Abstract
A rice straw -cellulose utilizing mold was isolated from rotted rice straw residues. The efficient rice straw degrading microorganism was identified as Trichoderma reesei. The results showed that different carbon sources in liquid culture such as rice straw, carboxymethyl cellulose, filter paper, sugar cane bagasse, cotton stalk and banana stalk induced T. reesei cellulase production whereas glucose or Potato Dextrose repressed the synthesis of cellulase. T. reesei cellulase was produced by the solid state culture on rice straw medium. The optimal pH and temperature for T. reesei cellulase production were 6 and 25 ºC, respectively. Rice straw exhibited different susceptibilities towards cellulase to their conversion to reducing sugars. The present study showed also that, the general trend of rice straw bioconversion with cellulase was more than the general trend by T. reesei. This enzyme effectively led to enzymatic conversion of acid, alkali and ultrasonic pretreated cellulose from rice straw into glucose, followed by fermentation into ethanol. The combined method of acid pretreatment with ultrasound and subsequent enzyme treatment resulted the highest conversion of lignocellulose in rice straw to sugar and consequently, highest ethanol concentration after 7 days fermentation with S. cerevisae yeast. The ethanol yield in this study was about 10 and 11 g.L-1.
cellulase; Trichoderma reesei; rice straw residues; enzymatic hydrolysis; bioethanol
INDUSTRIAL MICROBIOLOGY
RESEARCH PAPER
Bioethanol production from rice straw residues
Elsayed B. Belal
Agricultural Microbiology, Dept. of Agric. Botany, Fac. of Agric., Kafrelsheikh University, Kafr El-Sheikh, Egypt
Send correspondence to Send correspondence to: E.B. Belal Agricultural Microbiology, Dept. of Agric. Botany, Fac. of Agric., Kafrelsheikh Univ 33516 Kafr El-Sheikh, Egypt E-mail: elsayedb@yahoo.com
ABSTRACT
A rice straw -cellulose utilizing mold was isolated from rotted rice straw residues. The efficient rice straw degrading microorganism was identified as Trichoderma reesei. The results showed that different carbon sources in liquid culture such as rice straw, carboxymethyl cellulose, filter paper, sugar cane bagasse, cotton stalk and banana stalk induced T. reesei cellulase production whereas glucose or Potato Dextrose repressed the synthesis of cellulase. T. reesei cellulase was produced by the solid state culture on rice straw medium. The optimal pH and temperature for T. reesei cellulase production were 6 and 25 ºC, respectively. Rice straw exhibited different susceptibilities towards cellulase to their conversion to reducing sugars. The present study showed also that, the general trend of rice straw bioconversion with cellulase was more than the general trend by T. reesei. This enzyme effectively led to enzymatic conversion of acid, alkali and ultrasonic pretreated cellulose from rice straw into glucose, followed by fermentation into ethanol. The combined method of acid pretreatment with ultrasound and subsequent enzyme treatment resulted the highest conversion of lignocellulose in rice straw to sugar and consequently, highest ethanol concentration after 7 days fermentation with S. cerevisae yeast. The ethanol yield in this study was about 10 and 11 g.L-1.
Key words: cellulase, Trichoderma reesei, rice straw residues, enzymatic hydrolysis, bioethanol.
Introduction
Ethanol from renewable resources has been of interest in recent decades as an alternative fuel to the current fossil fuels. Lignocellulosic biomass like wood and agricultural crops residues, e.g., straw and sugar beet pulp are potential raw materials for producing several high-value products like fuel ethanol and biodiesel. lignocelluloses contains up to 80% from the polysaccharides (Kaparaju et al., 2009). These renewable raw materials look promising for replacing environmentally unfriendly fossil hydrocarbon raw materials and hence, creating "green" products. In contrast to traditional fuels, bioethanol does not contribute to the greenhouse effect, being a CO2 neutral resource.
Rice straw is a by-product of rice production and great bioresource. It is one of the abundant lignocellulosic waste materials in the world. It is annually produced about 4 million tons in Egypt. Rice straw can potentially produce 205 billion liter bioethanol per year in the world, which is about 5% of total of consumption. It is the largest amount from a single biomass feedstock. Rice straw predominantly contains cellulose 32-47%, hemicelluloses 19-27%, lignin 5-24% and ashes 18.8%. The pentoses are dominant in hemicelluloses which contains xylose. Xylose is the most important sugar followed by arabinose and hexoses. The carbohydrate of rice straw involves glucose 41-43.4%, xylose 14.8-20.2%, arabinose 2.7-4.5%, mannose 1.8% and galactose 0.4% (Roberto et al., 2003). Changes in how agricultural field residues are managed further complicate farming economies. In the past, disposal of straw by burning was an accepted practice. This practice is now being challenged due to concern over the health effects of smoke from burning fields. In these waste products, the polysaccharides, cellulose and hemicellulose are intimately associated with lignin in the plant cell wall (Ballerini et al., 1994). The lignin component acts as a physical barrier and must be removed to make the carbohydrates available for further hydrolysis processes. Therefore, the pretreatment is a necessary process for utilization of lignocellulosic materials to obtain ultimately high degree of fermentable sugars. Bioconversion of cellulosic biomass into fermentable sugar, for production of ethanol using microorganisms, especially cellulose degrading fungi, makes bioethanol production economic, environmental friendly and also renewable. Several pre-treatment processes have been developed for lignocelluloses, which function by an enlargement of the inner surface area. This is accomplished partly by solubilization of the hemicelluloses and partly by degradation of the lignin. The pre-treatments are: Milling and grinding, pyrolysis, high-energy radiation, high pressure steaming, alkaline or acid hydrolysis, hydrogen peroxide treatment, hydrothermal treatment, steam explosion, wet oxidation and biological treatment such as enzyme or microbial conversion: (Fan et al., 1982; McGinnis et al., 1983; Hormeyer et al., 1988; Olson and Hahn-Hagerdahl, 1997; Bollok 1999; Soni et al., 2010).
Studies are currently being made on conversion of the residues by microbial and enzymatic degradation to usable products such as enzymes and sugar syrups which uses in different applications (Andren et al., 1976). Cellulolytic enzymes play an important role in natural biodegradation process in which plant lignocellulosic materials are efficiently degraded by cellulolytic fungi and bacteria. In industry, these enzymes have found novel applications in the production of fermentable sugars and ethanol (Olson and Hahn-Hagerdahl, 1997; Levy et al., 2002; Nunes et al., 2011). Fungal cellulases are inducible enzymes that are usually excreted into the environment (Bhat and Bhat, 1997) and depend on cellulose type (amorphous or crystalline) acting on the organism (Ortega et al., 2001). The role of the fungi Acremonium spp., Chaetomium spp., Trichoderma reesei, Trichoderma viride, Penicillium pinophilum, Phanerochaete chrysosporium (Sporotrichum pulverulentum), Fusarium solani, Talaromyces emersonii, Trichoderma koningii, Fusarium oxysporum, Aspegillus niger and Rhizopus oryzae in the cellulose degradation process in various environments has been well documented (Toyama et al., 1981; Teeri, and Koivula, 1995; Bhat and Bhat, 1997; Schülein 1997; Murashima et al., 2002; Kovacs et al., 2009).
