Abstract
Bio-ethanol production from cane molasses (diluted to 15 % sugar w/v) was studied using the bacterium, Zymomonas mobilis MTCC 92 entrapped in luffa (Luffa cylindrica L.) sponge discs and Ca-alginate gel beads as the immobilizing matrices. At the end of 96 h fermentation, the final ethanol concentrations were 58.7 ± 0.09 and 59.1 ± 0.08 g/l molasses with luffa and Ca-alginate entrapped Z. mobilis cells, respectively exhibiting 83.25 ± 0.03 and 84.6 ± 0.02 % sugar conversion. There was no statistical significant difference (Fischer's LSD) in sugar utilization (t = 0.254, p <0.801) and ethanol production (t =-0.663, p <0.513) between the two immobilization matrices used. Further, the immobilized cells in both the matrices were physiologically active for three more cycles of operation with less than 15 % decrease in ethanol yield in the 4th cycle, which was due to some leakage of cells. In conclusion, luffa sponge was found to be equally good as Ca-alginate as a carrier material for bacterial (Z. mobilis. cell immobilization for ethanol production. Further, it has added advantages such as it is cheap, non-corrosive and has no environmental hazard.
Bio-ethanol; Cell immobilization; Fermentation; Molasses; Zymomonas mobilis MTCC 92
INDUSTRIAL MICROBIOLOGY
Ethanol fermentation of sugarcane molasses by Zymomonas mobilis MTCC 92 immobilized in Luffa cylindrica L. sponge discs and Ca-alginate matrices
Shuvashish BeheraI; Rama C. MohantyI; Ramesh C. RayII, * * Corresponding Author. Mailing address: Microbiology Laboratory, Central Tuber Crops Research Institute (Regional Centre), bhubaneswar-751019, Orissa, India.; E-mail: rc_rayctcri@rediffmail.com
IDepartment of Botany, Utkal University, Vanivihar, Bhubaneswar -751004, Orissa, India
IIMicrobiology Laboratory, Central Tuber Crops Research Institute (Regional Centre), bhubaneswar-751019, Orissa, India
ABSTRACT
Bio-ethanol production from cane molasses (diluted to 15 % sugar w/v) was studied using the bacterium, Zymomonas mobilis MTCC 92 entrapped in luffa (Luffa cylindrica L.) sponge discs and Ca-alginate gel beads as the immobilizing matrices. At the end of 96 h fermentation, the final ethanol concentrations were 58.7 ± 0.09 and 59.1 ± 0.08 g/l molasses with luffa and Ca-alginate entrapped Z. mobilis cells, respectively exhibiting 83.25 ± 0.03 and 84.6 ± 0.02 % sugar conversion. There was no statistical significant difference (Fischer's LSD) in sugar utilization (t = 0.254, p <0.801) and ethanol production (t =-0.663, p <0.513) between the two immobilization matrices used. Further, the immobilized cells in both the matrices were physiologically active for three more cycles of operation with less than 15 % decrease in ethanol yield in the 4thcycle, which was due to some leakage of cells. In conclusion, luffa sponge was found to be equally good as Ca-alginate as a carrier material for bacterial (Z. mobilis. cell immobilization for ethanol production. Further, it has added advantages such as it is cheap, non-corrosive and has no environmental hazard.
Key words: Bio-ethanol, Cell immobilization, Fermentation, Molasses, Zymomonas mobilis MTCC 92
INTRODUCTION
The continuous depletion of the fossil fuel reserves and consequent escalation in their price has stimulated an extensive evaluation of alternative technologies and substrates to meet the global energy demand. As a consequence, alternative sources of energy like methane, hydrogen and ethanol are increasingly being considered as potential substitutes to fossil fuels. Of the three, ethanol is being considered to be the most promising alternative liquid fuel; since it can be readily produced from agriculture based renewable materials like sugarcane juice, molasses, cassava and sweet potato starch and bagasse.
Molasses is an agro-industrial by-product during processing of sugarcane and sugarbeet into sugar, often used in alcohol distilleries due to the presence of high levels of fermentative sugars such as glucose, sucrose and fructose (16), being an optimal carbon source for the microorganism metabolism. Sugarcane molasses is abundantly available in countries like Brazil, India, China and Cuba, and its low cost US$ 25-30 per tonne in India is an important factor for the economical viability for ethanol production by fermentation.
