Abstract
Cellulase production was evaluated in two reference strains (T. reesei Rut-C30 and T. reesei QM9414), two strains isolated from a sugarcane cultivation area (Trichoderma sp. IPT778 and T. harzianum rifai IPT821) and one strain isolated in a program for biodiversity preservation in São Paulo state (Myceliophthora thermophila M77). Solid state cultures were performed using sugarcane bagasse (C), wheat bran (W) and/or soybean bran (S). The highest FPA was 10.6 U/gdm for M77 in SC (10:90) at 80% moisture, which was 4.4 times higher than production in pure W. C was a strong inducer of cellulase production, given that the production level of 6.1 U/gdm in WC (40:60) was 2.5 times higher than in pure W for strain M77; T. reesei Rut-C30 did not respond as strongly with about 1.6-fold surplus production. S advantageously replaced W, as the surplus production on SC (20:80) was 2.3 times relative to WC (20:80) for M77.
cellulase; productivity; solid media; sugarcane bagasse; Myceliophtora sp
Filamentous fungi and media for cellulase production in solid state cultures
B.V. KilikianI; L.C. AfonsoI; T.F.C. SouzaI; R.G. FerreiraI; I.R. PinheiroII
IDepartamento de Engenharia Química, Universidade de São Paulo, São Paulo, SP, Brazil
IIDepartamento de Engenharia Rural, Centro de Ciências Agrárias, Universidade Federal do Espírito Santo, Guararema, ES, Brazil
Send correspondence to Send correspondence to B.V. Kilikian Chemical Engineering Department, University of São Paulo, São Paulo Caixa Postal 61548, 05424-970 São Paulo, SP, Brazil E-mail: kilikian@usp.br
ABSTRACT
Cellulase production was evaluated in two reference strains (T. reesei Rut-C30 and T. reesei QM9414), two strains isolated from a sugarcane cultivation area (Trichoderma sp. IPT778 and T. harzianum rifai IPT821) and one strain isolated in a program for biodiversity preservation in São Paulo state (Myceliophthora thermophila M77). Solid state cultures were performed using sugarcane bagasse (C), wheat bran (W) and/or soybean bran (S). The highest FPA was 10.6 U/gdm for M77 in SC (10:90) at 80% moisture, which was 4.4 times higher than production in pure W. C was a strong inducer of cellulase production, given that the production level of 6.1 U/gdm in WC (40:60) was 2.5 times higher than in pure W for strain M77; T. reesei Rut-C30 did not respond as strongly with about 1.6-fold surplus production. S advantageously replaced W, as the surplus production on SC (20:80) was 2.3 times relative to WC (20:80) for M77.
Key words: cellulase, productivity, solid media, sugarcane bagasse, Myceliophtora sp.
Introduction
Cellulases are enzymes largely focused on by researchers and industries as they are used in various economically relevant processes. The hydrolytic action of cellulases on cellulose, a linear polysaccharide polymer with many glucose monosaccharide units, renders free monosaccharides not only for liquid fuel production, but also for the production of other chemicals, some of them potential substitutes for petroleum derivatives (Bozell and Petersen, 2010).
The proposal to convert sugars from biomass into liquid fuels, mainly ethanol and petroleum derivatives, is not new. Since the first oil crisis in the 1970s, governments and scientists have invested in alternatives sources of petroleum, which have relied mostly on biomass. Oil price regularization and its availability reduced the interest in biomass for more than 20 years, until geopolitical instabilities and environmental concerns renewed interest in biomass utilization (Zaldivar et al., 2001; Zanin et al., 2000).
Regarding the applications of cellulases in processes for which products must have low and competitive prices, their production process must be defined for each specific region and final use. There is a consensus among researchers that in order to make the market price of ethanol produced from biomass viable, cellulase production must be done "in situ", that is, at the ethanol production plant. In this case, is important to explore the application of sugarcane bagasse for cellulase synthesis in Brazil (Barta et al., 2008; Frederick et al., 2008; Merino and Cherry, 2007).