Therefore, in the present study an attempt has been made for the optimization of the cellulase production from fungal strain and its application in the bioconversion of rice straw residues into glucose for the production of second generation bioethanol.
Materials and Methods
Media
Mineral salt medium (MSL) as described by Drews (1968), Luria Bertani Medium (LB), Trypticase soy broth (TSB) and Potato Dextrose Agar (PDA) were used in the present study.
Sampling and cellulolytic microorganisms isolation
Samples of rotted rice straw were collected from stored rice straw by the farmers from different regions in Kafr EL-Sheikh Governorate, Egypt.
In laboratory,1gof rotted -milled rice straw was added to the conical flask containing 99 mL of MSL medium and mixed for 30 min on a rotary shaker (150 rpm) at room temperature. Ten-fold dilutions were prepared and then 100 µL of each dilution were spread on plates containing MSA (mineral salt agar) + carboxymethyl cellulose (10 gµL as a sole source of carbon) (pH 7; for bacteria), (pH 5.5; for fungi) using a glass spreader. Petri plates were then incubated at 25 ºC for 7 days. The isolates were maintained on respective media slants. The plates were incubated at 25 ºC for 24 h monitored for appearance of clear zone. For observations, plates were stained with 1% Congo red dye (15 min), followed by destaining with 1 M NaCl solution for 20 min. Cellulolytic strains were selected on the basis of the hydrolysis zone surrounding the colonies as described by Teather and Wood (1982), Bradner et al. (1999), Peciulyte (2007) and Belal (2008). The cultures were identified based on the cultural, morphological and biochemical characteristic as described by (Rifai 1969; Domsch et al., 1980; Parry et al., 1983; Burgess et al., 1994).
Saccharification of rice straw by the isolated microorganisms in liquid culture
The isolated colonies were then tested for their ability to grow and degrade rice straw. Rice straw was milled and sieved to 40 mesh. One gram of milled rice straw was inserted into each 500 mL Erlenmeyer flask with cotton stopper. One hundred mL of the mineral salt medium was transferred into a 500 mL Erlenmeyer flask, and after autoclaving was inoculated with 3 mL from fungal spores suspension containing 106 spores/mL or bacterial cell suspension containing 107 cfu/mL. The cultures were incubated at 150 rpm and 25 ºC for 14 days. After 14 days of cultivation, culture aliquots were filtered through cheese cloth and centrifuged at 5000 rpm to remove solids and filtered using sterile membrane filter (0.2 mm). The supernatant was used as the crude enzyme solution (Belal and El-Mahrouk, 2010). The protein concentration of enzyme was determined according to Lowry et al. (1951). The supernatants were assayed for their enzymatic activity. Cellulase activity was determined by incubating 0.5 mL of the supernatant (at a concentration of 300 /g protein/mL) with 0.5 mL of 0.6 mg of filter paper No.1 in 0.05 M citrate buffer (pH 4.8). It was incubated with agitation at 50 ºC for 12 h. After incubation, the reaction was terminated by adding 3 mL of 1% 3,5-dinitrosalicylic acid (DNS) reagent to 1 mL of the reaction mixture and heated for 10 min. In these tests, reducing sugars were estimated calorimetrically according to Miller (1959), using glucose as standards. One unit of cellulase activity is defined as the amount of enzyme that releases 1 µmol reducing sugars (measured as glucose) per ml per minute.
Effect of different substrates on Trichoderma reesei cellulase production in liquid culture
Different substrates such as carboxymethyl cellulose, filter paper, bagasse, rice straw, cotton stalk, banana stalk, glucose were used in mineral salt medium to evaluate their effect on T. reesei and cellulase production. Each substrate was dried and milled. One gram of each substrate was inserted into each 500 mL Erlenmeyer flask with cotton stopper. One hundred mL of the mineral salt medium was transferred into a 500 mL Erlenmeyer flask, and after autoclaving was inoculated with 3 mL from fungal spores suspension containing 106 spores/mL (one-week-old colonies of fungi grown at 25 ºC on PDA plates). Potato dextrose (PD) was used as complex medium and it was carried at the same conditions. Mineral salt medium was inoculated with T. reesei in absence of any substrate. The cultures were incubated at 150 rpm and 25 ºC for 14 days. The activity of T. reesei cellulase and reducing sugars were determined as described above.
Cellulase production in solid state fermentation
Solid state fermentation method was used since it consumes lesser power but produce more concentrated product. Thirty grams of pretreated rice straw were inserted into each 500 mL Erlenmeyer flasks with cotton stopper. Mineral salt liquid medium was used as supplement where 60% (6 mL g-1 substrate) of the solution was added into the flask. Each flask was finally inoculated with 10 mL from fungal spores suspension containing 106 spores/mL. The spore suspension was obtained from 7 day-old pure culture. After mixing, flasks were incubated at 25 ± 1 ºC under static conditions for 32 days (Belal 2003).
Determination of optimal pH and temperature for T. reesei cellulase production was investigated in solid state fermentation. The optimum pH for cellulase production was estimated at various pH values 4.5, 5, 5.5, 6, 6.5, 7, 7.5 and 8 and with appropriate buffer at 25 ºC for 32 days. For determination of optimum temperature for cellulase production, the reactions were carried out at 20, 25, 30, 35 and 40 ºC at pH 6.5. The mycelia were grown at the test pH or temperature under the conditions mentioned above. Cellulase activity was determined from the culture filtrate. Cellulase extraction was done by adding 300 mL of 0.05 M citrate buffer into each flask and the mixture incubated at 25 ºC on an orbital shaker, at 200 rpm min1 for 1 h. The suspended slurry was filtered through cheese cloth and centrifuged at 5000 rpm for 20 min and filtered using sterile membrane filter (0.2 mm). The supernatant was used as the crude enzyme solution (Belal and El-Mahrouk 2010). The protein concentration of enzyme was determined according to Lowry et al. (1951). Cellulase activity was determined as described above.
