Abstract
The objective of this study was to detect C. difficileA/B toxins and to isolate strains of C. perfringensand C. difficile from diarrheic and non-diarrheic dogs in Brazil. Stool samples were collected from 57 dogs, 35 of which were apparently healthy, and 22 of which were diarrheic. C. difficileA/B toxins were detected by ELISA, and C. perfringensand C. difficilewere identified by multiplex PCR. C. difficileA/B toxins were detected in 21 samples (36.8%). Of these, 16 (76.2%) were from diarrheic dogs, and five (23.8%) were from non-diarrheic dogs. Twelve C. difficile strains (21.1%) were isolated, of which ten were A+B+and two were A-B-. All non-toxigenic strains were isolated from non-diarrheic animals. The binary toxin gene cdtBwas found in one strain, which was A+B+and was derived from a non-diarrheic dog. C. perfringensstrains were isolated from 40 samples (70.2%). Of these, 18 (45%) were from the diarrheic group, and 22 (55%) belonged to the non-diarrheic group. All isolates were classified as C. perfringenstype A and there was an association between the detection of the cpegene and the presence of diarrhea. Interestingly, ten strains (25%) were positive for the presence of the cpb2gene. The high rate of detection of the A/B toxins in non-diarrheic dogs suggests the occurrence of subclinical disease in dogs or carriage of its toxins without disease. More studies are needed to elucidate the epidemiology of C. difficileand C. perfringensin dogs and to better our understanding of C. difficileas a zoonotic agent. This is the first study to report the binary toxin gene in C. difficilestrains isolated from dogs in Brazil.
nosocomial diarrhea; small animals; colitis; enteritis; canine
Detection of toxins A/B and isolation of Clostridium difficile and Clostridium perfringens from dogs in Minas Gerais, Brazil
Rodrigo Otávio Silveira SilvaI,* * Corresponding Author. Mailing address: R.O.S. Silva. Escola de Veterinária, Universidade Federal de Minas Gerais, Minas Gerais, Belo Horizonte, Brazil. E-mail: rodrigo.otaviosilva@gmail.com. ; Renata Lara Resende SantosI; Prhiscylla Sadanã PiresI; Luiz Carlos PereiraI; Silvia Trindade PereiraI; Marina Carvalho DuarteI; Ronnie Antunes de AssisII; Francisco Carlos Faria LobatoI
IEscola de Veterinária, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil
IILaboratório Nacional Agropecuário de Minas Gerais, Pedro Leopoldo, MG, Brazil
ABSTRACT
The objective of this study was to detect C. difficileA/B toxins and to isolate strains of C. perfringensand C. difficile from diarrheic and non-diarrheic dogs in Brazil. Stool samples were collected from 57 dogs, 35 of which were apparently healthy, and 22 of which were diarrheic. C. difficileA/B toxins were detected by ELISA, and C. perfringensand C. difficilewere identified by multiplex PCR. C. difficileA/B toxins were detected in 21 samples (36.8%). Of these, 16 (76.2%) were from diarrheic dogs, and five (23.8%) were from non-diarrheic dogs. Twelve C. difficile strains (21.1%) were isolated, of which ten were A+B+and two were A-B-. All non-toxigenic strains were isolated from non-diarrheic animals. The binary toxin gene cdtBwas found in one strain, which was A+B+and was derived from a non-diarrheic dog. C. perfringensstrains were isolated from 40 samples (70.2%). Of these, 18 (45%) were from the diarrheic group, and 22 (55%) belonged to the non-diarrheic group. All isolates were classified as C. perfringenstype A and there was an association between the detection of the cpegene and the presence of diarrhea. Interestingly, ten strains (25%) were positive for the presence of the cpb2gene. The high rate of detection of the A/B toxins in non-diarrheic dogs suggests the occurrence of subclinical disease in dogs or carriage of its toxins without disease. More studies are needed to elucidate the epidemiology of C. difficileand C. perfringensin dogs and to better our understanding of C. difficileas a zoonotic agent. This is the first study to report the binary toxin gene in C. difficilestrains isolated from dogs in Brazil.