According to Galbe et al. (2007), culture medium costs can be a significant fraction of the enzyme cost if an expensive substrate is applied. Although cheap substrates such as sugarcane bagasse are less important to the enzyme cost, they must be carefully chosen as the substrate influences enzyme productivity, an important fraction of the enzyme cost.
Productivity defines the size of the reactors, which, in turn, are a major part of the capital investment. As stated by Himmel et al. (1999), productivity of cellulase in submerged culture (SmC) must be as high as 200 U/L/h, which drives the fraction of the enzyme cost to no higher than US$0.20 per gallon of ethanol.
Cellulases, as well as other enzymes, are excellent microbial products for solid state cultivation when produced by filamentous fungi, because hyphae have the natural ability to cover the solid nutritive surface of the substrate and even to enter its pores, and thus become strongly attached to the substrate (Raimbault, 1998). The easy growth of filamentous fungi on solid media relies on the high capacity of hydrolytic enzyme synthesis in the media along with a high content of polymerized sugars, which are the inducers of gene expression of these enzymes (Sachslehner et al., 1998; Sternberg and Mandels, 1979). The ordinary content of cellulose in abundant natural crop residues, such as sugarcane bagasse and rice straw, is about 40% w/w (Cen and Xia, 1999). When these residues are used as substrates for solid state cultures (SSC), the cellulose concentration in the medium is around 6-28%, considering that moisture levels vary between 30 and 85% (Krishna, 2005), while for SmC, the maximum cellulose concentration is about 0.5 to 6% (Chahal, 1985). Therefore, the induction of cellulase synthesis has reduced power in SmC relative to SSC.
Substrates of solid state cultures, besides being strong inducers of cellulase synthesis, also induce hemicellulases such as xylanases and ligninases, if the solid substrate is composed of hemicellulose and lignin (Sachslehner et al., 1998). Therefore, SSC can result in a more diverse pool of hydrolytic enzymes than SmC. Finally, solid state media are interesting to make good use of the huge amounts of sugarcane bagasse available in Brazil. According to UNICA (Brazil's Union of Sugar Cane Industries), in 2012/2013, the south-central region of Brazil alone processed about 533 million tons of sugar cane, producing around 150 million tons of bagasse.
The objective of this study was to evaluate filamentous fungi and substrates for cellulase production in SSC, in media consisting mainly of sugarcane bagasse, in addition to wheat bran and soybean bran; wheat bran was also applied solely in order to allow comparisons with published data. Besides investigating microorganisms evaluated in various publications, Trichoderma reesei Rut-C30 and Trichoderma reesei QM9414, novel microorganisms were evaluated, such as Trichoderma sp. IPT778, Trichoderma harzianum rifai IPT821, and the recently isolated Myceliophthora thermophila M77. Data were analyzed regarding production (U/gdm), productivity (U/gdm/h), and stability of cellulases.
Materials and Methods
Microorganisms and inoculums
Five microorganisms, Trichoderma reesei Rut-C30 (ATCC 56765), Trichoderma reesei QM9414 (ATCC 26921), Trichoderma sp. IPT778, Trichoderma harzianum rifai IPT821 -the latter two isolated in a sugar cane cultivation area, SP, Brazil -and Myceliophthora thermophila M77, recently isolated through an environmental program for biodiversity preservation in the State of São Paulo (BIOTA FAPESP), were maintained in stock on a solid medium made of wheat bran and sugarcane bagasse (20:80 w/w). One gram of the stock medium containing the spores of each microorganism was suspended in 100 mL of NaCl solution 0.9% (w/w) and this suspension was applied as the inoculum for the SSC.
Culture media
Culture media were made of combinations of sugarcane bagasse (C), wheat bran (W) and soybean bran (S), the compositions on a dry mass basis (w:w) were as follows: W (100); WC (90:10); WC (80:20); WC (40:60); WC (60:40); SC (10:90) and SC (20:80).