Bioconversion of rice straw residues into bioethanol using T. reesei cellulase
Microorganism
T. reesei as efficient rice degrading microorganism was used for solid state fermentation as described above.
Enzymatic saccharification
One gram pretreated rice straw with 0.495 U/ g rice straw of crude in 0.05 M citrate buffer at pH 4.8 was added into in 100 mL Erlenmeyer flasks with magnetic bar. Final volume was adjusted to 50 mL using citrate buffer. It was incubated at 50 ºC for 12 h with agitation. The experiment was performed with 0.3 mg/mL chloramphenicol. After incubation, the reaction was terminated by adding 3 mL of 1% 3,5-dinitrosalicylic acid (DNS) reagent to 1 mL of the reaction mixture and heated for 10 min. In these tests, reducing sugars were estimated calorimetrically according to Miller (1959), using glucose as standards. One unit of cellulase activity is defined as the amount of enzyme that releases 1 µmol reducing sugars (measured as glucose) per mL per min.
Alkali pre-treatment
About 50 g milled dried rice straw were suspended in 5% NaOH in ratio of 1 : 10 (w/v) rice straw and NaOH. After that the samples were incubated in water bath 85 ºC for 1 h. (Yoswathana and Phuriphipat, 2010). Finally, pretreated sample was pressed through cheese cloth. The amount of reducing sugar in juice was measured as described above.
Acid pretreatment
About 50 g chopped dried rice straw was suspended in acid solution (1% Sulfuric acid) in ratio of 1: 10 (w/v) rice straw and Sulfuric acid. The mixtures were autoclaved at 121 ºC for 15 min. (Yoswathana and Phuriphipat, 2010). After that, the treated sample was pressed through cheese cloth and the amount of reducing sugar in juice was measured as above.
Alkali /enzyme pretreatment
The previous alkali pretreatment condition was carried out for alkali/enzyme pre-treatment. The NaOH treated sample was pressed through cheese cloth. The juice was kept and the remaining pulp was mixed with citrate buffer (1:10 w/v) containing enzyme (0.495 U/ g rice straw). The pH of sample was adjusted at pH 4.8. Sample was incubated in a water bath at 50 ºC for 12 h. The reaction was terminated as described above. After that the sample was pressed through cheese cloth and the juice was carried out for sugar content measurement.
Acid /enzyme pretreatment
The previous acid pretreatment condition was carried out for acid/enzyme pre-treatment. For enzymatic hydrolysis the sample was treated as described in alkali/enzyme pretreatment (Yoswathana and Phuriphipat, 2010).
Ultrasound pretreatment
Samples of the mixture of acid pre-treated rice straw (1% acid) and citrate buffer (pH 4.8) at a weight ratio of 1:10, was placed in glass beaker, and were subjected to ultrasound pretreatment before the addition of enzyme. The sample was treated with ultrasoound at 40 W for 10 min and the temperature during ultrasonic treatment was 50 ºC. After ultrasound treatment the sample was subjected to enzyme (0.495 U/ g rice straw) treatment as described above. The reaction was terminated as described above. After that the sample was pressed through cheese cloth and the amount of reducing sugar was measured as mentioned above. One treatment was subjected to ultrasound without any treatment with acid or enzyme. All treatments were conducted in triplicate (Yoswathana and Phuriphipat, 2010).
Detoxification
The pretreated samples with acid, ultrasound and enzyme were mixed with wood activated charcoal (20:1 w/w sample: Charcoal) and then agitate for 2 days on magnetic stirrer at room temperature. The samples were filtered through filter paper No. 5 (Whatman, Germany) after charcoal treatment to remove the charcoal (Yoswathana and Phuriphipat, 2010). The filtrate was subjected for sugar measurement as described above.
Fermentation
Bioethanol fermentation was conducted in liquid state fermentation. The yeast Saccharomyces cerevisae was used for fermentation. The yeast inoculum was prepared in YEPD broth. A loopful of twenty four hours old culture was inoculated and incubated at 30 ºC on rotary shaker (200 rpm) for twenty four hours. The initial yeast count in fermentation sample was 8x108 cfu /mL.
The production medium used for ethanol fermentation was composed of: glucose (sugars solution obtained from saccharified rice straw), 0.1% KH2PO4, 0.5% (NH4)2SO4, 0.05% MgSO4.7H2O and 0.1% yeast extract, pH was adjusted to pH 5. The medium is introduced in 250 mL capacity flasks containing 100 mL of the fermentation medium. The pH of the medium was adjusted to 5. This inoculum was used at 10 percent to inoculate saccharified rice straw from the pretreated samples. All experiments were incubated at 30 ºC on rotary shaker (200 rpm) for 7days. The ethanol content was measured after 7days fermentation (Sandhu et al., 1998; Patel et al., 2007).
Ethanol estimation
One mL of the fermented wash was taken in 500 mL pyrex distillation flask containing 30 mL of distilled water. The distillate was collected in 50 mL flask containing 25 mL of potassium dichromate solution (33.76 g of K2Cr2O7 dissolved in 400 mL of distilled water with 325 mL of sulphuric acid and volume raised to 1 litre). About 20 mL of distillate was collected in each sample and the flasks were kept in a water bath maintained at 62.5 ºC for 20 min.
The flasks were coaled to room temperature and the volume raised to 50 mL. Five mL of this was diluted with 5 mL of distilled water for measuring the optical density at 600 nm using spectrophotometer (Caputi et al., 1968). A standard curve was prepared under similar set of conditions by using standard solution of ethanol containing 2 to 14% (v/v) ethanol in distilled water and then ethanol content of each sample was estimated (Yoswathana and Phuriphipat, 2010).
Results and Discussion
There are several important aspects that should be considered for the development of any bioprocess in solid state fermentation. These include selection of suitable microorganism and substrate, optimization of process parameters and separation of the product. In order to achieve high enzyme yield, efforts are made to develop a suitable medium and to work out the favourable environmental conditions for the proper growth and maximum secretion of enzyme. Development of such medium requires using the right selection of cheaper and readily available components.
