Abstract
Considering the importance of lignocellulose macrophyte-derived for the energy flux in aquatic ecosystems and the nutrient concentrations as a function of force which influences the decomposition process, this study aims to relate the enzymatic activity and lignocellulose hydrolysis in different trophic statuses. Water samples and two macrophyte species were collected from the littoral zone of a subtropical Brazilian Reservoir. A lignocellulosic matrix was obtained using aqueous extraction of dried plant material (≈40 °C). Incubations for decomposition of the lignocellulosic matrix were prepared using lignocelluloses, inoculums and filtered water simulating different trophic statuses with the same N:P ratio. The particulate organic carbon and dissolved organic carbon (POC and DOC, respectively) were quantified, the cellulase enzymatic activity was measured by releasing reducing sugars and immobilized carbon was analyzed by filtration. During the cellulose degradation indicated by the cellulase activity, the dissolved organic carbon daily rate and enzyme activity increased. It was related to a fast hydrolysable fraction of cellulose that contributed to short-term carbon immobilization (ca. 10 days). After approximately 20 days, the dissolved organic carbon and enzyme activity were inversely correlated suggesting that the respiration of microorganisms was responsible for carbon mineralization. Cellulose was an important resource in low nutrient conditions (oligotrophic). However, the detritus quality played a major role in the lignocelluloses degradation (i.e., enzyme activity) and carbon release.
Keywords
Anaerobic decomposition; Cellulose; Carbon cycle; Aquatic macrophytes
Introduction
The macrophytes are, after death, an important detritus source due to their role in carbon and nutrient cycles in aquatic systems. Decomposition is a key process for the maintenance of the heterotrophic activity11 Cheesman A, Turner BL, Inglett PW, Reddy KR. Phosphorus transformation during decomposition of wetland macrophytes. Environ Sci Technol. 2010;44:9265-9271. releasing both hydrosoluble and non-soluble compounds.22 Bianchini I Jr, Cunha-Santino MB. Model parameterization for aerobic decomposition of plant resources drowned duringman-made lakes formation. Ecol Model. 2011;222:1263–1271. The release of hydrosoluble fraction (i.e., dissolved material) occurs by leaching polar compounds of the detritus and may vary from 24 h to 15 days33 Silva DS, Cunha-Santino MB, Marques EE, Bianchini Jr. The decomposition of aquatic macrophytes: bioassays versusin situ experiments. Hydrobiology. 2011;665:219–227.; the non-soluble material (i.e., lignocellulosic material) is a fraction showing slow decay constant rates contributing with diagenetic processes.
The role of the dissolved fraction as a source of carbon to the microbial loop is well documented.44 Azam F, Fenchel T, Field GJ, Gray JS, Meyer-Reil LA, Thingstad F. The ecological role of water-column microbes in the sea. Mar Ecol. 1983;10:257-263.–66 Rossi L, Constantini ML, Carlino P, Lascio A, Rossi D. Autochthonous and allochthonous plant contributions to coastal benthic detritus deposits: a dual stable-isotope study in a volcanic lake. Aquat Sci. 2010;72:227-236. From this concept, heterotrophic bacteria are the main dissolved carbon recycler in the aquatic ecosystems and the energy is channeled to higher trophic levels. However, some studies77 Moran MA, Hodson RE. Formation and bacterial utilization of dissolved organic carbon derived from detrital lignocelluloses. Limnol Oceanogr. 1989;62:1034-1047.–99 Moran MA, Hodson RE. Contribution of degrading Spartina alterniflora lignocelluloses to the dissolved organic carbon pool of a salt marsh. Mar Ecol Prog Ser. 1990;62:161-168. have recognized that the lignocelluloses (i.e., POC) also sustain the aquatic food web due to the enzymatic action of microbial community and DOC production. The lignocelluloses contribution to the DOC pool may be considered an important pathway of carbon cycle in the detritus food web.
Lignocellulose material is highly resistant to the microbial community and other animals and is generally not used by grazers. The enzymatic activity of microorganisms reduces the molecular weight of the polymer by hydrolysis and/or solubilization during decomposition, enabling it to be used by the microbial cells. Cellulose is the most abundant component in the plant tissue and its depolymerization mainly involves three enzymes: 1,4-β-exoglucanases, 1,4-β-endoglucanases and 1,4-β-glucosidades.1010 Sinsabaugh RL, Shah JJF. Ecoenzymatic stoichiometry of recalcitrant organic matter decomposition: the growth rate hypothesis in reverse. Biogeochemistry. 2011;102:31-43. This polymer is relatively susceptible to degradation1111 Lynd LR, Weimer PJ, Van Zyl JH, Petrorius IS. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev. 2002;66:506-577.,1212 Sinsabaugh RL, Carneiro MM, Repert DA. Allocation of extracellular enzymatic activity in relation to litter composition N deposition and mass loss. Biogeochemistry. 2002;60:1-24. yielding by-products readily available to microbial metabolism.1010 Sinsabaugh RL, Shah JJF. Ecoenzymatic stoichiometry of recalcitrant organic matter decomposition: the growth rate hypothesis in reverse. Biogeochemistry. 2011;102:31-43.