Key words: nosocomial diarrhea, small animals, colitis, enteritis, canine.
Introduction
Clostridium difficileis a spore-forming, anaerobic, Gram-positive bacillus that has been recognized as an important bacterial pathogen in both humans and animals. It has been implicated as a cause of enteric disease in a variety animal species including adult horses, foals, piglets and rabbits (Silva et al., 2013a). In dogs, the importance of C.difficilehas not been fully determined. There are reports of a diagnosis of chronic and acute diarrhea caused by C.difficile, and also an outbreak of infection in dogs from a veterinary hospital has been described (Weese and Armstrong, 2003; Clooten et al., 2008). However, there remains doubt about the role of C.difficileas the primary or secondary agent causing diarrhea in dogs.
Most isolates of C. difficileproduce two types of toxins: toxin A, an enterotoxin, and toxin B, a cytotoxin (Voth and Ballard, 2005). Laboratory diagnosis of C. difficileinfection is based on the detection of these toxins by cell culture or by enzyme immunoassays (ELISAs) (Delmeé, 2001). In addition, it has been suggested that a binary toxin, called C. difficiletransferase (CDT), may be an additional important virulence factor (Stubbs et al., 2000). CDT consists of two independent unlinked protein chains that are encoded by two separate genes, designated cdtAand cdtB. According to Schwan et al.(2009), this toxin may increase the adherence and colonization of the bacterium. To date, few studies have evaluated the presence of the binary toxin genes in strains isolated from dogs.
C. perfringensis an anaerobic, spore-forming, Gram-positive bacillus that has been associated with outbreaks of acute and often severe diarrhea in human beings, broiler chicken, pigs, dogs and horses (Rudmann et al., 2003; Songer and Uzal, 2005; Silva et al., 2009; Eriksen et al., 2010; Waggett et al., 2010; Silva et al., 2013b). C. perfringensisolates are classified as one of five toxigenic types (A-E) based on the capacity to produce one or more of the four major toxins (alpha, beta, epsilon and iota). In addition to the major toxins, C. perfringenscan produce other toxins such as enterotoxin, which is associated with canine enteritis and large bowel diarrhea (Kruth et al., 1989; Sasaki et al., 1999; Weese et al., 2001), and beta-2 toxin, which is associated with diarrhea in pigs and horses (Herholz et al., 1999, Songer and Uzal, 2005; Silva et al., 2013c).
Isolation followed by screening for toxin genes leads to a better understanding of transmission patterns and risk factors. The evaluation of the distributions of these strains and the potential association with occurrence of diarrhea are important factors for elucidating the epidemiology of C. difficileand C. perfringens(Arroyo et al., 2007; Barbut et al., 2005). The objective of this study was to detect C. difficileA/B toxins and to isolate strains of C. perfringensand C. difficile in stool samples from diarrheic and nondiarrheic dogs.
Materials and Methods
Stool samples were collected from 57 dogs, of which 35 were apparently healthy, and 22 were diarrheic. The samples from diarrheic dogs were obtained direct from the rectum, in the Veterinary Hospital of Universidade Federal de Minas Gerais at the time of the consultation and were only collected from dogs for which the main motivation for the consultation was the occurrence of diarrhea. Samples from apparently healthy animals were collected in three city squares in Belo Horizonte city (Minas Gerais, Brazil), with the prior permission of the owner and when the animal was defecating. All samples were stored at -20 °C and were processed within 72 hours after collection.
C. difficile A/B toxins were detected using an ELISA kit (Ridascreen Clostridium difficiletoxins A/B, R-Biopharm, Germany). The reaction was carried out in accordance the manufacturer's instructions.