Sugarcane bagasse in natura (Usina Iracema, Iracemápolis, São Paulo, Brazil) with 50% moisture on a wet basis was stored at -20 ºC in order to prevent microbial proliferation (Roussos et al., 1991). Upon utilization, the sugarcane bagasse was dried at 50 ºC to 6% moisture, in order to allow the adjustment of initial moisture of the cultivation to 60%, after the addition of the spore suspension and a salt solution. The granulometry of dry sugarcane bagasse to formulate the culture medium was obtained through sieving and selection of the fraction retained between 10 and 20 mesh.
Wheat bran at 13% moisture on a wet basis was supplied by "Anaconda Industrial e Agrícola de Cereais" (São Paulo, Brazil) and soybean bran at 11% moisture was supplied by "Coama Agroindustrial Cooperativa" (Paraná, Brazil). The two types of bran were stored at room temperature as low moisture protects them from microbial proliferation. Upon use, they were dried to allow further moisture adjustment with the addition of the spore suspension and a salt solution, to an initial value of 60 or 80% on a wet basis. Both types of bran were sieved between 8 and 20 mesh.
Solid-state cultures
Solid-state cultures were made in 500 mL Erlenmeyer flasks with 7 g of culture medium plus a salt solution, and sterilized by autoclaving at 121 ºC for 20 min before inoculation with a sufficient volume of the spore suspension to obtain 107 spores/gdm. Initial moisture was adjusted with a salt solution (Urbánszki et al., 2000): 5 g/L KH2PO4; 5 g/L (NH4)2SO4; 1 g/L MgSO47H2O; 1 g/L NaCl; 5 mg/L FeSO47H2O; 1.6 mg/L MnSO4; 3.45 mg/L ZnSO47H2O and 2.0 mg/L CoCl26H2O.The flasks were kept in an incubator at 30 ºC (all fungi except for Myceliophthora thermophila M77) or at 45 ºC (Myceliophthora thermophila M77) for 120 hours. Cultivations were entirely made in duplicate, from the spore suspension to the cultures, except for cultures with soybean bran which were made in triplicate. Samples were collected once a day from the first to the third day and after five days of cultivation.
Analytical methods
Moisture determination
The moisture level was determined by OHAUS MB 35 Halogen Moisture Analyzer (USA) which operates on the thermogravimetric principle, in which the sample is quickly heated by a halogen drying unit for moisture evaporation. At the end of drying, the result is displayed as percent moisture content on a wet basis.
Enzyme extraction
Enzyme extraction was performed by shaking (180 rpm, 20 ºC, 60 min) a mixture of4gofthe cultivated medium with 40 mL of distilled water, 40 mL of citrate buffer pH 4.8 (for all the fungi except for Myceliophthora thermophila M77) or acetate buffer pH 5.0 (Myceliophthora thermophila M77), and one drop of Tween 80. After shaking, the sample was filtered under vacuum through S & S 5802 filter paper (1.2 µm) and the enzyme activity of the filtrate was measured.
Enzyme Activity
Measurements of filter paper activity (FPA) were made according to Ghose (1987) and expressed relative to the mass of the culture medium; that is, as a specific activity. CFPA is the specific cellulase activity in the medium for filter paper activity and was calculated as described in Eq. (1).
where Cgis the measured glucose concentration (g/L); M is the molecular weight of glucose (0.18 g/µmol); t is time interval of reaction of 60 min; u is the moisture of the culture medium (%) and D is the dilution of the liquid extract.
Enzyme productivity
Productivity, PR, is the enzyme production rate, (U/gdm/h), determined as described by Eq. (2), where t is the cultivation time for maximum enzyme activity.
Results and Discussion
Media and microorganisms for cellulase production
Microorganisms and media made mainly from sugarcane bagasse were evaluated in terms of cellulase production and productivity in solid state cultures. Five strains of filamentous fungi selected for this evaluation had either been investigated in previous publications or had been recently isolated with specific features of interest for cellulase production: (1) Trichoderma reesei Rut-C30 (ATCC 56765); (2) Trichoderma reesei QM9414 (ATCC 26921); (3) Trichoderma sp. IPT778; (4) Trichoderma harzianum rifai IPT821, and (5) Myceliophthora thermophila M77.