Function forces, including temperature and nutrient availability may influence the enzymatic activity and, consequently, the rate of DOC production. High temperatures play an important role in enzyme metabolism,1313 Gimenes KZ, Cunha-Santino MB, Bianchini I Jr. Cellulaseactivity in anaerobic degradation of aquatic macrophytetissues. Fundam Appl Limnol. 2013;183(1):24–39. but the influence of nutrients is still unclear.1414 Sarneel JM, Geurts JJM, Beltman B, et al. The effect of enrichment of either the bank or the surface water on shoreline vegetation and decomposition. Ecosystems. 2010;13:1275-1286.,1515 Li X, Cui B, Yang Q, et al. Detritus quality controls macrophyte decomposition under different nutrient concentrations in a eutrophic shallow lake North China. PLoS ONE. 2012;7:1-10. The degradation of cellulose in different trophic statuses contributes to understanding the biochemical aspects of carbon turnover in aquatic ecosystems. In this study, we address (i) the relationship between DOC release during macrophyte-cellulose decomposition and enzymatic activity; (ii) the main aspects of the DOC release/use; (iii) the role of the trophic status in the carbon turnover. We supposed that the DOC release controls the enzymatic activity and both are higher in abundant nutrient conditions (i.e., hypereutrophic).
Materials and methods
Sampling area
Water and macrophyte samples were collected in Barra Bonita Reservoir (22°29′–22°32′ S and 48°29′–48°34′ W), located in São Paulo State. Barra Bonita (325 km2 of area) is the first of six cascade reservoirs along the Tiete River and is considered eutrophic due to intense anthropic activity.1616 CETESB. Companhia de Tecnologia de Saneamento Ambiental. Relatório de Qualidade das águas Interiores do Estado de São Paulo; 2012. Accessed 10.08.2012. The littoral zone of the reservoir is mainly colonized by Paspalum repens P. J. Bergius (Poaceae) and Pistia stratiotes L. (Araceae). The main limnological characteristics of Barra Bonita Reservoir1717 Bottino, F, Cunha-Santino, MB, Bianchini Jr I. Decomposition of particulate organic carbon from aquatic macrophytes under different nutrient conditions. Aquat. Geochem. 2015; DOI 10.1007/s10498-015-9275-x.
https://doi.org/10.1007/s10498-015-9275-...
are high concentrations of total phosphorus (89–143 µg L-1), low concentrations of dissolved oxygen (4.5–5.0 mg L-1), total nitrogen concentration ranging from 3.0–3.5 mg L-1, neutral pH (7.0–7.5) and total organic carbon varying from 4.8 to 24 mg L-1.
Water and macrophyte sampling
Water samples (40 L) were collected from the littoral zone of the reservoir at an eutrophic site (N:P ratio: 55) with P. repens (22°28′19.27″ S and 48°29′20.41″ W); and at a hypereutrophic site (N:P ratio: 79) with P. stratiotes (22°26′20.1″ S and 48°31′18.2″ W) with a 5.0 L Van Dorn bottle at different depths (surface, middle and bottom). The water samples were mixed in a polyethylene container to obtain vertically integrated samples. Mature plant samples were manually collected in the littoral zone of the reservoir. In the laboratory, the water samples were filtered through a cellulose ester membrane (Ф = 0.45 µm) and the plants were washed with tap water to remove the adhered coarse material. The plant material was oven dried (40 °C), and the POC was obtained after cold aqueous extraction of the hydrosoluble fractions (4 °C, 24 h)1818 Møller J, Miller M, Kjøller A. A fungal–bacterial interaction on beech leaves: influence on decomposition and dissolved organic carbon quality. Soil Biol Biochem. 1999;31:374-376. from previously sterilized plants (120 °C, 1 atm, 15 min). The POC was considered the lignocellulose matrix and was used in the decomposition assays. The detritus quality is indicated by C:N C:P molecular ratios of initial POC (P. repens: C:N = 1:1.5; C:P 1:0.8; P. stratiotes: C:N: 1:0.37; C:P 1:0.6)
Experimental design
DOC and cellulose determination
Anaerobic decomposition assays were carried out using 180 (n = 90 per species) decomposition chambers (400 mL) containing POC and filtered water sample (proportion of 10.0 g DM L-1). The chambers were maintained in the dark at 25 °C (average water temperature measured in the reservoir) and 1 mL of indigenous inoculum (i.e., water and sediment from reservoir) was added to each chamber. The initial trophic conditions of both sampling stations were adopted as nutrient backgrounds to prepare the decomposition chambers simulating four trophic regimes1919 Vollenwider A. Scientific Fundamentals of the Eutrophication of Lakes and Flowing Waters with Particular Reference to Nitrogenand Phosphorus as Factors in Eutrophication. Organization for Economic Cooperation and Development; 1983:192 p. by dilution or by adding solutions of nitrogen and phosphorus. The N:P ratio of sampling stations was maintained in the incubations.