Zymomonas mobilis, a gram-negative anaerobic bacterium, has been attracting increasing attention in recent years for ethanol production, because of its better fermentation attributes, such as high specific rates of sugar uptake as well as that it converts sugar almost stoichiometrically to ethanol and CO2, grows more rapidly and demonstrates highest ethanol productivity during fermentation when compared to other fermentation organisms (13, 14, 32, 33). Further, the ethanol tolerance of Zymomonas spp. is as good as or even better (up to 16% v/v) than that of most Saccharomyces cerevisiae strains (10, 14) and it produces fewer by-products (26). However, its catabolizing capabilities are restricted to a few substrates, such as glucose, fructose, maltose and sucrose (35). Z. mobilis may have, therefore, a greater potential for industrial ethanol production from raw sugar, sugarcane juice, syrups and molasses (19, 37).
Bio-ethanol can be produced using either free or immobilized cells. Using immobilized cells is advantageous over free cells due to enhanced yields, ease to separate cell mass, reduce risk of contamination and better operational stability and cell viability for several cycles of operations (12, 25). Among the different immobilization technologies, entrapment of microbial cells within the polymeric matrices such as agar agar, Ca-alginate, gelatin, k -carrageenan, etc have been studied widely for ethanol production (1, 7); entrapment in Ca-alginate as beads is found most suitable (6, 17). Sodium alginate, the precursor of calcium alginate is readily available and is a non-toxic chemical. On the other hand, luffa sponge, a natural material consisting of a fibrous network, obtained from the mature dried fruits of Luffa cylindrica L., is used to immobilize various plant (20) and microbial (27, 28) cells. Immobilization in luffa sponge is cheap; the material is highly porous, resistant to autoclaving, pH and temperature variations and is supposed to be an ideal material for use in industrial fermentation in developing countries (23).
In this context, a comparative study was carried out on the production of ethanol from cane molasses by fermentation using Z. mobilis entrapped in Ca-alginate beads and luffa sponge discs. Further, the growth and fermentation kinetics between the two immobilization systems of bacterial cells have been studied.
MATERIALS AND METHODS
Molasses
The molasses used for ethanol fermentation was brought from Sakthi Sugars Pvt. Ltd, Dhenkanal, Orissa, India during the month of October, 2009. The molasses had the following compositions [g/100 ml (w/v)]: water, 20 ± 0.055; sucrose, 36 ± 0.06; fructose,11 ± 0.094; glucose, 9 ± 0.056; other reducing sugars, 4 ± 0.148; nitrogenous compounds, 3.5 ± 0.084; non-nitrogenous acids, 5 ± 0.195; ash, 7 ± 0.06; others, 4.5 ± 0.07. The sugar content of molasses (60 %, w/v) was brought into 15 % (w/v) by 1:3 dilutions with distilled water. Concentrated sulphuric acid (0.5 % v/v) was added to the molasses medium and heated to 80°C for 30 min and left overnight. Two layers were formed, the upper shining black, while the lower yellowish brown (due to the precipitates of trace metals). The clear supernatant (shiny layer) was used as fermentation medium with 15 % sugar content.
Microorganism and culture condition
Zymomonas mobilis MTCC 92, procured from the Institute of Microbial Technology, Chandigarh, India was maintained on yeast extract-glucose-salt-agar (YGSA) medium (g/l): yeast extract, 10; glucose, 20; MgCl2, 10; (NH4)2SO4, 10; KH2PO4, 10; Agar, 15 and the pH was adjusted to 6.5. The culture was stored at 4±0.5°C for further use.
Preparation of inoculum
The inoculum was prepared in 100 ml growth medium (as mentioned above but without agar) taken in sterilized (at 121°C for 20 min) 250 mL Erlenmeyer flask. The flask was inoculated with a loopful of the Z. mobilis MTCC 92 culture and incubated for 24 h at 30°C at 120 rpm in an orbital shaker incubator (Remi Pvt, Ltd, Bombay, India). Fourty ml of the bacterial inoculum [equivalent to 10 % of the fermentation medium (400 ml)] was separately immobilized with Ca-alginate and luffa sponge discs as the matrices described in the following section.
Immobilization of whole cells in different matrices
Calcium alginate: Forty ml of the bacterial inoculum (prepared as above) was centrifuged at 8,000 rpm for 20 min in a refrigerated centrifuge (Model C-24, Remi Pvt., Ltd, Bombay, India), washed and then the pellets were suspended with 10 ml of deionized water. The cell suspension was used for cell immobilization. The Z. mobilis cell suspension (1x105 CFU/ml) was added to 4 % (w/v) sodium alginate solutions in 1:1 (v/v) ratio and mixed thoroughly. The cell-alginate mixture was then cast into beads by dropping from a hypodermic syringe into cold sterile 0.1 M CaCl2 solution. These beads had a diameter of approximately 3.0 mm and were hardened by keeping n the dilute (0.1 M) CaCl2 solution for 24 h at 4°C with gentle agitation (7, 17). Finally, these beads were washed with sterile distilled water to remove excess Ca2+ ions and un-entrapped cells, before being used for the fermentation process.