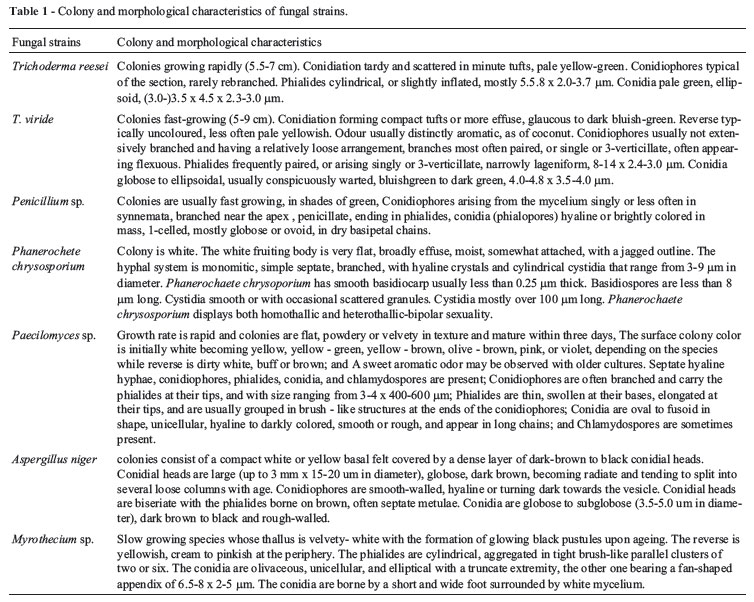
Rotted rice straw residues were used as source for isolation of the cellulolytic microorganisms in the present work. Eight microorganisms were isolated by using clear zone formation on MSA (mineral salt agar) containing carboxymethyl cellulose as a sole source of carbon. A preliminary classification based on cultural and morphological characteristics of the isolates revealed that the rice straw residues -degrading microorganisms belong to the group of fungi as well as to the group of bacteria. Among 8 isolated strains, seven fungal strains were identified as Trichoderma reesei, T. viride, Penicillium sp., Phanerochete chrysosporium, Pacilomyces sp., Aspergillus niger and Myrothecium sp. (Table 1).
One out of 8 rice straw -degrading microorganisms was gram -positive, motile, rod shaped bacterium and spore former. Results of identification (Table 2) showed that, the rice straw degrading bacterial strain was identified as Bacillus pumilus.
Fungi are well-known agents of decomposition of organic matter in general and cellulose substrates in particular (Lynd et al., 2002; Soni et al., 2010).
Obviously, fungi play an outstanding role in degrading rice straw, since the majority of strains belong to this group. It is known that many genera of fungi play an important role in degradation of anthropogenic substrates. The bacterial isolate were also routinely streaked onto plates of nutrient agar or nutrient agar for bacterial strains but the fungal strains were further purified by using acidic complex medium (PDA).
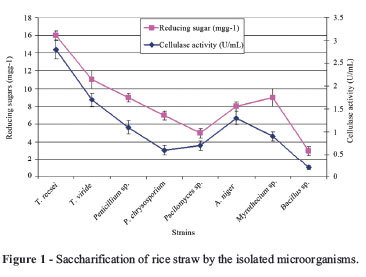
Results in Figure 1 showed that the strains were tested for their growth ability on MSL supplemented with rice straw as a sole source of carbon. Among 8 isolated strains, one strain was identified as T. reesei exhibited higher cellulase and reducing sugars productivity from rice straw than the other isolated strains. It indicates that this strain have the highest degradability for rice straw than the other strains. Trichoderma is known as a very good producer of cellulases, perhaps due to the different adaptability of fungi to the anthropogenic substrates and different resistance to the factors affecting fungal populations during the recycling procedures. Our results are in agreement with previous findings reported by Teather and Wood (1982), Bradner et al. (1999) and Peciulyte (2007).
Therefore, Trichoderma reesei as efficient for productivity of extracellular cellulase and reducing sugars was selected for the further studies.
Cellulase production with various carbon sources
T. reesei was grown in mineral salt medium with various carbon sources such as carboxymethyl cellulose, filter paper, bagasse, rice straw, cotton stalk, banana stalk, glucose at 25 ºC for 14 days with shaking at 150 rpm. PD was used as complex medium.
The obtained results in Figure 2 indicate that the extracellular cellulase was produced only during growth of T. reesei on carboxymethyl cellulose, filter paper and all agricultural residues (bagasse, rice straw, cotton stalk, and banana stalks) in MSL medium as carbon sources. The results demonstrated that a maximum cellulase activity was obtained when carboxymethyl cellulose followed by filter paper, bagasse, rice straw, cotton stalk were used as substrate. On the other hand in PD as a complex medium or in MSL + glucose, enzyme secretion is not induced. According to Schlegel (1992) most enzymes systems involved in substrate degradation are inductive enzymes. In this study i found that when glucose was used as a carbon source, no activity was detected whereas higher amounts were produced when the carboxymethyl cellulose, filter paper and all agricultural residues (bagasse, rice straw, cotton stalk, and banana stalks) were used as a carbon source. PD was also suppressed T. reesei cellulase production. The choice of an appropriate substrate is of great importance for the successful production of cellulase. The substrate not only serves as a carbon source but also produces the necessary inducing compounds for the microorganism. This results are in agreement with my previous findings and other investigators while secretion of Poly(s-caprolactone) hydrolase was only induced in the culture supernatant with Poly(s-caprolactone) as aliphatic homopolyester or BTA 45:55 (Ecoflex) as copolyester as substrates but was not induced on glucose or GYM as complex medium (Lin and Kolattukudy, 1978; Oda et al., 1995; Murphy et al., 1996; Belal 2003; Belal 2008).
Effect of pH and temperature on cellulase production in solid state fermentation
Environmental factors do not only influence the rice straw residues to be degraded, they also have a crucial influence on the microbial population and on the activity of the different microorganisms themselves and also the amount of the enzyme production depends on the biomass. Factors such as temperature and pH (Karpouzas and Walker, 2000), have important effects on the microbial degradation of rice straw and so these conditions must be considered when the biodegradability of rice straw is tested. Belal (2008) found that Trichoderma viride cellulase affected by pH and temperature. Studies were performed in solid state fermentation to optimize different fermentation conditions (pH and temperature) for cellulase production from T. resei. The production of cellulase by T. reesei F-418 was studied in solid-state fermentation (SSF). Solid state fermentation has numerous advantages over submerged fermentation (SmF), including superior productivity, simple technique, low capital investment, low energy requirement and less wastewater output and better product recovery (Asgher et al., 2006).
Optimum pH
The influence of pH on biomass yield of T. reesei cellulase and reducing sugars production is shown in Figure 3. Generally, the optimum pH was 6 for Trichoderma reesei. The maximum T. reesei cellulase and reducing sugars production were recorded at pH6. Trichoderma reesei grew at quite wide pH range (from 4 to 8). This variation is very useful to use these isolates in degradation test in different environments at different pH. Therefore, it can expect that this strain can tolerate the pH change during the degradation process thereby increase the degradation potential for this strain. The optimal pH for fungal cellulases varies from species to species, though in most cases the optimum pH ranges from 3.0 to 6.0 (Garg and Neelakantan, 1981; Niranjane et al., 2007).
Optimum temperature
The temperature for cellulase production of T. reesei was optimized. The effect of different temperatures on T. reesei cellulase production is shown in Figure 4. The optimum temperature for maximal cellulase production was found to be 25 ºC at pH 6. Further increase in temperature resulted in decrease in cellulase production. A temperature 25 ºC appears to be the optimum for T. reesei cellulase production.