On each sampling day (days 10, 20, 30, 60, 90, and 120), three chambers of each plant and trophic condition were filtered (Ф = 0.45 µm) and fractioned into POC and DOC. The DOC was filtered successively (Ф = 0.45 µm and 0.2 µm) and the carbon concentration was measured using a carbon analyzer (Shimadzu TOC-L CPH, Japan). The DOC daily rate was calculated according to Eq. (1). The DOC daily rate was considered as mineralization or immobilization. The difference between the concentrations of filtered dissolved carbon (0.45 and 0.2 µm) was assumed as formation of organic compounds including microbial biomass (immobilization). Cellulose content of remaining POC was measured by gravimetric analysis with a prior acid digestion (H2SO4- 72%, 3 h).2020 Silva DJ, Queiroz AC. Análise de alimentos: métodos químicos e biológicos. Universidade Federal de Viçosa; 2004.
where DOC, DOC daily rate (mg L-1 d-1); [DOC], DOC concentration (mg L-1); t, time (days).Enzyme assay – cellulolytic activity determination
From each decomposition chamber, enzyme extracts were prepared using 20.0 mL of DOC and 1 g of POC (fresh mass). The samples were homogenized (Ultra-Turrax model T10, Germany), sonicated with an ultrasound (model Unique, Brazil) and centrifuged (3000 × g, 15 min, 4 °C; Heraeus Instruments, Megafuge 3.0R, Germany). The cellulase activity was determined spectrophotometrically (Ultrospec 2100 Pro, Sweden) by measuring the concentration of released reducing sugar2121 Somogyi M. Notes on sugar determination. J Bioecol Chem. 1952;195:19-23. acting on specific substrates2222 Mandels M, Andreotti R, Roche C. Measurement of saccharifying cellulose. Biotechnol Bioeng Symp. 1976;6:21-33. (a pure cellulose filter – Whatman no. 1). The measurement of 1 µmol of glucose per minute of reaction per milliliter corresponds to 1 international unit per milliliter.
Statistical analysis
The nonparametric test of Kruskal–Wallis (significance level p < 0.05) followed by the Dunn's multiple comparison test were applied to the DOC and to the cellulase data to evaluate differences in the nutrient treatments. The differences between the plants were assessed using Kruskal–Wallis.
Results
The remaining content of cellulose from detritus of P. repens and P. stratiotes at the end of the decomposition experiments are presented in Table 1. The oligotrophic condition showed the major cellulose decay for the P. repens detritus and it decreased according to an increase in the trophic status (Table 1). For P. stratiotes, the highest decay occurred in oligotrophic and hypereutrophic environments that showed similar cellulose content after 120 days (Table 1).