Luffa sponge discs: The luffa sponge discs were obtained from mature dried fruits of L. cylindrica. The sponge was cut into discs of 2.5-cm diameter, 4 mm thick and washed in boiling water four times. The luffa sponge discs were then oven-dried at 70°C. Fourty ml of the bacterial inoculum (1 x 105 CFU/ml) was directly poured into six luffa sponge discs contained in 500-ml beaker. After the cells (inoculum) were trapped within the matrix of the sponge, these immobilized luffa discs were taken out and washed thoroughly with fresh sterilized YGSA broth to remove the free cells before being used for the fermentation process.
Fermentation medium: Four hundred milliliters of treated cane molasses with 15 % (w/v) sugar was taken into individual 500 ml Erlenmeyer flasks. The flasks were cotton plugged and steamed at 90°C in a water bath for 15-20 min. After cooling (NH4)2SO4 was added to the slurry (as a source of nitrogen) for growth of the bacteria at the rate of 1g/l and subsequently the pH was adjusted to 6.5. Then the medium (400 ml) was inoculated with either immobilized luffa sponge discs (6 nos.) or Ca-alginate gel beads. The triplicate flasks (n=3) for each treatment was separately incubated for 96 h at the room temperature (30 ± 2° C).
Cell leakage: The cells leaked from the matrices (Ca-alginate beads and luffa sponge discs) into fermentation medium were determined by plate count using yeast extract-glucose-salt-agar (YGSA) medium, incubated at 30°C for 24 h.
Analytical methods
At 24 h interval, fermented broths (in triplicate flasks) were removed and the contents were analyzed for total sugar and ethanol. The ethanol content of the fermented broth was determined by measuring specific gravity of the distillate according to the procedure described by Amerine and Ough (2). In this procedure, the weight of a certain volume of an alcohol distillate was compared to the weight of exactly the same volume of distilled water. The ratio of the weights of the two (alcohol: water) gave the specific gravity of the distillate. The total sugar was assayed by Anthrone method (21). The pH was measured by a pH meter (Systronics, Ahmadabad, India) using glass electrode. The cell concentrations in immobilized systems were calculated as follows. The luffa sponge was dried at 80°C for 24 h prior to immobilization, and the initial weight was determined. After cell immobilization, the sponge containing the immobilized cells was washed gently with distilled water and dried at 80°C for 24 h. The immobilized cell concentration was calculated from the difference in the weight of the luffa sponge before and after immobilization (8). The biomass of calcium alginate immobilized cells was determined by dissolving the gel beads in a 4% (w/v) EDTA solution and reading absorbance at 550 nm against a suitable blank in a UV-VIS spectrophotometer (Model No. CE 7250, Cecil Instrument, UK). The corresponding dry weight was obtained from a standard curve of absorbance versus dry weight (7). The immobilized bacterial cells, separated after fermentation, were reused for successive three batches. The fermentation kinetics was studied as per the formulae described below (4).
(1) Final ethanol (P): Ethanol formed (ml) per liter was multiplying with 0.9.
(2) Final biomass concentration (X, g/L): Cell biomass developed (g) per liter of hydrolysate.
(3) Cell yield (Yx/s, g/g): Final biomass concentration (X, g/L) was divided with volumetric substrate uptake (Qs, g L-1h-1)
(4) Volumetric product productivity (Qp, g L-1h-1): Product formed (g) per liter of hydrolysate per hour.
(5) Volumetric substrate uptake (Qs, g L-1h-1): Substrate (glucose) uptake (g) per liter of hydrolysate per hour.
(6) Ethanol yield (Yp/s, g g-1): Mass of product (ethanol) formed per Mass of substrate (glucose) consumed was multiplied with 100.
(7) Conversion rate (%) into ethanol: Final ethanol (P. was divided with Sugar utilization (Initial sugar-final sugar)
Statistical analysis
The data of ethanol production using immobilized cells of Z. mobilis was analyzed using one way ANOVA. Where significant difference in ANOVA (p <0.05) was detected by the Fisher's Least Significance Difference (LSD) multiple comparison test which was applied to compare the factor level difference. The analysis was performed using MSTAT-C (version 2.0, Michigan State University, Michigan, USA).