Wheat bran was applied because it has been reported on in several publications, and was thus considered the reference medium. Also, its high protein content around 17% and high starch content around 19%, on a dry basis, make it an excellent culture medium to provide amino acids, nitrogen, and carbon sources for cellular growth, besides being an excellent support for growth owing to its cellulose and hemicellulose content as high as 39% (Brijwani et al., 2010; Sun et al., 2008). Soybean bran was applied as it is more available than wheat bran in Brazil, and is also a good source of amino acids and organic nitrogen. According to the supplier, the soybean bran applied to the cultures herein contained 46.55% protein, 2.16% lipids, 4.95% fiber, and 12.6% moisture.
The mean values and standard deviations from two or three runs for maximum specific cellulase activity, CFPAmax (U/gdm), and cellulase productivity, PR (U/gdm/h), in each culture medium are depicted in Figures 1 to 5, respectively, for each microorganism.
The data in Figure 1 for Trichoderma reesei Rut-C30 show that the inclusion of sugarcane bagasse (40%) into a medium made from wheat bran resulted in a 60% increase in specific cellulase activity, that is, from 2.5 to 4.0 U/gdm, while productivity decreased by 40%, from 0.05 to 0.03 /gdm/h. The higher enzyme productivity in medium made of 100% wheat bran was probably due to faster cell growth in a medium enriched with organic nitrogen and amino acids, while cultures on media made with sugarcane bagasse resulted in a higher CFPAmax. Media made with more than 40% (w/w) sugarcane bagasse resulted in lower CFPAmax.
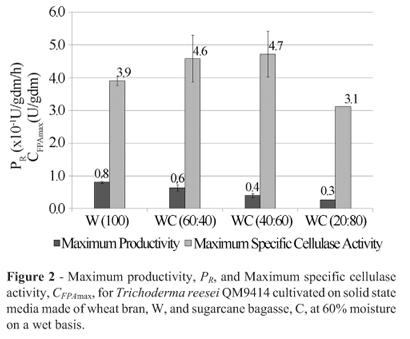
The data in Figure 2 for Trichoderma reesei QM9414 show almost the same behavior in media with 40 or 60% sugarcane bagasse and wheat bran, resulting an 18% increase in CFPAmax relative to the medium made with pure wheat bran, while PRdecreased by 25%, from 0.08 to 0.06 U/gdm/h. It is worth noting that although the general behavior was the same as that of Trichoderma reesei Rut-C30, the significance of the variations was less pronounced, indicating that the strain Trichoderma reesei QM9414 was less sensitive to those media than Trichoderma reesei Rut-C30.
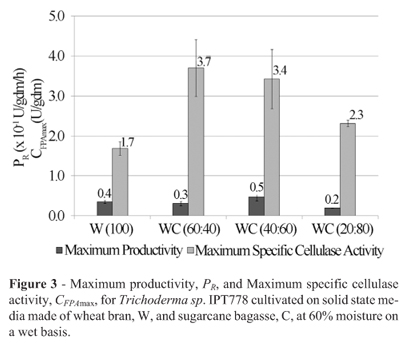
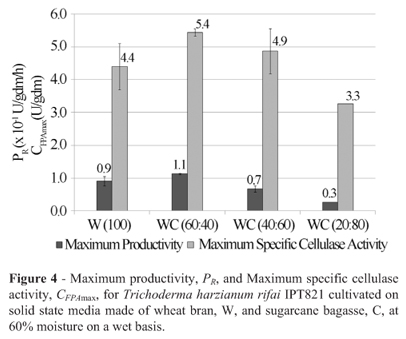
Almost the same results as for Trichoderma reesei Rut-C30 and Trichoderma reesei QM9414 were found for Trichoderma sp. IPT778 (Figure 3) and Trichoderma harzianum rifai IPT821 (Figure 4). However, the improvement in CFPAmax for strain Trichoderma sp. IPT778 was remarkable, 117%, from 1.7 to 3.7 U/gdm upon the inclusion of 40% sugarcane bagasse into the medium; this was an extremely inducible strain.