Cellulose (%) on the remaining Particulate Organic Carbon (POC) (after 120 days) in the detritus of Paspalum repens and Pistia stratiotes. N:P ratios of sampling stations with Paspalum repens and Pistia stratiotes: 55 and 79, respectively. C:N:P ratios of Paspalum repens and Pistia stratiotes detritus: 1:1.5:0.8 and 1:0.3:0.6, respectively.
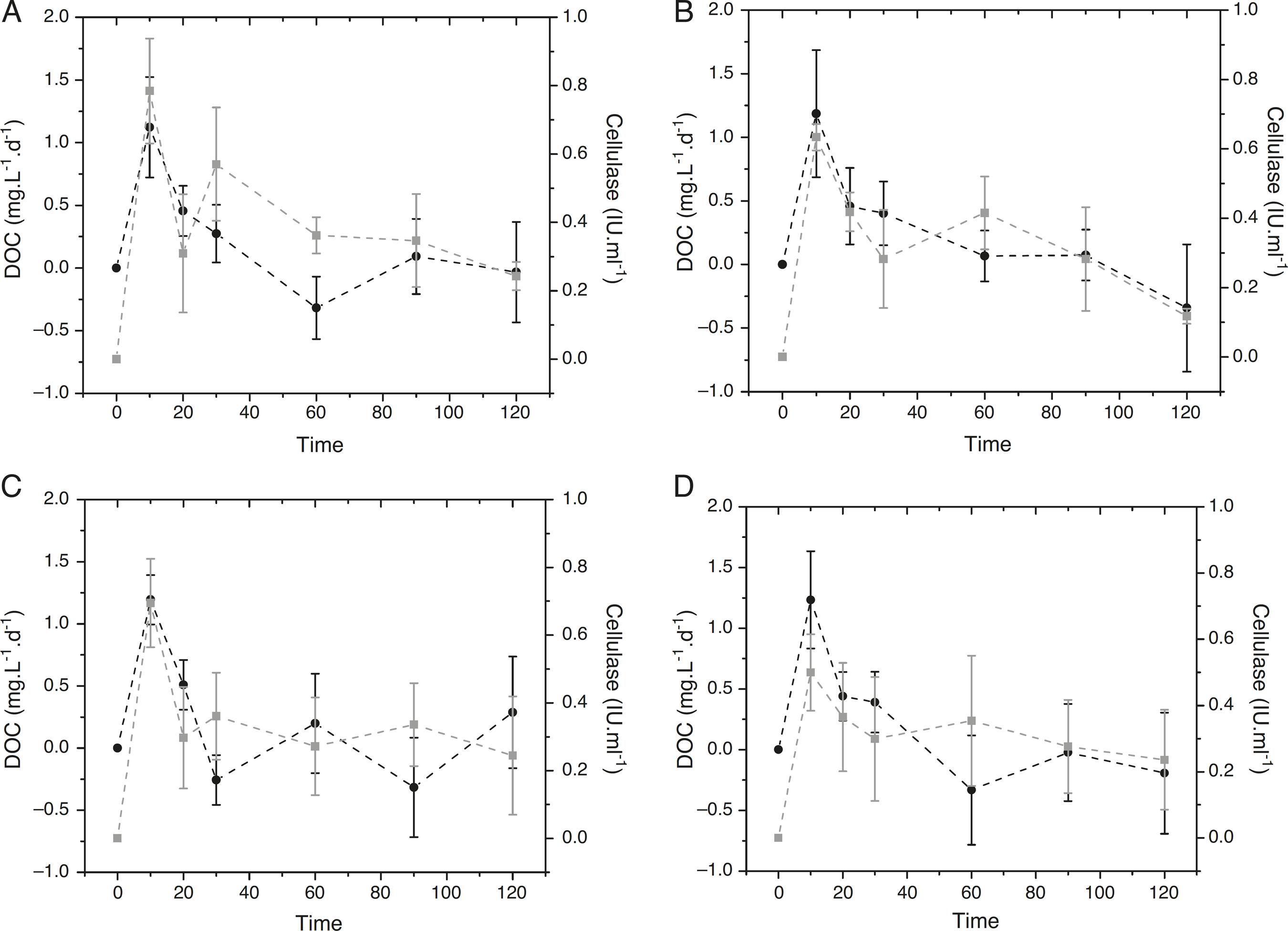
Cellulase activity on P. repens detritus increased over 60 days for all trophic statuses (Fig. 1). In the eutrophic and hypereutrophic conditions, the enzyme activity showed a slight decrease from day 20 to day 30 (0.62–0.47; 0.64–0.54 IU mL-1, respectively). The highest cellulase activity occurred in the oligotrophic status on day 60 (0.81 IU mL-1). The values showed a sharp decrease until day 120 (0.25 IU mL-1). Mesotrophic and eutrophic statuses showed a similar variation pattern of enzymatic activity; for the hypereutrophic condition, the cellulase activity decreased from day 90 (0.7–0.3 IU mL-1). Statistical analysis of temporal enzyme activities pointed out no significant differences among the trophic states (p < 0.05).
Temporal variation of Dissolved Organic Carbon (DOC) daily rate (black) and cellulase activity (gray) of Paspalum repens in four trophic conditions: (A) oligotrophic, (B) mesotrophic, (C) eutrophic, and (D) hypereutrophic.
During the cellulose degradation indicated specifically by cellulase activity, the DOC daily rate derived from P. repens detritus increased almost proportionally to the enzyme activity. After approximately 20 days, the DOC daily rate and enzyme activity were inversely correlated (Fig. 1) showing the predominance of the consumption process (i.e., mineralization or immobilization). The DOC daily rate decreased over time in all trophic statuses. No statistical differences were observed among the treatments (p > 0.05).
For P. stratiotes detritus, the cellulase activity increased until day 10 for all trophic conditions (0.78, 0.63, 0.69, 0.5 IU mL-1 for oligotrophic, mesotrophic, eutrophic and hypereutrophic, respectively) (Fig. 2). The values declined until day 30 for mesotrophic and hypereutrophic statuses while a high peak of cellulase occurred for oligotrophic and eutrophic (0.57 and 0.36 IU mL-1, respectively). On day 60, the highest enzymatic activity was recorded for the mesotrophic status (0.41 IU mL-1) decreasing until day 120 for mesotrophic and hypereutrophic conditions. Oligotrophic and eutrophic statuses showed the highest cellulase activity on day 90 (0.35 IU mL-1). Statistical analysis of temporal enzyme activities pointed out no significant differences among the treatments (p < 0.05).
Temporal variation of Dissolved Organic Carbon (DOC) daily rate (black) and cellulase activity (gray) of Pistia stratiotes in four trophic conditions: (A) oligotrophic, (B) mesotrophic, (C) eutrophic, and (D) hypereutrophic.