To select C. difficilespores, equal volumes of stool samples and ethanol 96% (v/v) were mixed, and after incubation for 3 minat room temperature (Silva et al., 2013c), aliquots of 5 mLwere inoculated on plates containing cycloserine-cefoxitin-fructose agar (CFFA, Hi-media, India) supplemented with 7% horse blood. These plates were incubated anaerobically at 37 °C for 72 hours. All colonies with suggestive morphology, Gram stain appearance and typical horse-manure odor (Fedorko and Williams et al., 1997) were collected and suspended in 40 mL of sterile Milli-Q water. DNA extraction was performed according to the method of Baums et al.(2004), and samples were stored at 4 °C until use in the PCR assay. Genes encoding toxin A (tcdA), toxin B (tcdB) and the binary toxin (cdtB) were detected by multiplex PCR as previously described by Silva et al.(2011).
For isolation of C.perfringens, 0.08 to 0.12 g of feces was serially diluted by factors of 10, ranging from 10-1to 10-6. Aliquots of approximately 5 mLof each dilution were plated on sulfite polymyxin sulfadiazine agar (SPS, Difco Laboratories, Detroit, USA) and were incubated anaerobically at 37 °C for 24 hours. After incubation, approximately three to five characteristic colonies were collected and suspended in 40 mLof sterile Milli-Q water. The DNA extraction was performed according to the method of Baums et al.(2004), and samples were stored at 4 °C until use in the PCR assay. Genes encoding the beta-2 toxin (cpb2), enterotoxin (cpe) and major C. perfringenstoxins (alpha, beta, epsilon and iota) were detected by multiplex PCR (Vieira et al., 2008). For all PCR reactions, amplifications were carried out in a thermocycler (Thermal Cycler Px2 - Thermo Electron Corporation, Milford, USA), and the products were visualized under UV light in a 2% agarose gel stained with ethidium bromide (Sigma-Aldrich, Saint Louis, USA). Chi-square tests were used to evaluate possible association between dependent variables and clinical groups. P values of <0.05 were considered significant.
Results and Discussion
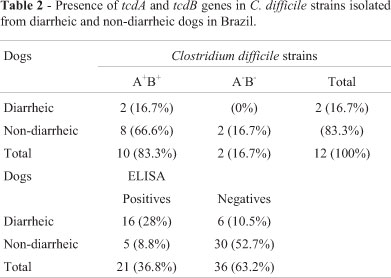
In the detection of C. difficileA/B toxins, 21 samples (36.8%) were positive (Table 1). Of these, 16 (76.2%) were from diarrheic dogs, and five (23.8%) were from non-diarrheic dogs. There was a significant association (p < 0.05) between the detection of toxin A/B and the presence of diarrhea.
The results corroborate those of previous studies, which have reported an association between the A/B toxins and diarrhea in dogs (Weese et al., 2001; Marks et al., 2002; Clooten et al., 2008). However, the rate of positive animals found in the present study was much greater than that reported previously. Marks et al.(2002) found only 3.8% (5/130) positives samples, of which four (80%) belonged to the group of diarrheic dogs. In another study, Weese et al.(2001) found 15.5% (22/142) positives samples, a proportion greater than that reported by Marks et al.(2002) but lower than that found in this study.
It is interesting to note the presence of non-diarrheic animals positive for A/B toxins. This result corroborates those of previous studies (Weese et al., 2001; Marks et al., 2002; Clooten et al., 2008) and suggests the occurrence of subclinical disease in dogs or carriage of A/B toxins without disease. There have been no studies histologically examining the intestine of non-diarrheic dogs positive for toxins A and B. However, in piglets, the occurrence of subclinical animals positive for A/B toxins is common, and when their intestines are evaluated microscopically, these animals show characteristics lesions caused by C.difficile(Yaeger et al., 2007). Further studies are needed to determine the importance of the detection of toxins A and B in dogs without diarrhea. These results suggest the possibility that C. difficileis a primary or secondary diarrhea-causing agent in dogs.
Twelve (21%) strains of C. difficilewere isolated, two from diarrheic dogs and ten from non-diarrheic dogs (Table 2). The isolation rate may have been influenced by the high proportion of samples from young dogs (under one year of age): 18 (31.6%) samples were from young dogs, and 39 (68.4%) were from adult animals. In addition, the large number of young dogs in the group of diarrheic animals is noteworthy; they accounted for 60% of samples from this group but accounted for only 17% of the non-diarrheic group. According to Struble et al.(1994), the risk of colonization by C. difficileincreases proportionately with age in dogs. The present study corroborates this statement, as all strains were isolated from adult animals, the youngest of which was aged two years.