RESULTS AND DISCUSSION
Fermentation of cane molasses (with 15 % sugar, w/v) by the bacterium Z. mobilis MTCC 92 immobilized in luffa sponge discs and Ca-alginate gel beads was compared, and the sugar utilization and ethanol production profile by these two methods are given in Fig. 1. Preliminary studies (data not shown) have shown that 15 % sugar content in molasses is optimum for ethanol production using Z. mobilis as the fermenting organism. This is in agreement with the previous studies (3, 24).
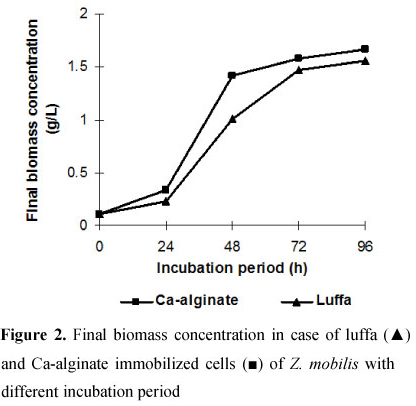
In the present study, ethanol production started in the log phase of the growth and maximum ethanol production was achieved during the stationary phase (96 h) (Fig. 1).
This was evident from the study of cell growth pattern in both the immobilized systems as shown in Figure 2. There was a fall of 47.05 and 47.93 % in total sugar concentration over the initial content with simultaneous production of 19.2 ± 0.095 and 21 ± 0.06 g/l ethanol up to 24 h of fermentation with the cells immobilized in luffa sponge discs and Ca-alginate gel beads, respectively. The decrease in sugar reserve might be also due to its utilization in part, for initial growth and metabolism of the bacteria in addition to its conversion into ethanol (7). For 48, 72 and 96 h of fermentation, the sugar concentrations were 55.11 ± 0.044, 29.2 ± 0.133 and 9 ± 0.055 g/l molasses with simultaneous increase in ethanol concentration to 36.37 ± 0.081, 46.91 ± 0.36 and 58.7 ± 0.09 g/l molasses, respectively with the luffa immobilized Z. mobilis cells (Fig. 1A). During the same period, sugar concentrations were 54 ± 0.076, 28.2 ± 0.057 and 10.3 ± 0.149 g/l molasses with production of ethanol to 37.51 ± 0.17, 48.34 ± 0.083 and 59.1 ± 0.08 g/l molasses, respectively when Ca-alginate immobilized bacterial cells were used for fermentation (Fig. 1B). Thus, at the end of the fermentation period (96 h), there was 83.25 ± 0.03 and 84.6 ± 0.02 % sugar conversion into ethanol with immobilized Z. mobilis cells entrapped in luffa sponge discs and Ca-alginate gel beads, respectively. Similar results were obtained in our earlier study using mahula (Madhuca latifolia L) flowers as the substrate in which 77.9 and 79.2 % sugar conversion into ethanol was obtained by free and Ca-alginate entrapped Z. mobilis cells, respectively (7). Further, there was no statistical significant difference (Fischer's LSD) in sugar utilization (t = 0.254, p <0.801) and ethanol production (t = -0.663, p <0.513) between the two immobilization matrices used. Similar results on ethanol yield from molasses have been reported by other authors using either free or immobilized cells. Cazetta et al. (11) found the best conditions for ethanol production were 200 g/l of total reducing sugars in the molasses, temperature of 30°C and time of fermentation of 48 h, achieving 55.8 g/l ethanol. The fermentation organism was Z. mobilis. Mariam et al. (22) found maximum ethanol yield (52.9 g/l) by Ca-alginate immobilized yeast (S. cerevisiae. cells from cane molasses after 120 h of incubation with a maximum sugar consumption of 147.6 g/l. Also, Yansong et al. (39) reported 13.6 % (v/v) ethanol in the fermentation of cane molasses after 72 h of incubation with an initial molasses concentration of 50 % in a fermenter using S. cerevisiae 1912. Further, Roukas et al. (34) used immobilized cells of S. cerevisiae in Ca-alginate beads for the fermentation of non-sterilized beet molasses and found 31.2 % was the maximum ethanol yield with 73.3 % sugar utilization at initial sugar concentration of 250 g/l.