The increase in the DOC daily rate was closely related to the cellulase activity until day 10. After that the pattern was opposite (i.e., increase of DOC consumption) and the DOC daily rate decay increased over time, except for the eutrophic condition (high daily rate on day 60: 0.19%). A raise of DOC occurred on day 90 for oligotrophic, mesotrophic and hypereutrophic statuses (0.09%, 0.07%, 0.02%, respectively). In general, the increase in DOC was the dominant process on the mesotophic status while its consumption due to mineralization or immobilization was the main route to a hypereutrophic condition. Statistical analysis of the DOC daily rate showed no significant differences among the trophic statuses (p < 0.05).
In general, the immobilization of DOC (i.e., microbial biomass formation) was higher for P. repens (Fig. 3A). The maximum carbon immobilization occurred from day 20 to 60, mainly for the hypereutrophic condition. For P. stratiotes, the highest biomass formation was on day 60 for eutrophic and hypereutrophic statuses (Fig. 3B).
Total Organic Carbon (TOC) variation representing the biomass formation from Dissolved Organic Carbon (DOC) (in terms of carbon concentration) of Paspalum repens (A) and Pistia stratiotes (B).
Discussion
After 120 days of decomposition, our results showed a high loss of cellulose content in P. repens detritus mainly in poor-nutrient conditions (i.e., oligotrophic) suggesting that the particulate detritus is an important carbon resource for microorganisms in nutrient-limited environments. The lignin–celullose–hemicellulose matrix interactions and also the detritus quality (indicated by C:N:P ratios) may reflect the major decay of cellulose from P. stratiotes detritus in eutrophic conditions. The access of microorganisms to the fibers depends on their structural arrangement, which is different for each plant and can also be different for each structure (e.g. leaves, stem) of the same plant.1111 Lynd LR, Weimer PJ, Van Zyl JH, Petrorius IS. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev. 2002;66:506-577. Furthermore, the biochemical degradation of an organic resource is improved in a nutrient-rich environment due to the high extracellular enzyme activity.2323 Keiblinger KM, Schneider T, Roschitzki B, et al. Effects of stoichiometry and temperature perturbations on beech leaf litter decomposition enzyme activities and protein expression. Biogeosciences. 2012;9:4551-4577. The significant differences between the cellulose decay of both plants show the role of the detritus quality (indicated here by C:N C:P ratio) in the degradation of fibers.
In general, the enzymatic activity in P. repens detritus increased over time with a slight decrease over 30 days when the DOC immobilization was the predominant process. We suppose that the DOC daily rate increase (until day 10) was faster than the microbial uptake and the microorganisms responsible for this process produced the highest cellulase enzymatic activity in the first days of incubation. After 10 days, the main route of the DOC was immobilization and the increase in cellulase activity may be related to the high heterotrophic activity due to the increase in biomass. Low DOC availability in the system probably stimulated the microbial community to enzymatically attack the lignocellulosic detritus to obtain carbon.2424 Shackle VJ, Freeman C, Reynolds B. Carbon supply and the regulation of enzyme activity in constructed wetlands. Soil Biol Biochem. 2000;32:1935-1940.
The input of carbon in a system increases microbial respiration, enzyme activity production and changes the carbon availability, and therefore, the microbial community structure.2525 Blagodastskaya E, Khomyakov N, Myachina O, Bogomolova I, Bladodatsky S, Kuzyakov Y. Microbial interactions affect priming induced by cellulose. Soil Biol Biochem. 2014;74:39-49. The degradation of less recalcitrant resources (ca. hemicelluloses glycosidic bonds) in the first days of decomposition decreases the carbon limitation supporting the heterotrophic metabolism and the attack to the cellulose fibrils.1010 Sinsabaugh RL, Shah JJF. Ecoenzymatic stoichiometry of recalcitrant organic matter decomposition: the growth rate hypothesis in reverse. Biogeochemistry. 2011;102:31-43.,2626 Tabolt JM, Treseder KK. Interactions among lignin cellulose and nitrogen drive litter chemistry-decay relationships. Ecology. 2012;93:345-354. The whole cellulose activates the enzymatic chain of cellulase production decreasing the DOC availability due to the formation of biomass in a no-limiting nutrient environment.2525 Blagodastskaya E, Khomyakov N, Myachina O, Bogomolova I, Bladodatsky S, Kuzyakov Y. Microbial interactions affect priming induced by cellulose. Soil Biol Biochem. 2014;74:39-49.,2727 Attermeyer K, Hornick T, Kayler ZE, et al. Enhanced bacterial decomposition increasing addition of autochthonous to allochthonous carbon without any effect on bacterial community composition. Biogeosciences. 2014;11:1479-1489. The input of soluble cellulose improved the DOC formation (day 10) and its rapid assimilation, mainly by bacteria (biomass formation), increased the cellulolytic activity. It is more evident in nutrient-rich incubations (from mesotrophic to hypereutrophic). Thus, the presence of phosphorus and nitrogen in the medium may improve the microbial activity increasing the carbon demand reflecting in the enzymatic production.