In the present study, ten isolates were A+B+and two were A-B-. No variant strains were found. Weese et al.(2001) obtained C. difficilefrom 1.4% (2/142) of dog stool samples, a lower rate than that observed in the present study. On the other hand, Marks et al.(2002) and Struble et al.(1994) recovered C. difficilefrom 13.1% (17/130) and 18.4% (28/152) dogs, respectively, and 70.6% and 50% of these strains were toxigenic (A+B+), respectively. The absence of variant strains (A-B+) corroborates the results of previous reports (Struble et al., 1994; Weese et al., 2001; Marks et al., 2002; Clooten et al., 2008). To date, these strains have been isolated only from dogs that had visited human hospitals (Lefebvre et al., 2006).
In this study, all non-toxigenic strains were isolated from healthy animals, corroborating the findings of Alvarez-Perez et al.(2009). According to Clooten et al.(2008), previous colonization by a non-toxigenic strain reduces the risk of developing diarrhea associated with C. difficilein dogs. The same trend has been observed in piglets and in humans (Buggy et al., 1983; Kyne et al., 2000; Silva et al., 2011). As a result, a non-toxigenic strain for competitive exclusion is under development for use in at-risk humans (Songer, 2010).
The binary toxin gene was found in one strain, which was A+B+and was derived from a non-diarrheic animal. This was the first study to report the presence of the binary toxin gene in stool samples from dogs in Brazil. In humans, Persson et al.(2008), reported CDT+in approximately 26% of human strains, 97.3% of which were A+B+. In domestic animals, the presence of the binary toxin was found in approximately 4% of samples from horses (Arroyo et al., 2007) and in more than 50% of samples from piglets (Norman et al., 2009; Silva et al., 2011). Little is known about the clinical relevance and pathogenic role of CDT in C.difficileinfections, and most studies have focused on human patients. As CDT is a potent cytotoxin, Gonçalves et al.(2004) suggested that it might prepare the way for toxins A and B. Alternatively, CDT can also act in synergy with other toxins, depolymerizing the cytoskeleton by a complementary mechanism. It is also important to note that, until now, there is no description of cdtB+strains in humans in Brazil.
Differences in isolation rates and toxinotype frequencies may result from differences in management practices, the clustering of cases (Arroyo et al., 2007) and differences in geographical distribution. A recent study has suggested prevalent variation of certain genotypes of C. difficilein different geographic regions (Avbersek et al., 2009). In addition, the carrier state of C. difficileseems to vary among asymptomatic individuals by species and within the same species, depending on age and other population characteristics (Keel and Songer et al., 2006). The presence of a CDT+strain observed in the present study highlights the importance of future work to evaluate the distribution and role of C. difficilein dogs.
Recently, C. difficilewas isolated from ready-to-eat retail meats and salads. Many of these strains were of ribotypes associated with C. difficileinfection in humans and food animals (Bakri et al., 2005, Rodriguez-Palacios et al., 2007; Songer et al., 2009). It is also important to note that the two most common toxigenic ribotypes in dogs, which account for 90% of isolates, are also recognized as causes of C. difficiledisease in humans (Arroyo et al., 2007). All of these reports raise the possibility of C. difficiletransmission from human fecal contamination to dogs or even as a zoonotic disease, but more studies are needed for confirmation.
A low percentage of animals were positive for both the isolation and detection of A/B toxins simultaneously. This result corroborates those found in other studies with dogs (Struble et al., 1994; Marks et al., 2002) and is related to the difficulty of isolating this bacteria species. In many cases, toxins A/B are found in fecal content, but the bacterium itself is not isolated. The vegetative form of C.difficilecan persist for a short time under aerobic conditions and is dependent on sporulation for it to remain viable in the feces (Buggy et al., 1983). In addition, some strains cannot grow on CCFA due to the sensitivity to one or both antibiotics used in selective media (Songer and Uzal, 2005).