Myceliophthora thermophila M77 (data in Figure 5) was cultivated in the same media applied for the four aforementioned microorganisms, plus SC (10:90) and SC (20:80) at 60% moisture, and SC (10:90) at 80% moisture. Regarding the media made with wheat bran and wheat bran plus sugarcane bagasse, a similar behavior for Myceliophthora thermophila M77 as for the other microorganisms was seen, with enzyme production in media with C 40% and C 60% higher than in medium with W 100%. The highest CFPAmax in media with C 60% was 154% greater than in W 100%, as it increased from 2.4 to 6.1 U/gdm. In this case, the PR also improved, from 0.05 to 0.13 U/gdm/h, a 160% increase.
Myceliophthora thermophila M77 cultures made in SC (10:90) and SC (20:80) media resulted in higher cellulase production than in media with W and C, in the same proportions, as clearly illustrated in Figure 5. Therefore, S was better than W for enzyme production, a performance that could be due to the higher protein content in S, which probably enhanced growth and protein synthesis. Besides, medium with S as low as 10% was remarkably better for cellulase production compared to media made of 10% W.
Increasing the moisture of the SC (10:90) medium from 60 to 80% resulted in a CFPAmax of 10.6 U/gdm, the highest production among all the results presented, while PRalso remained on the order of the highest values, 0.09 U/gdm/h. Comparing CFPAmax in medium made with SC (10:90) with 80% moisture with CFPAmax achieved using medium W (100) at 60% moisture, the increase was 341%. Although the soybean bran price on the Brazilian market is higher than the wheat bran price -US$412.00/ton and US$212.00/ton, respectively (Corretora Mercado, 2012) half of the mass of the former results in a significantly higher enzyme production.
On the basis of these results, it is possible to conclude that soybean bran is an excellent substrate for cellulase production under high productivities. Sugarcane bagasse is excellent as a cellulase synthesis inducer, besides having a high water retention capacity (Oriol et al., 1988), which is positive for microbial growth. Myceliophthora thermophila M77 is an important microorganism for cellulase production as shown by its high sensitivity to induction imposed by cellulose, and, as a thermophilic fungi, it produces thermostable enzymes, as reported by Zanphorlin et al. (2010) when assaying the enzymes of this fungus.
Sugarcane bagasse besides being an excellent inducer for cellulase synthesis may be an important factor in scaleup of solid state cultivation systems since its addition to the medium alters the structure of the substrate, minimizing compaction of medium and improving its ability for gas exchange, thus contributing to the microorganism growth and enzyme production (Raimbault, 1998).
Specific cellulase activity and productivity in SSC and SmC
Published data on specific cellulase activity, CFPA(U/gdm), and productivity, PR(U/gdm/h), in solid and liquid media cultures of some microorganisms, including the data from the present paper, are presented in Table 1. Specific cellulase activity in the culture media and productivity are important features for calculating enzyme cost. In order to allow comparisons of CFPAand PRin solid and liquid media, both were presented related to media volume, U/mL, by means of Eq. (3), where u is the initial moisture (mass fraction) on a wet basis and dB is the bulk density of the culture medium:
The huge differences among the specific cellulase activities in SSC, from 4 to 247 (U/gdm), cannot be explained on the basis of different microorganisms and media if one considers the range of values presented in Table 1. These variations may be due to differences in the filter paper activity measurement, although many authors refer to Ghose (1987) or Mandels et al. (1976). Following the method of Ghose (1987) rigorously, at least two dilutions of the sample with cellulases must be made. Besides, some authors make modifications in the temperature or in the time interval of the reaction (Silva et al., 2005). The lack of a complete standardization of the method impairs free comparisons. Other aspects that deserve consideration are the high values of CFPAin SmC and SSC made in 1984 and 1985, 30 U/mL and 172 U/gdm, respectively. Both results were obtained with T. reesei Rut-C30 and are far higher than current values; this makes it reasonable to suppose that there have been mutations in the capacity of this microorganism concerning cellulase production. The instability of filamentous fungi for enzyme production is probably associated with the time interval of 25 years and the different methods of strain preservation (Kilikian et al., 1992).