Hence optimum pH 6 and optimum temperature 25 ?C were used in all the subsequent experiments
Production of bioethanol from rice straw
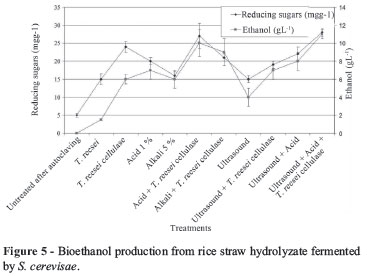
Figure 5 illustrates the obtained reducing sugars resulted from different treatment with T. reesei, T. reesei cellulase, Acid 1%, Alkali 5% and ultrasound for rice straw. The individual treatment and the combination of the treatments resulted in high yield of reducing sugars. This results show the positive effect of T. reesei, T. reesei cellulase, Acid 1%, Alkali 5% and ultrasound treatment during pretreatment of rice straw on polysaccharide conversion into sugar. In case of the treatment with Acid 1%, Alkali 5% and ultrasound and subsequent with T. reesei cellulase were found to be effective. Autoclaving for sterilization has affected and resulted in increase in sugar content.
The production of cellulase is a key factor in the hydrolysis of cellulosic material and it is essential to make the process economically viable. Reduction in the cost of cellu-lase production can be achieved by the use of cheap and easily available substrates. T. reesei cellulase was produced by the solid state culture. This enzyme effectively led to enzymatic conversion of acid, alkali and ultrasonic pretreated cellulose from rice straw into glucose, followed by fermentation into ethanol. As shown in Figure 5, a maximum increase in glucose concentration was achieved after acid pre-treatment or ultrasound and subsequent enzyme. The combined method of acid pretreatment with ultrasound and subsequent enzyme treatment resulted the highest conversion of rice straw to sugar and consequently, highest ethanol concentration after 7 days fermentation with S. cerevisae. The ethanol yield was also observed to be the highest in these treatments. The ethanol yield in this study was 11 g.L-1.
The production of ethanol from any lignocellulosic biomass generally involves four process -feedstock pretreatment, enzymatic saccharification, fermentation and ethanol recovery. Lignocellulosic residues including rice straw, sugarcane bagasse, wheat straw, corn stover, spruce and municipal solid waste have been researched by several workers for microbial and enzymatic bioconversion with commercial or in-house produced cellulase into glucose employing various pretreatment protocols including acid, alkali and steam (Li et al., 2007; Patel et al., 2007; Kovacs et al., 2009; Rabelo et al., 2009; Yoswathana and Phuriphipat, 2010). Following pretreatment, plant cell wall polysaccharides are more susceptible to enzymatic hydrolysis that breaks them into monomeric (single) sugars that can be fermented into ethanol (Lynd et al., 1999).
Cellulase production from rice straw with fungi like Trichoderma reesei through solid state fermentation is important because in this way production of cellulase can be increased, which further help to produce cellulose. This an important enzyme required for breakdown of polysaccharides into monosaccharide, those can further converted into ethanol and other alcohols through fermentation process. Cellulase has a lot of industrial applications including production of food and medicines and help to breakdown the waste plants materials to clean up the environment.
The positive effect of acid pre-treatment or ultrasonic and subsequent enzyme treatment could be explained as better access of enzyme to lignocellulose materials in rice straw.
The ultrasound pre-treatment and subsequent enzyme treatment resulted in yield of reducing sugars. This phenomenon was attributed to the effect of ultrasound on the structure of cellulose and its crystalline arrangement. Namely, cellulose granules contain both ordered crystalline regions and amorphous regions, in which polymer chains are less well ordered and more susceptible to attack by cellulase action. Thereby, the ultrasound pretreatment may be effective.
The acid pre-treatment or ultrasound and subsequent enzyme treatment yielded high amount of reducing sugar which were fermented to yield ethanol. The final glucose concentration decreased, due to enzyme inhibition by glucose accumulation. Similarly, Kolusheva and Marinova (1991) concluded that elevated concentration of glucose significantly decreased the starch hydrolysis rate and affected the enzyme inhibition. Vlasenko et al. (1997) have found that after acid pretreatment (10% solid mater, 0.8% acid and 160 ºC) followed by enzyme treatment the yield of glucose was 43 g L-1 or 43% conversion of cellulose to glucose. The results in present study are low than (Vlasenko et al., 1997) reported. This is may be due to lower temperature (121 ºC compare to 160 ºC) during acid treatment or because of different conditions during enzyme treatment. The decrease of sugar content in acid treated samples is may be to convert of monomeric sugars (xylose, glucose) to furfural and hydroxymethyl furfural. These substances are toxic substances for yeast and can inhibit the yeast growth. During detoxification of samples part of sugar could be adsorbed on activated charcoal leading reduction of total sugar in sample.
In the present study, Alkali rice straw was used for production of cellulase by T. reesei F-418. The purpose of the alkaline pretreatment was delignification, the removal of lignin is necessary for cellulose to become readily available for the enzymes, which permit the yeast to convert the glucose into ethanol (Wyman 1994). Combined acid pretreatment and ultrasonic followed by enzyme treatment resulted highest sugar yield. This results show the effectiveness of combination of physical and chemical pre-treatment prior to enzymatic hydrolysis of lignocellulose material to sugar. Abedinfar et al. (2009) have investigated the fermentation of rice straw (pretreated with diluted acid and subsequent enzyme treatment) rice straw using Mucor indicus and Rhizopus oryzae. They have found an ethanol yield of 0.36-0.43 g g-1 using Mucor indicus which was comparable with the corresponding yield by S. cerevisae (0.37-0.45). Rhizopus oryzae produce 0.33-0.41 gµL ethanol. The ethanol yield in this study was about 0.42 gµL. That is in accordance with ethanol yield reported from literature (Abedinfar et al., 2009). The remaining sugar of 35-45% w/v is may be xylose. Xylose is pentose sugar that can not be digested by S. cerevisae. Bioconversion offers a cheap and safe method of not only disposing the agricultural residues by solid substrate fermentation (SSF) of rice straw under optimum conditions, but also it has the potential to convert lignocellulosic wastes into usable forms such as enzymes and reducing sugars that could be used for ethanol production. This enzyme system effectively led to enzymatic conversion of ultrasonic and acid pretreated cellulose from rice straw into glucose, followed by into ethanol. These results help to reduce the environmental pollution which caused by agricultural residues.
Acknowledgement
I would like to thank Kafrelsheikh University (Researches Support Fund) for financial support of this project. The author is grateful to Dr. Z. Morsy, Mr. M. Salem, Mr. A. Khedr and Mr. A. Gad for their helpful to achieve this work.