C. perfringensstrains were isolated from 40 samples (70.2%). Of these, 18 (45%) belonged to diarrheic dogs, and 22 (55%) belonged to non-diarrheic dogs. This result is similar to those obtained by Marks et al.(2002) and Weese et al.(2001), who obtained C.perfringensin 78% and 80% of dog stool samples, respectively. As in the present study, both Weese et al.(2001) and Marks et al.(2002) found no significant differences in the isolation rates between the diarrheic group and the non-diarrheic group.
All isolates obtained were classified as C. perfringenstype A, whereas the cpegene, responsible for encoding the enterotoxin, was found in 15 strains (37.5%), six (17.1%) were from apparently healthy animals, and nine (40.9%) were from diarrheic dogs (Table 3). This result corroborates those of previous studies that have reported that type A is the most frequent C. perfringenstype in dogs (Sasaki et al., 1999; Marks et al., 2002; Siqueira et al., 2012). Moreover, there was a significant association (p >0.05) between the detection of the cpegene and the presence of diarrhea, result similar to those reported by Marks et al.(2002).
Interestingly, ten strains (25%) were positive for the presence of cpb2gene. In other domestic animals, such as pigs and horses, C. perfringenscpb2+strains have been associated with diarrhea and typhlocolitis, respectively (Herholz et al., 1999; Songer and Uzal, 2005; Silva et al., 2013c). More studies are needed to elucidate the role and the importance of beta-2 toxin in C.perfringensdiarrhea in dogs.
The next step of this study is to evaluate the minimum inhibitory concentrations of antibiotics commonly used in small animal clinics against C. difficileand C. perfringensstrains. In addition, PCR-ribotyping could be useful to elucidate the epidemiology of C. difficilein dogs and would add more information about the role of C. difficileas zoonotic agent. This is the first study about C.perfringensand C. difficilefrom dogs in Brazil.
Acknowledgments
This work was supported by funds from Capes, Fapemig, CNPq and INCT.
Submitted: June 6, 2011
Approved: July 2, 2012
- Alvarez-Perez S, Blanco JL, Bouza E, Alba P, Gibert X, Maldonado J, Garcia ME (2009) Prevalence of Clostridium difficilein diarrhoeic and non-diarrhoeic piglets. Vet Microbiol 137:302-305.
- Arroyo LG, Staempfli H, Weese JS (2007) Molecular analysis of Clostridium difficileisolates recovered from horses with diarrhea. Vet Microbiol 120:179-183.
- Avbersek J, Janezic S, Pate M, Rupnik M, Zidaric V, Logar K, Vengust M, Zemljic M, Pirs T, Ocepek M (2009) Diversity of Clostridium difficilein pigs and other animals in Slovenia. Anaerobe 15:252-255.
- Bakri MM, Brown DJ, Butcher JP, Sutherland AD (2009) Clostridium difficilein ready-to-eat salads, Scotland. Emerg Infect Dis 15:817-818.
- Barbut F, Decré D, Lalande V, Burghoffer B, Noussair L, Gigandon A, Espinasse F, Raskine L, Robert J, Mangeol A, Branger C, Petit JC (2005) Clinical features of Clostridium difficile-associated diarrhoea due to binary toxin (actin-specific ADP ribosyltransferase)-producing strains. J Med Microbiol 54:181-185.
- Baums CG, Schotte U, Amtsberg G, Goethe R (2004) Diagnostic multiplex PCR for toxin genotype of Clostridium perfringensisolates. Vet Mic 100:11-16.
- Baverud V (2002) Clostridium difficileinfections in animals with special reference to the horse. A review. Vet Q 24:203-219.
- Berry AP, Levett PN (1986) Chronic diarrhea in dogs associated with Clostridium difficileinfection. Vet Rec 118:102-103.
- Buggy BP, Wilson KH, Fekety R (1983) Comparison of methods for recovery of Clostridium difficilefrom an environmental surface. J Clin Microbiol 18:348-352.