Medium composition of course influences CFPA, but it is clear that for one specific microorganism cultivated in some media, over a restricted time interval of stock culture preservation such as a few months, enzyme activity will not show large variations as shown by the data presented in Figures 1 to 5.
Despite the doubts on the accuracy of the reported values of specific cellulase activity measured using FPA,it is possible to conclude, based on values reported in the last 10 years, that SSC results in cellulase productivities as high or even higher than in SmC. The data from Dillon et al. (2006), for instance, show productivity in SSC 4.6 times greater than in SmC for the same microorganism cultivated in the same laboratory, and therefore using the same FPA measurement procedure.
However, these values are not high enough for the economic viability of cellulase production, taking into account that a significant drop in cost has been found with a productivity increase from 50 to 200 U/L/h as stated by Himmel et al. (1999). Further increases in productivity have a minor impact on enzyme cost. Table 1 shows that a value of 200 U/L/h was not reported by any reference; only Hendy et al. (1984) reached a CFPA close to this, 180.7 U/L/h.
Finally, it is important to note that enzyme activity for SSC, was always based on measures made in liquid extracts, which probably does not contain the entirety of the enzymes produced using solid media (Rodriguez et al., 2006).
Stability of cellulase activity as a function of culture medium
The general behavior of an increase of specific cellulase activity in media followed by a decrease is not fully understood. It is known that in microbial production of enzymes, proteases are frequently synthesized, which may reduce the activity of the target enzyme. According to Haab et al. (1990), high levels of protease in the extracellular culture environment are correlated with the appearance of products of proteolytic cellulase degradation. The data in Figure 6 illustrate the stability of cellulase as a function of the culture medium composition. The novel microorganism, Myceliophtora thermophila M77 cultivated in media with soybean bran was included owing to its good results, while Trichoderma harzianum rifai IPT821 was included owing to the illustrative response to wheat bran. Cellulases of Myceliophtora thermophila M77 were stable in both media with 10% soybean bran, while the increase in S to 20% resulted in a decrease in CFPA from the second day of culture. On the other hand, a medium with 20% W supported a stable cellulase activity produced by Trichoderma harzianum rifai IPT821. Owing to the instability of cellulase activity as a result of the action of proteases, the higher protein content in S relative to W probably means a higher induction in protease synthesis. Above 20% W, which is to say 40, 60 and 100%, there was always a significant decrease in cellulase activity, from the second or third day up to the fifth day of culture.
The kinetics of cellulase production were also determined for all other cultures and showed different levels of instability for a given media and microorganism, which means that cellulase stability, depends on the specific strain in addition to medium composition. However, for the best case scenario among the cultures presented in this paper, Myceliophtora thermophila M77 on a medium made of SC (10:90) at 80% moisture, CFPAwas not only stable, but it continued to increase on the fifth day of culture.
Conclusions
Highest FPA, 10.6 U/gdm, was achieved with Myceliophthora thermophila M77 on soybean bran (S) and sugarcane bagasse (C), (10:90), initial moisture 80%. This activity was 4.4 times higher than production on pure wheat bran (W). C was a strong inducer of cellulase production, given that the maximum CFPAin W and C (40:60), 6.1 U/gdm, was 2.5 times higher than on pure W. T. reesei Rut-C30 did not respond as strongly, with a 1.6-fold surplus production. S advantageously replaced W, as the surplus production on S and C (20:80) was 2.3 times relative to W and C (20:80) for M77.