For P. stratiotes, the enzymatic activity showed an increasing pattern from day 20 to day 30 and a decrease in the DOC daily rate after 10 days. The sharp decrease in the DOC daily rate indicates its mineralization and loss of fiber content (approximately 82% of cellulose lost to the eutrophic condition). The high enzymatic activity, mainly in the oligotrophic status, suggests that even in a nutrient-limited environment, the microbial community uses resources to depolymerize extensive carbon molecules, probably using the detritus as a resource of carbon. The nutrient-rich condition supported the microorganisms energy demands and the carbon immobilization.2424 Shackle VJ, Freeman C, Reynolds B. Carbon supply and the regulation of enzyme activity in constructed wetlands. Soil Biol Biochem. 2000;32:1935-1940. This assumption confirms that for P. stratiotes, both the trophic status and detritus quality influenced the carbon flow.
The degradation of the cellulose from macrophytes detritus depends on the production of extracellular enzymes by bacteria and fungi acting synergistically (Cellulosome Concept2828 Shoham Y, Lamed R, Bayer EA. The cellulosome concept as an efficient microbial strategy for the degradation of insoluble polysaccharides. Trends Microbiol. 1999;7:275-328.). In anaerobic conditions, microorganisms utilize a complex system of enzymes to hydrolyze cellulose releasing readily degradable compounds (monosaccharides and oligosaccharides) and high molecular-weight compounds.2929 Quiroz-Castañeda RE, Folch-Mallol JL. Hydrolysis of biomassmediated by cellulases for the production of sugars. In: Quiroz-Castañeda RE, Folch-Mallol JL, eds. Sustainable Degradation of Lignocellulosic Biomass – Techniques Applications and Commercialization. Mexico: Dr Anuj Chandel, Morelos;2013:119–155. Bacteria and fungi acting in the cellulose substratum increase the degradation of the cellulose. Fungi attack recalcitrant substances (i.e., high molecular weight compounds) releasing intermediate by-products to the bacteria uptake and, consequently, increasing the cellulase activity.3030 Romaní AM, Fischer H, Mille-Lindblom C, Tranvik LJ. Interactions of bacteria and fungi on decomposing litter: differential enzyme activities. Ecology. 2006;87:2559-2569. The relationship between enzyme activity and DOC immobilization and mineralization presented here show the importance of a recalcitrant carbon source (i.e., cellulose) for the heterotrophic metabolism. Some studies77 Moran MA, Hodson RE. Formation and bacterial utilization of dissolved organic carbon derived from detrital lignocelluloses. Limnol Oceanogr. 1989;62:1034-1047.,3131 Docherty KM, Young KC, Maurice PA, Bridgham SD. Dissolved organic matter concentration and quality influences upon structure and function of freshwater microbial communities. Microb Ecol. 2006;22:378-388.,3232 Cunha A, Almeida A, Coelho FJRC, Gomes NCM, Oliveira V, Santos AL. Bacterial extracellular enzymatic activity inglobally changing aquatic ecossystems. In: Méndez-Vilas A, ed. Current Research Technology and Education Topics in Applied Microbiology and Microbial Biotechnology. Spain: Formatex Research Center; 2010:124–135. have shown the role of the lignocellulosic matrix in the DOC production and its function in the energy flow.
Cellulose breakdown results in fermentative sugars and the fungi growth improves this process due to the processing of lignocellulosic detritus.3333 Barik SK, Mishra S, Ayyappan S. Decomposition patterns of unprocessed and processed lignocellulosics in a freshwater fish pond. Aquat Ecol. 2000;34:185-204.,3434 Bansal P, Vowell BJ, Hall M, Realff MJ, Lee JH, Bommarius AS. Ellucidation of cellulose accessibility hydrolysability and reactivity as the major limitations in the enzymatic hydrolysis of cellulose. Bioresour Technol. 2012;107:243-250. During cellulose degradation, the microbial community shifts to a highly specialized community producing a range of 11 enzymes acting synergistically on the substratum in an anaerobic condition.3535 Schwarz WH. The cellulosome and cellulose degradation by anaerobic bacteria. Appl Microbiol Technol. 2011;56:634-649. The enzyme activity supports the release of the soluble carbon from the cellulose high molecular-weight carbon (but not recalcitrant) enhancing the heterotrophic metabolism.2424 Shackle VJ, Freeman C, Reynolds B. Carbon supply and the regulation of enzyme activity in constructed wetlands. Soil Biol Biochem. 2000;32:1935-1940. In this regard, this study assumes that the hydrolysable fraction of the cellulose fiber was responsible for the high DOC daily rates and increasing biomass, mainly during P. repens degradation. The high amount of proteins in that macrophyte species3636 FAO – Food and Agriculture Organization of the United Nations. Use of Algae and Aquatic Macrophytes as Feed in Small-Scale Aquaculture; 1997. Accessed 19.01.15. is closely related to the complex enzyme activation as it binds proteins and nitrogen compounds together.