- Chouicha N, Marks SL (2006) Evaluation of five enzyme immunoassays compared with the cytotoxicity assay for diagnosis of Clostridium difficile-associated diarrhea in dogs. J Vet Diagn Invest 18:182-188.
- Clooten J, Kruth S, Arroyo L, Weese JS (2008) Prevalence and risk factors for Clostridium difficilecolonization in dogs and cats hospitalized in an intensive care unit. Vet Microbiol 129:209-214.
- Delmeé M (2001) Laboratory diagnosis of Clostridium difficiledisease. Clin Microbiol Infect 7:411-416.
- Eriksen J, Zenner D, Anderson SR, Grant K, Kumar D (2010) Clostridium perfringensin London, July 2009: Two weddings and an outbreak. Euro Surveill 15:19598.
- Fedorko DP, Williams EC (1997) Use of cycloserine-cefoxitin-fructose agar and L-proline-aminopeptidase (PRO Discs) in the rapid identification of Clostridium difficile J Clinic Microbiol 35:1258-1259.
- Gonçalves C, Decré D, Barbut F, Burghoffer B, Petit JC (2004) Prevalence and characterization of a binary toxin (actin-specific ADP-ribosyltransferase) from Clostridium difficile J Clinic Microbiol 42:1933-1999.
- Herholz C, Miserez R, Nicolet J, Frey J, Popoff M, Gibert M, Gerber H, Straub R (1999) Prevalence of beta2-toxigenic Clostridium perfringensin horses with intestinal disorders. J Clinic Microbiol 37:358-361.
- Keel MK, Songer JG (2006) The comparative pathology of Clostridium difficile-associated disease. Vet Pathol 43:225-240.
- Kruth SA, Prescott JF, Welch MK, Brodsky MH (1989) Nosocomial diarrhea associated with enterotoxigenic Clostridium perfringensinfection in dogs. J Am Vet Med Assoc 195:331-334.
- Kyne L, Warny M, Qamar A, Kelly CP (2000) Asymptomatic carriage of Clostridium difficileand serum levels of IgG antibody against toxin A. N Engl J Med 342:390-397.
- Lefebvre SL, Arroyo LG, Weese JS (2006) Epidemic Clostridium difficilestrain in hospital visitation dog. Emerg Infect Dis 12:1036-1037.
- Marks SL, Kather EJ, Kass PH, Melli AC (2002) Genotypic and phenotypic characterization of Clostridium perfringensand Clostridium difficilein diarrheic and healthy dogs. J Vet Intern Med 15:533-540.
- Norman KN, Harvey RB, Scott HM, Hume ME, Andrews K, Brawley AD (2009) Varied prevalence of Clostridium difficilein an integrated swine operation. Anaerobe 15:256-260.
- Persson S, Torpdahl M, Olsen KEP (2008) New multiplex PCR method for the detection of Clostridium difficile toxin A (tcdA) and toxin B (tcdB) and the binary toxin (cdtA/cdtB) genes applied to a Danish strain collection. Clinic Microbiol Infect 14:1057-1064.
- Rodriguez-Palacios A, Staempfli HR, Duffield T, Weese JS (2007) Clostridium difficilein retail ground meat, Canada. Emerg Infect Dis 13:485-487.
- Rudmann DG, Vandereide SL (2003) Necrotizing enterotyphlocolitis in dogs treated with a potent antimuscarinic. Vet Pathol 40:710-713.
- Sasaki J, Goryo M, Asahina M, Makara M, Shishido S, Okada K (1999) Hemorrhagic enteritis associated with Clostridium perfringenstype A in a dog. J Vet Med Sci 61:175-177.
- Schwan C, Stecher B, Tzivelekidis T, van Ham M, Rohde M, Hardt WD, Wehland J, Aktories K (2009) Clostridium difficiletoxin CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathog 5:e1000626.