Abbreviations
SmC, submerged cultures; SSC, solid state cultures; C, sugar cane bagasse; W, wheat bran; S, soybean bran; FPA, filter paper activity; CFPA, specific cellulase activity; CFPAmax, maximum specific cellulase activity; PR, cellulase productivity; T., Trichoderma; gdm, gram of dry matter.
Acknowledgments
The authors greatly acknowledge the financial grant for this research provided by the Brazilian National Council of Scientific and Technological Development (CNPq), the Coordination for the Improvement of Higher Education Personnel (CAPES) and the Studies and Projects Funder Agency (FINEP). We thank Dr. Eleni Gomes from UNESP (SP/BRAZIL) for providing the fungus Myceliophtora thermophila M77 and Dr. Maria Filomena de Andrade Rodrigues from IPT (SP/BRAZIL) for providing Trichoderma sp. IPT778 and Trichoderma harzianum rifai IPT821.
Submitted: March 18, 2012; Approved: April 1, 2013.
All the content of the journal, except where otherwise noted, is licensed under a Creative Commons License CC BY-NC.
- Awafo VA, Chahal DS, Simpson BK (2000) Evaluation of combination treatments of sodium hydroxide and steam explosion for the production of cellulase-systems by two T. reesei mutants under solid-state fermentation conditions. Bioresour. Technol. 73:235-245.
- Barta Z, Sassner P, Zacchi G, Reczey K (2008) Techno-economic aspects of on-site cellulase production. Hung. J. Ind. Chem. 36:5-9.
- Bozell JJ, Petersen GR (2010) Technology development for the production of biobased products from biorefinery carbohydrates -The US Department of Energy's "Top 10" revised. Green Chem 12:539-554.
- Brijwani K, Oberoi HS, Vadlani PV (2010) Production of a cellulolytic enzyme system in mixed-culture solid-state fermentation of soybean hulls supplemented with wheat bran. Process Biochem. 45:120-128.
- Cen P, Xia L (1999) Production of cellulase by solid-state fermentation. Adv Biochem Eng Biotechnol 65:69-92.
- Chahal DS (1985) Solid-State Fermentation with Trichoderma reesei for Cellulase production. Appl Environ Microbiol 49:205-210.
- Corretora Mercado. Available at: http://www.clicmercado.com.br/novo/website/index.asp Acessed: January, 2012.
» link - Dillon AJP, Zorgi C, Camassola M, Henriques JAP (2006) Use of 2-deoxyglucose in liquid media for the selection of mutant strains of Penicillium echinulatum producing increased cellulose and β-glucosidase activities. Appl. Microbiol. Biotechnol. 70:740-746.
- Frederick WJ, Lien SJ, Courchene CE, DeMartini NA, Ragauskas AJ, Iisa K (2008) Production of ethanol from carbohydrates from loblolly pine: a technical and economic assessment. Bioresour Technol 99:5051-5057.
- Galbe M, Sassner P, Wingren A, Zacchi G (2007) Process engineering economics of bioethanol production, in: Olssen, L., Biofuels. Springer Berlin/Heidelberg 303-328.
- Gamarra NN, Villena GK, Gutiérrez-Correa M (2010) Cellulase production by Aspergillus niger in biofilm, solid-state, and submerged fermentations. Appl. Microbiol. Biotechnol 87:545-551.
- Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257-268.
- Haab D, Hagspiel K, Szakmary K, Kubicek CP (1990) Formation of the extracellular proteases from Trichoderma reesei QM9414 involved in cellulase degradation. J Biotechnol. 16:187-198.
- Hendy N, Wilke C, Blanch H (1984) Enhanced cellulase production using solka floc in a fed-batch fermentation. Biotechnol Lett 6:667-672.
- Himmel ME, Ruth MF, Wymans CE (1999) Cellulase for commodity products from cellulosic biomass. Curr Opin Biotechnol 10:358-364.
- Kilikian BV, Facciotti MCR, Schimidell Netto W (1992) Analysis of the kinetic pattern of glucoamylase production regarding the Aspergillus awamori preservation time. Rev Microbiol 23:123-127.