Accessibility and hydrolysability are the main factors controlling the cellulose breakdown.3434 Bansal P, Vowell BJ, Hall M, Realff MJ, Lee JH, Bommarius AS. Ellucidation of cellulose accessibility hydrolysability and reactivity as the major limitations in the enzymatic hydrolysis of cellulose. Bioresour Technol. 2012;107:243-250. From this point of view, the cellulose hydrolysis decreased until there was a degree of conversion of cellulose of 30% and a clogging system diminished the affinity between cellulose and cellulase. The results suggest that the detritus quality, including its recalcitrance (i.e., amount of fibers) were important to predict the relationship between cellulase and cellulose and its conversion to DOC. The higher cellulose content in P. repens detritus1717 Bottino, F, Cunha-Santino, MB, Bianchini Jr I. Decomposition of particulate organic carbon from aquatic macrophytes under different nutrient conditions. Aquat. Geochem. 2015; DOI 10.1007/s10498-015-9275-x.
https://doi.org/10.1007/s10498-015-9275-...
limited its conversion to DOC (lower rates than P. stratiotes), but the cellulase activity may be due to the enzymes’ clogging system.
In summary, the cellulose macrophyte-derived showed a fast hydrolysable fraction (ca. 10 days), which contributed to carbon immobilization. Mineralization was the long-term predominant process mainly due to the respiratory activity of microorganisms. Moreover, the hydrolysable fraction of cellulose improved the immobilization process, which was stimulated by both the nutrient condition and the detritus quality. High nutrient concentrations increased the respiratory activity of microorganisms and, therefore, the CO2 formation. The trophic status showed minor importance for cellulose breakdown and the availability of resources in the environment limits the attack of microorganisms to the detritus. The intrinsic characteristics of detritus (i.e., C:N C:P ratios) were important for the degradation of fibers. The outcomes show an alternative route of the carbon cycle in aquatic ecosystems and contribute to understanding the process occurring within the sediments.
-
Associate Editor: Solange Ines Mussatto
Acknowledgements
We would like to thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for their financial support (São Paulo Research Foundation, Process number 2012/21829-0).
References
-
1Cheesman A, Turner BL, Inglett PW, Reddy KR. Phosphorus transformation during decomposition of wetland macrophytes. Environ Sci Technol 2010;44:9265-9271.
-
2Bianchini I Jr, Cunha-Santino MB. Model parameterization for aerobic decomposition of plant resources drowned duringman-made lakes formation. Ecol Model 2011;222:1263–1271.
-
3Silva DS, Cunha-Santino MB, Marques EE, Bianchini Jr. The decomposition of aquatic macrophytes: bioassays versusin situ experiments. Hydrobiology 2011;665:219–227.
-
4Azam F, Fenchel T, Field GJ, Gray JS, Meyer-Reil LA, Thingstad F. The ecological role of water-column microbes in the sea. Mar Ecol 1983;10:257-263.
-
5Fenchel T. The microbial loop – 25 years later. J Exp Mar Biol Ecol 2008;366:99-103.
-
6Rossi L, Constantini ML, Carlino P, Lascio A, Rossi D. Autochthonous and allochthonous plant contributions to coastal benthic detritus deposits: a dual stable-isotope study in a volcanic lake. Aquat Sci 2010;72:227-236.
-
7Moran MA, Hodson RE. Formation and bacterial utilization of dissolved organic carbon derived from detrital lignocelluloses. Limnol Oceanogr 1989;62:1034-1047.
-
8Anesio AM, Abreu PC, Biddanda BA. The role of free and attached microorganisms in the decomposition of estuarine macrophyte detritus. Estuar Coast Shelf Sci 2003;56:197-201.
-
9Moran MA, Hodson RE. Contribution of degrading Spartina alterniflora lignocelluloses to the dissolved organic carbon pool of a salt marsh. Mar Ecol Prog Ser 1990;62:161-168.
-
10Sinsabaugh RL, Shah JJF. Ecoenzymatic stoichiometry of recalcitrant organic matter decomposition: the growth rate hypothesis in reverse. Biogeochemistry 2011;102:31-43.
-
11Lynd LR, Weimer PJ, Van Zyl JH, Petrorius IS. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 2002;66:506-577.
-
12Sinsabaugh RL, Carneiro MM, Repert DA. Allocation of extracellular enzymatic activity in relation to litter composition N deposition and mass loss. Biogeochemistry 2002;60:1-24.
-
13Gimenes KZ, Cunha-Santino MB, Bianchini I Jr. Cellulaseactivity in anaerobic degradation of aquatic macrophytetissues. Fundam Appl Limnol 2013;183(1):24–39.
-
14Sarneel JM, Geurts JJM, Beltman B, et al. The effect of enrichment of either the bank or the surface water on shoreline vegetation and decomposition. Ecosystems 2010;13:1275-1286.
