Colistin and polymyxin B: a re-emergence. Indian J Crit Care Med 2009;13:49-53.
-
30Li J, Turnidge J, Milne R, Nation RL, Coulthard K. In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob Agents Chemother 2001;45:781-785.
-
31Li J, Rayner CR, Nation RL, et al. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii Antimicrob Agents Chemother 2006;50:2946-2950.
-
32Dixon RA, Chopra I. Leakage of periplasmic proteins from Escherichia coli mediated by polymyxin B nonapeptide. Antimicrob Agents Chemother 1986;29:781-788.
-
33Zasloff M. Antimicrobial peptides of multicellular organisms. Nature 2002;415:389-395.
-
34Kitano K, Tomasz A. Triggering of autolytic cell wall degradation in Escherichia coli by beta-lactam antibiotics. Antimicrob Agents Chemother 1979;16:838-848.
-
35Hocquet D, Berthelot P, Roussel-Delvallez M, et al. Pseudomonas aeruginosa may accumulate drug resistance mechanisms without losing its ability to cause bloodstream infections. Antimicrob Agents Chemother 2007;51:3531-3536.
Publication Dates
-
Publication in this collection
Apr-Jun 2016
History
-
Received
17 Nov 2013 -
Accepted
7 Oct 2014