References
Abedinfar S, Karimi K, Khananhmadi M, Taherzadeh M (2009) Ethanol production by Mucor indicus and Rhizopus oryzae from rice straw by separate hydrolysis and fermentation. Biomass Bioenergy 33:828-833.
Andren RK, Mandels M, Modeiros JE (1976) Production of sugars from waste cellulose by enzymatic hydrolysis: Primary evaluation of substrates, Process Biochem, Ocobetr, p. 2-11 (C F Toyama S, Yonaha K, and Ishihara M (1981) Degradation of bagsse cellulose by Acremonium sp. W-398.Sci Bull Coll AGr Univ Ryukyus, 28:89-99).
Asgher M, Asad MJ, Legge RL (2006) Enhanced lignin peroxidase synthesis by Phanerichaete chrysosporium in solid state bioprocessing of a lignocellulosic substrate. World J Microbiol Biotechnol 22:449-453.
Ballerini D, Desmarquest JP, Pourquie J (1994) Ethanol production from lignocellulosics: Large scale experimentation and economics. Biores Tec 50:17-23.
Belal EBA (2003) Investigation on the biodegradation of polyesters by isolated mesophilic microbes. Dissertation, Technical University Braunschweig, Germany.
Belal EB (2008) Biodegradation of wastepaper by Trichoderma viride and using bioprocessed materials in biocontrol of damping -off of pea caused by Pythium debaryanum.J Agric Res Kafrelsheikh Univ 34:567-587.
Belal EB, El-Mahrouk ME (2010) Solid -State fermentation of rice Straw residues for its use as growing medium in ornamental nurseries. Acta Astronautica 67:1081-1089.
Bhat M, Bhat S (1997) Cellulose degrading enzymes and their potential industrial applications. Biotechnol Adv Vol 15, p. 583-620.
Bollok M (1999) Studies on ethanol production on lignocellulosics: Agricultural and Chemical Technology. Technical University of Budapest, Hungary.
Bradner JR, Gillings M, Nevalainen KMH (1999) Qualitative assessment of hydrolytic activities in Antarctic microfungi grown at different temperatures on solid media. World J Microbiol Biotechnol 15:131-132.
Burgess LW, Summerell BA, Bullocks SG, Backhouse KPD (1994) Laboratory Manual for Fusarium Research. 3rd edition. University of Sydney, 133 pp.
Caputi AJ, Ueda M, Brown T (1968) Spectrophotometric determination of ethanol in wine. Am J Enol Vitic 19:160-165.
Domsch KH, Gams W, Andersson T-H (1980) In: Compendium of soil fungi, 1. Academic Press, London, .
Drews G (1968) Mikrobilogishes Praktikum fuer Natuwissenschaftler, Springer, Verlag, Berlin-Heidelberg -New York, in Alef, K. (1991). Methodenhandbuch Bodenmikrobiologie Bayreuth, Deutschland.
Fan LT, Lee YH, Gharpuray MM (1982) The nature of lignocellulosics and their pretreatments for enzymatic hydrolysis. Adv Biochem Eng 23:157-187.
Garg SK and S Neelakantan (1981) Effect of cultural factors on cellulase activity and protein production by Aspergilllus terreus. Biotechnol Bioeng 23:1653-1659.
Hormeyer HF, Schwals W, Bonn G, Bobleter O (1988) Hydrothermolysis of birchwood as pretreatment for enzymatic saccharification. Holzforschung 42:96-98.
Kaparaju P, Serrano M, Thansen AB, Kongian P, Angelidai I (2009) Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresour Technol 100:2562-2568.
Karpouzas DG, Walker A (2000) Factors influencing the ability of Pseudomonas putida strain epl and epll to degrade the organophosphate ethoprophos. J Appl Microbiol 89:40-48.
Kolusheva T, Marinova A (2007) A study of the optimal conditions for starch hydrolysis through thermostable a-amylase. Journal of the University of Chemical Technology and Metallurgy, 42:93-96.
Kovacs K, Marcelli S, Szakacs G, Zacchi G (2009) Enzymatic hydrolysis of steam-preatreaated lignocellulosic materials with Tricohderma aatroviride enzymes produced in-house. Biotechnology for Biofuels Doi: 10.1186/1754-6834-2-14.
Kuzmanova S, Vandeska E, Dimitrovski A (1991) Production of mycelial protein and cellulolytic enzymes from food waste. J of Indust Microbiol Biotechn 7:257-261.
Levy I, Shani Z, Shoseyov O (2002) Modification of polysaccharides and plant cell wall by endo-1,4-b-glucanase and cellulose-binding domains. Biomol Eng 19:17-30.
Li A, Antizaar-Ladislaao B, Khraaisheh M (2007) Bioconversion of municipal solid waste to glucose for bio-ethaanol production. Bioprocess Biosyst Eng 30:189-196.
Lin TS, Kolattukudy PE (1978) Induction of a biopolyester hydrolase (cutinase) by low levels of cutin monomers in Fusarium solani f. sp. Pisi. J Bacteriol 133:942-951.
Lowry OH, Rsebrough NJ, Farr AL, Rundal RL (1951) protein measurements with the folin phenol reagent. J Biol Chem 193:265-275.
Lynd LR, Wyman CE, Gerngross TU (1999) Biocommodity engineering. Biotechnol Prog 15:777-793.
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial Cellulose Utilization: Fundamentals and Biotechnology. Microbiol and Mol Biol Rev 66: P. 506-577.
McGinnis GD, Wilson WW, Prince SE, Cheng CC (1983) Conversion of biomass into chemicals with high-temperature wet oxidation. Ind Eng Chem Prod Res Dev 22:633-639.
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Annl Chem 31:426-428.
Murashima K, Nishimura T, Nakamura Y, Koga J, Moriya T, Sumida N, Yaguchi T, Kono T (2002) Purification and characterization of new endo-1,4-D-glucanases from Rhizopus oryzae. Enzyme Microb Technol 30:319-326.
Murphy CA, Cameron JA, Huang SJ, Vinopal RT (1996) Fusarium polycaprolactone depolymerase is cutinase. Appl Environ Microbiol 62:456-460.
Niranjane AP, P Madhou, Stevenson TW (2007) The effect of carbohydrate carbon sources on the production of cellulase by Phlebia gigantean. Enzyme Microbial Technol 40:1464-1468.
Nunes MF, Cunha-Santino MBD, Junior IB (2011) Xylanase and cellulase activities during anaerobic decomposition of three aquatic macrophytes. Braz J Microbiol 42:75-83.
Oda Y, Asari H, Urakami T, Tonomura K (1995) Microbial Degradation of Poly(3-Hydroxybutyrate) and Polycaprolactone by filamentous fungi. J Ferm Bioeng 80:265-269.