-
15Li X, Cui B, Yang Q, et al. Detritus quality controls macrophyte decomposition under different nutrient concentrations in a eutrophic shallow lake North China. PLoS ONE 2012;7:1-10.
-
16CETESB. Companhia de Tecnologia de Saneamento Ambiental. Relatório de Qualidade das águas Interiores do Estado de São Paulo; 2012. Accessed 10.08.2012.
-
17Bottino, F, Cunha-Santino, MB, Bianchini Jr I. Decomposition of particulate organic carbon from aquatic macrophytes under different nutrient conditions. Aquat. Geochem. 2015; DOI 10.1007/s10498-015-9275-x.
» https://doi.org/10.1007/s10498-015-9275-x -
18Møller J, Miller M, Kjøller A. A fungal–bacterial interaction on beech leaves: influence on decomposition and dissolved organic carbon quality. Soil Biol Biochem 1999;31:374-376.
-
19Vollenwider A. Scientific Fundamentals of the Eutrophication of Lakes and Flowing Waters with Particular Reference to Nitrogenand Phosphorus as Factors in Eutrophication Organization for Economic Cooperation and Development; 1983:192 p.
-
20Silva DJ, Queiroz AC. Análise de alimentos: métodos químicos e biológicos Universidade Federal de Viçosa; 2004.
-
21Somogyi M. Notes on sugar determination. J Bioecol Chem 1952;195:19-23.
-
22Mandels M, Andreotti R, Roche C. Measurement of saccharifying cellulose. Biotechnol Bioeng Symp 1976;6:21-33.
-
23Keiblinger KM, Schneider T, Roschitzki B, et al. Effects of stoichiometry and temperature perturbations on beech leaf litter decomposition enzyme activities and protein expression. Biogeosciences 2012;9:4551-4577.
-
24Shackle VJ, Freeman C, Reynolds B. Carbon supply and the regulation of enzyme activity in constructed wetlands. Soil Biol Biochem 2000;32:1935-1940.
-
25Blagodastskaya E, Khomyakov N, Myachina O, Bogomolova I, Bladodatsky S, Kuzyakov Y. Microbial interactions affect priming induced by cellulose. Soil Biol Biochem 2014;74:39-49.
-
26Tabolt JM, Treseder KK. Interactions among lignin cellulose and nitrogen drive litter chemistry-decay relationships. Ecology 2012;93:345-354.
-
27Attermeyer K, Hornick T, Kayler ZE, et al. Enhanced bacterial decomposition increasing addition of autochthonous to allochthonous carbon without any effect on bacterial community composition. Biogeosciences 2014;11:1479-1489.
-
28Shoham Y, Lamed R, Bayer EA. The cellulosome concept as an efficient microbial strategy for the degradation of insoluble polysaccharides. Trends Microbiol 1999;7:275-328.
-
29Quiroz-Castañeda RE, Folch-Mallol JL. Hydrolysis of biomassmediated by cellulases for the production of sugars. In: Quiroz-Castañeda RE, Folch-Mallol JL, eds. Sustainable Degradation of Lignocellulosic Biomass – Techniques Applications and Commercialization Mexico: Dr Anuj Chandel, Morelos;2013:119–155.
-
30Romaní AM, Fischer H, Mille-Lindblom C, Tranvik LJ. Interactions of bacteria and fungi on decomposing litter: differential enzyme activities. Ecology 2006;87:2559-2569.
-
31Docherty KM, Young KC, Maurice PA, Bridgham SD. Dissolved organic matter concentration and quality influences upon structure and function of freshwater microbial communities. Microb Ecol 2006;22:378-388.
-
32Cunha A, Almeida A, Coelho FJRC, Gomes NCM, Oliveira V, Santos AL. Bacterial extracellular enzymatic activity inglobally changing aquatic ecossystems. In: Méndez-Vilas A, ed. Current Research Technology and Education Topics in Applied Microbiology and Microbial Biotechnology Spain: Formatex Research Center; 2010:124–135.
-
33Barik SK, Mishra S, Ayyappan S. Decomposition patterns of unprocessed and processed lignocellulosics in a freshwater fish pond. Aquat Ecol 2000;34:185-204.
-
34Bansal P, Vowell BJ, Hall M, Realff MJ, Lee JH, Bommarius AS. Ellucidation of cellulose accessibility hydrolysability and reactivity as the major limitations in the enzymatic hydrolysis of cellulose. Bioresour Technol 2012;107:243-250.
-
35Schwarz WH. The cellulosome and cellulose degradation by anaerobic bacteria. Appl Microbiol Technol 2011;56:634-649.
-
36FAO – Food and Agriculture Organization of the United Nations. Use of Algae and Aquatic Macrophytes as Feed in Small-Scale Aquaculture; 1997. Accessed 19.01.15.
Publication Dates
-
Publication in this collection
Apr-Jun 2016
History
-
Received
2 Apr 2015 -
Accepted
14 Oct 2015