- Krishna C (2005) Solid-State Fermentation Systems -An Overview. Crit Rev Biotechnol 25:1-30.
- Mandels M, Andreotti R, Roche C (1976) Measurement of saccharifying cellulase. Biotechnol. Bioeng Symp 6:21-33.
- Mekala NK, Singhania RR, Sukumaran RK, Pandey A (2008) Cellulase Production Under Solid-State Fermentation by Trichoderma reesei RUT C30: Statistical Optimization of Process Parameters. Appl Biochem Biotechnol 151:122-131.
- Merino ST, Cherry J (2007) Progress and challenges in enzyme development for biomass utilization. Adv Biochem Eng Biotechnol 108:95-120.
- Oriol E, Raimbault M, Roussos S, Viniegra-Gonzales G (1988) Water and water activity in the solid state fermentation of cassava starch by Aspergillus niger Appl Microbiol Biotechnol 27:498-503.
- Raimbault M (1998) General and microbiological aspects of solid substrate fermentation. Elec J Biotechnol 1:174-188.
- Rodriguez JA, Mateos JC, Nungaray J, Gonzalez V, Bhagnagar T, Roussos S, Cordova J, Baratti J (2006) Improving lipase production by nutrient source modification using Rhizopus homothallicus cultured in solid state fermentation. Process Biochem. 4:2264-2269.
- Roussos S, Raimbault M, Viniegra-González G, Saucedo-Castañeda G, Lonsane BK (1991) Scale-up of cellulases production by Trichoderma harzianum on a mixture of sugar cane bagasse and wheat bran in solid state fermentation system. Micol Neotrop Appl 4:83-98.
- Sachslehner A, Nidetzky B, Kulbe KD, Haltrich D (1998) Induction of Mannanase, Xylanase, and Endoglucanase Activities in Sclerotium rolfsii Appl. Environ. Microbiol. 64:594-600.
- Silva R, Lago ES, Merheb CW, Macchione MM, Park YK, Gomes E (2005) Production of xylanase and CMCase on solid state fermentation in different residues by Thermoascus aurantiacus miehe. Braz J Microbiol 36:235-241.
- Sternberg D, Mandels GR (1979) Induction of cellulolytic enzymes in Trichoderma reesei by sophorose. J Bacteriol 139:761-769.
- Sun X, Liu Z, Qu Y, Li X (2008) The Effects of Wheat Bran Composition on the Production of Biomass-Hydrolyzing Enzymes by Penicillium decumbens Appl Biochem Biotechnol 146:119-28.
- Tangnu SK, Blanch HB, Wilke CR (1981) Enhanced Production of Cellulase, Hemicellulase, and beta-Glucosidase by Trichoderma reesei (Rut C-30). Biotechnol Bioeng 23:1837-1849.
- Urbánszki K, Szakacs G, Tengerdy RP (2000) Standardization of the filter paper activity assay for solid substrate fermentation. Biotechnol Lett 22:65-69.
- Zaldivar J, Nielsen J, Olsson L (2001) Fuel ethanol production from lignocellulose: a challenge for metabolic engineering and process integration. Appl Microbiol Biotechnol 56:17-34.
- Zanin GM, Santana CC, Bon EPS, Giordano RCL, De Moraes FF, Andrietta SR, De Carvalho Neto CC, Macedo IC, Lahr Fo D, Ramos LP, Fontana JD (2000) Brazilian Bioethanol Program. Appl Biochem Biotechnol 84:1147-1161.
- Zanphorlin LM, Facchini FA, Vasconcelos F, Bonugli-Santos RC, Rodrigues A, Sette LD, Gomes E, Bonilla-Rodriguez GO (2010) Production, Partial Characterization, and Immobilization in Alginate Beads of an Alkaline Protease from a New Thermophilic Fungus Myceliophthora sp J Microbiol 48:331-336.
Send correspondence to
Publication Dates
-
Publication in this collection
11 Apr 2014 -
Date of issue
2014
History
-
Received
18 Mar 2012 -
Accepted
01 Apr 2013