Olson L, Hahn-Hagerdahl B (1997) Fermentation of lingocellulose hydrolisates for ethanol production. Enzyme Microb Technol 18:312-331.
Ortega N, Busto MD, Perez-Mateos M (2001) Kinetics of cellulose saccharification by Trichoderma reesei cellulases. Int BiodeteriorBiodegradation 47:7-14.
Parry JM, PCB Turnbull and TR Gibson (1983) A colour Atlas of Bacillus Species. Wolf Medical Books, London.
Patel SJ, Onkarapp AR, Shobha KS (2007) Comparative Study of Ethanol Production from Microbial Pretreated Agricultural Residues. J Appl Sci Environ Manage 11:137-141.
Peciulyte D (2007) Isolation of cellulolytic fungi from wastepaper gradual recycling materials. Ekologija 53:11-18.
Rabelo SC, Filhho RM, Costa AC (2009) Lime pretreatment of sugarcane bagasse for bioethanol production. Appl Biochem Biotechnol 53:139-150.
Rifai MA (1969) A revision of the genus Trichoderma. Common Wealth Mycol., Inst. Myco. Papers No. 116, 56 pp.
Roberto IC, Mussatto SI, Rodrigues RCLB (2003) Dilute-acid hydrolysis for optimization of xylose recovery from rice straw in a semi-pilot reactor. Ind Crops Prod 7:171-176.
Sandhu H, Bajaj KL, Arneja JS (1998) Biochemical studies on bioconversion of rice straw to ethanol. Ind J of Ecol 25:62-65.
Schlegel H G (ed.) (1992) Allgemeine Mikrobiolgie. 7th edition. Georg Thieme Verlag, Stuttgart.
Schülein M (1997) Enzymatic properties of cellulases from Humicola insolens. J Biotechnol 57:71-81.
Soni SK, Batra N, Bansal N, Soni R (2010) Bioconversion of sugarcane bagasse into second generation bioethanol after enzymatic hydrolysis with in-house produced cellulase from Aspegillus sp. S4B2F. Biores Tech 5:741-758.
Teather RM, Wood PJ (1982) Use of congo red-polysacharide interactions in enumeration and characterization of cellulolytic bacteria in the bovine rumen. Appl Environm Microbiol 43:777-780.
Teeri T, Koivula A (1995) Cellulose degradation by native and engineered fundal cellulases. Carbohydr Eur 12:28-33.
Toyama S, Yonaha K, Ishihara M (1981) Degradation of bagsse cellulose by Acremonium sp. W-398.Sci Bul Coll AGr Univ Ryukyus 28:89-99.
Vlasenko EY, Ding H, Labavitch JM, Shoemaker SP (1997) Enzymatic hydrolysis of pretreated rice straw. Califor Inst Food Agric Res 59:109-119.
Wyman CE (1994) Ethanol from lignocellulosic biomass: technology economics, and opportunities. Bioresource Techonol 50:3-15.
Yoswathana N, Phuriphipat P (2010) Bioethanol Production from Rice Straw. Ene Rese J 1:26-31.
Submitted: September 13, 2011
Approved: July 2, 2012.
All the content of the journal, except where otherwise noted, is licensed under a Creative Commons License CC BY-NC.
- Abedinfar S, Karimi K, Khananhmadi M, Taherzadeh M (2009) Ethanol production by Mucor indicus and Rhizopus oryzae from rice straw by separate hydrolysis and fermentation. Biomass Bioenergy 33:828-833.
- Andren RK, Mandels M, Modeiros JE (1976) Production of sugars from waste cellulose by enzymatic hydrolysis: Primary evaluation of substrates, Process Biochem, Ocobetr, p. 2-11 (C F Toyama S,
- Yonaha K, and Ishihara M (1981) Degradation of bagsse cellulose by Acremonium sp. W-398.Sci Bull Coll AGr Univ Ryukyus, 28:89-99).
- Asgher M, Asad MJ, Legge RL (2006) Enhanced lignin peroxidase synthesis by Phanerichaete chrysosporium in solid state bioprocessing of a lignocellulosic substrate. World J Microbiol Biotechnol 22:449-453.
- Ballerini D, Desmarquest JP, Pourquie J (1994) Ethanol production from lignocellulosics: Large scale experimentation and economics. Biores Tec 50:17-23.
- Belal EBA (2003) Investigation on the biodegradation of polyesters by isolated mesophilic microbes. Dissertation, Technical University Braunschweig, Germany.
- Belal EB (2008) Biodegradation of wastepaper by Trichoderma viride and using bioprocessed materials in biocontrol of damping -off of pea caused by Pythium debaryanumJ Agric Res Kafrelsheikh Univ 34:567-587.
- Belal EB, El-Mahrouk ME (2010) Solid -State fermentation of rice Straw residues for its use as growing medium in ornamental nurseries. Acta Astronautica 67:1081-1089.
- Bhat M, Bhat S (1997) Cellulose degrading enzymes and their potential industrial applications. Biotechnol Adv Vol 15, p. 583-620.
- Bollok M (1999) Studies on ethanol production on lignocellulosics: Agricultural and Chemical Technology. Technical University of Budapest, Hungary.
- Bradner JR, Gillings M, Nevalainen KMH (1999) Qualitative assessment of hydrolytic activities in Antarctic microfungi grown at different temperatures on solid media. World J Microbiol Biotechnol 15:131-132.
- Burgess LW, Summerell BA, Bullocks SG, Backhouse KPD (1994) Laboratory Manual for Fusarium Research. 3rd edition. University of Sydney, 133 pp.
- Caputi AJ, Ueda M, Brown T (1968) Spectrophotometric determination of ethanol in wine. Am J Enol Vitic 19:160-165.
- Domsch KH, Gams W, Andersson T-H (1980) In: Compendium of soil fungi, 1. Academic Press, London,
- Drews G (1968) Mikrobilogishes Praktikum fuer Natuwissenschaftler, Springer, Verlag, Berlin-Heidelberg -New York,
- in Alef, K. (1991). Methodenhandbuch Bodenmikrobiologie Bayreuth, Deutschland.
- Fan LT, Lee YH, Gharpuray MM (1982) The nature of lignocellulosics and their pretreatments for enzymatic hydrolysis. Adv Biochem Eng 23:157-187.
- Garg SK and S Neelakantan (1981) Effect of cultural factors on cellulase activity and protein production by Aspergilllus terreus. Biotechnol Bioeng 23:1653-1659.
- Hormeyer HF, Schwals W, Bonn G, Bobleter O (1988) Hydrothermolysis of birchwood as pretreatment for enzymatic saccharification. Holzforschung 42:96-98.