- Silva ROS, Salvarani FM, Cruz Junior ECC, Pires PS, Santos RLR, Assis RA, Guedes RMC, Lobato FCF (2011) Detection of enterotoxin A and cytotoxin B, and isolation of Clostridium difficilein piglets in Minas Gerais, Brazil. Ciência Rural 41:1430-1435.
- Silva ROS, Salvarani FM, Assis RA, Martins NRS, Pires PS, Lobato FCF (2009) Antimicrobial susceptibility of Clostridium perfringens strains isolated from broiler chickens. Braz J Microbiol 40:261-263.
- Silva ROS, Guedes RMC, Lobato FCF (2013a) Clostridium difficileinfection: Main features and occurrence in domestic species in Brazil. Ciência Rural 43:73-80.
- Silva ROS, Ribeiro MG, Palhares MS, Borges AS, Maranhão RPA, Silva MX, Lucas TM, Lobato FCF (2013b) Detection of A/B toxin and isolation of Clostridium difficileand C. perfringensfrom foals. Equine Vet J, doi 10.1111/evj.12046.
- Silva ROS, Lobato FCF, Pires PS, Gabardo MP, Paladino ES, Guedes RMC (2013c) Neonatal diarrhea in piglets associated with cpb-2 positive Clostridium perfringens Braz J Vet Pathol 6:94-98.
- Siqueira FF, Almeida MO, Barroca TM, Horta CC, Carmo AO, Silva RO, Pires PS, Lobato FC, Kalapothakis E (2012) Characterization of polymorphisms and isoforms of the Clostridium perfringensphospholipase C gene (plc) reveals high genetic diversity. Vet Microbiol 159:397-405.
- Songer JG, Trinh HT, Killgore GE, Thompson AD, McDonald LC, Limbago BM (2009) Clostridium difficilein retail meat products, USA, 2007. Emerg Infect Dis 15:819-821.
- Songer JG, Uzal FA (2005) Clostridial enteric infections in pigs. J Vet Diagn Invest 17:528-536.
- Songer JG (2010) Clostridia as agents of zoonotic disease. Vet Microbiol 140:399-404.
- Struble AL, Tang YJ, Kass PH, Gumerlock PH, Madewell BR, Silva Jr J (1994) Fecal shedding of Clostridium difficilein dogs: a period prevalence survey in a veterinary medical teaching hospital. J Vet Diagn Invest 6:342-347.
- Stubbs S, Rupnik M, Gibert M, Brazier J, Duerden B, Popoff M (2000) Production of actin-specific ADP-ribosyltransferase (binary toxin) by strains of Clostridium difficile FEMS Microbiol Lett 186:307-312.
- Vieira AAS, Guedes RMC, Salvarani FM, Silva ROS, Assis RA, Lobato FCF (2008) Genotipagem de Clostridium perfringensisolados de leitões diarréicos. Arq Instit Biol 75:513-516.
- Voth DE, Ballard JD (2005) Clostridium difficiletoxins: mechanism of action and role in disease. Clin Microbiol 18:247-263.
- Waggett BE, McGorum BC, Wernery U, Shaw DJ, Pirie RS (2010) Prevalence of Clostridium perfringensin faeces and ileal contents from grass sickness affected horses: comparisons with 3 control populations. Equine Vet J 42:494-499.
- Weese JS, Armstrong J (2003) Outbreak of Clostridium difficile-associated disease in a small animal veterinary teaching hospital. J Vet Int Med 17:813-816.
- Weese JS, Staempfli HR, Prescott JF, Kruth SA, Greenwood SJ, Weese HE (2001) The roles of Clostridium difficileand enterotoxigenic Clostridium perfringensin diarrhea in dogs. J Vet Intern Med 15:374-378.
- Yaeger MJ, Kinyon JM, Songer JG (2007) A prospective, case control study evaluating the association between Clostridium difficiletoxins in the colon of neonatal swine and gross and microscopic lesions. J Vet Diagn Invest 19:52-59.
Publication Dates
-
Publication in this collection
26 Mar 2013 -
Date of issue
2013
History
-
Received
06 June 2011 -
Accepted
02 July 2012