- Kaparaju P, Serrano M, Thansen AB, Kongian P, Angelidai I (2009) Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresour Technol 100:2562-2568.
- Karpouzas DG, Walker A (2000) Factors influencing the ability of Pseudomonas putida strain epl and epll to degrade the organophosphate ethoprophos. J Appl Microbiol 89:40-48.
- Kolusheva T, Marinova A (2007) A study of the optimal conditions for starch hydrolysis through thermostable a-amylase. Journal of the University of Chemical Technology and Metallurgy, 42:93-96.
- Kovacs K, Marcelli S, Szakacs G, Zacchi G (2009) Enzymatic hydrolysis of steam-preatreaated lignocellulosic materials with Tricohderma aatroviride enzymes produced in-house. Biotechnology for Biofuels Doi: 10.1186/1754-6834-2-14.
- Kuzmanova S, Vandeska E, Dimitrovski A (1991) Production of mycelial protein and cellulolytic enzymes from food waste. J of Indust Microbiol Biotechn 7:257-261.
- Levy I, Shani Z, Shoseyov O (2002) Modification of polysaccharides and plant cell wall by endo-1,4-b-glucanase and cellulose-binding domains. Biomol Eng 19:17-30.
- Li A, Antizaar-Ladislaao B, Khraaisheh M (2007) Bioconversion of municipal solid waste to glucose for bio-ethaanol production. Bioprocess Biosyst Eng 30:189-196.
- Lin TS, Kolattukudy PE (1978) Induction of a biopolyester hydrolase (cutinase) by low levels of cutin monomers in Fusarium solani f. sp. Pisi. J Bacteriol 133:942-951.
- Lowry OH, Rsebrough NJ, Farr AL, Rundal RL (1951) protein measurements with the folin phenol reagent. J Biol Chem 193:265-275.
- Lynd LR, Wyman CE, Gerngross TU (1999) Biocommodity engineering. Biotechnol Prog 15:777-793.
- Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial Cellulose Utilization: Fundamentals and Biotechnology. Microbiol and Mol Biol Rev 66: P. 506-577.
- McGinnis GD, Wilson WW, Prince SE, Cheng CC (1983) Conversion of biomass into chemicals with high-temperature wet oxidation. Ind Eng Chem Prod Res Dev 22:633-639.
- Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Annl Chem 31:426-428.
- Murashima K, Nishimura T, Nakamura Y, Koga J, Moriya T, Sumida N, Yaguchi T, Kono T (2002) Purification and characterization of new endo-1,4-D-glucanases from Rhizopus oryzae Enzyme Microb Technol 30:319-326.
- Murphy CA, Cameron JA, Huang SJ, Vinopal RT (1996) Fusarium polycaprolactone depolymerase is cutinase. Appl Environ Microbiol 62:456-460.
- Niranjane AP, P Madhou, Stevenson TW (2007) The effect of carbohydrate carbon sources on the production of cellulase by Phlebia gigantean Enzyme Microbial Technol 40:1464-1468.
- Nunes MF, Cunha-Santino MBD, Junior IB (2011) Xylanase and cellulase activities during anaerobic decomposition of three aquatic macrophytes. Braz J Microbiol 42:75-83.
- Oda Y, Asari H, Urakami T, Tonomura K (1995) Microbial Degradation of Poly(3-Hydroxybutyrate) and Polycaprolactone by filamentous fungi. J Ferm Bioeng 80:265-269.
- Olson L, Hahn-Hagerdahl B (1997) Fermentation of lingocellulose hydrolisates for ethanol production. Enzyme Microb Technol 18:312-331.
- Ortega N, Busto MD, Perez-Mateos M (2001) Kinetics of cellulose saccharification by Trichoderma reesei cellulases. Int BiodeteriorBiodegradation 47:7-14.
- Parry JM, PCB Turnbull and TR Gibson (1983) A colour Atlas of Bacillus Species. Wolf Medical Books, London.
- Patel SJ, Onkarapp AR, Shobha KS (2007) Comparative Study of Ethanol Production from Microbial Pretreated Agricultural Residues. J Appl Sci Environ Manage 11:137-141.
- Peciulyte D (2007) Isolation of cellulolytic fungi from wastepaper gradual recycling materials. Ekologija 53:11-18.
- Rabelo SC, Filhho RM, Costa AC (2009) Lime pretreatment of sugarcane bagasse for bioethanol production. Appl Biochem Biotechnol 53:139-150.
- Rifai MA (1969) A revision of the genus Trichoderma. Common Wealth Mycol., Inst. Myco. Papers No. 116, 56 pp.
- Roberto IC, Mussatto SI, Rodrigues RCLB (2003) Dilute-acid hydrolysis for optimization of xylose recovery from rice straw in a semi-pilot reactor. Ind Crops Prod 7:171-176.
- Sandhu H, Bajaj KL, Arneja JS (1998) Biochemical studies on bioconversion of rice straw to ethanol. Ind J of Ecol 25:62-65.
- Schlegel H G (ed.) (1992) Allgemeine Mikrobiolgie. 7th edition. Georg Thieme Verlag, Stuttgart.
- Schülein M (1997) Enzymatic properties of cellulases from Humicola insolens J Biotechnol 57:71-81.
- Soni SK, Batra N, Bansal N, Soni R (2010) Bioconversion of sugarcane bagasse into second generation bioethanol after enzymatic hydrolysis with in-house produced cellulase from Aspegillus sp. S4B2F. Biores Tech 5:741-758.
- Teather RM, Wood PJ (1982) Use of congo red-polysacharide interactions in enumeration and characterization of cellulolytic bacteria in the bovine rumen. Appl Environm Microbiol 43:777-780.
- Teeri T, Koivula A (1995) Cellulose degradation by native and engineered fundal cellulases. Carbohydr Eur 12:28-33.
- Toyama S, Yonaha K, Ishihara M (1981) Degradation of bagsse cellulose by Acremonium sp. W-398.Sci Bul Coll AGr Univ Ryukyus 28:89-99.
- Vlasenko EY, Ding H, Labavitch JM, Shoemaker SP (1997) Enzymatic hydrolysis of pretreated rice straw. Califor Inst Food Agric Res 59:109-119.
- Wyman CE (1994) Ethanol from lignocellulosic biomass: technology economics, and opportunities. Bioresource Techonol 50:3-15.
- Yoswathana N, Phuriphipat P (2010) Bioethanol Production from Rice Straw. Ene Rese J 1:26-31.
Send correspondence to:
Publication Dates
-
Publication in this collection
13 June 2013 -
Date of issue
2013
History
-
Received
13 Sept 2011 -
Accepted
02 July 2012