The growth and fermentation kinetics of the immobilized bacterial cells were also studied (Table 1). The ethanol concentration (P. obtained with Ca-alginate immobilized cells of Z. mobilis (59.1 ± 0.08 g/l) was marginally higher (0.7%) than that of luffa immobilized cells (58.7 ± 0.09 g/l), whereas the volumetric substrate uptake (Qs. was found to be 0.68 % more in case of luffa (1.47 ± 0.044 g/l/h) than that of Ca-alginate (1.46 ± 0.05 g/l/h) immobilized cells. The volumetric product productivity (Qp =0.616 ± 0.008 g/l/h) and ethanol yield (Yp/s =0.421 ± 0.014 g/g) obtained with cells immobilized in Ca-alginate matrix was found to be 0.81 and 1.18 %, respectively higher than that of Qp (0.611 ± 0.012 g/l/h) and Yp/s (0.416 ± 0.017 g/g) of Z. mobilis immobilized in luffa sponge discs. In our earlier study, ethanol concentration, volumetric substrate uptake, ethanol yield and volumetric product productivity were 22.425 ± 0.13 g/l, 0.493 ± 0.007 g/l/h, 0.473 ± 0.01 g/g and 0.233 ± 0.018 g/l/h, respectively by the Ca-alginate entrapped Z. mobilis cells at the end of fermentation of mahula flowers (7). The difference between these two studies might be due to the difference in the substrate (fermentation medium), and sugar concentration as well as growth medium of the starter culture. Likewise, the final sugar to ethanol conversion rate (%) with Ca-alginate immobilized cells was 1.6 % higher than with luffa immobilized cells.
The immobilized cells were further recycled for three more times limiting the duration of each fermentation cycle upto 96 h as most of the sugars in molasses were consumed during these period. The cells not only survived but were also active physiologically in these three cycles of fermentation. The ethanol production in the 2nd, 3rd and 4thcycle with the luffa immobilized cells of Z. mobilis were 56.83 ± 0.14, 53.91 ± 0.058 and 50.32 ± 0.043 g/l molasses, respectively showing 3.17, 8.14 and 14.26 % decrease over the 1st cycle (58.69 ± 0.092 g/l molasses). During the same period, the ethanol productions were 57.72 ± 0.119, 55.43 ± 0.072 and 53.22 ± 0.031 g/l molasses, respectively with the Ca-alginate immobilized cells. The decrease in ethanol concentration during these cycles of fermentation might be due to marginal leakage of cells from the matrices. In the successive fermentation with luffa, a gradual cell leakage (0.06-0.14 mg/ml) was observed which was almost similar to that with Ca-alginate matrix (0.04-0.11 mg/ml). Further, in the first cycle of operation in both the cases (Ca-alginate and luffa sponge disc) the leakage of cells from immobilized support was negligible (<5%); hence the observed ethanol production was presumed because of the action of immobilized cells. Similar results were obtained on ethanol production from cane molasses using alginate-luffa as the carrier matrix for the immobilization of yeast cells (31). In their study, the ethanol production was same during the 1st and 2nd cycles of operation (91.7 g/l cane molasses), with a marginal decrease (0.5 %) in the 3rd cycle (90.6 g/l cane molasses).
Berekaa et al. (9) reported the repeated use of immobilized cells in luffa sponge for production of poly-y-glutamate (PGA). The application of fed-batch cultivation of Bacillus cells adsorbed on sponge led to increase of production till the 3rd run and reached 55.5 g/l, then slightly decreased in the 4th run. Kar et al. (18) reported the reusability of
Streptomyces erumpens cells immobilized in luffa sponge disc for production of thermostable alpha-amylase in submerged fermentation. The cells not only survived but also active physiologically on the luffa support for three cycles of fermentation yielding 3830, 3655 and 3575 units of enzyme in cycle 1, 2, and 3, respectively. The decrease in enzyme production was because of the leakage of cells in successive cycle. Likewise, Usha et al. (38) found the leakage of cells from the Ca-alginate and PUF (polyurethane foam) immobilized matrix for the H-acid (1-amino 8-hydroxy naphthalene 3, 6-disulfonic acid) degradation with Alcaligenes latus. They observed that the cell leakage was higher (1 x 103 CFU/ml) in Ca-alginate matrix than in PUF (2 x 101 CFU/ml) after 19 cycles and 25 cycles, respectively. However, Bangrak et al. (5) produced ethanol with the reusability of immobilized yeast cells entrapped in loofa-reinforced alginate carriers. The immobilized cell reactor was successfully operated for 30 days without any loss in ethanol productivity.
In this study, luffa matrix was found to be equally suitable as Ca-alginate matrix for cell immobilization of Z. mobilis for ethanol production from cane molasses. This is due to the low value of differences between these two matrices with every aspects of kinetics. Ogbonna et al. (29, 30) reported that luffa sponge alone could be used to achieve 99 % immobilization of flocculating yeasts (S. cerevisiae. cells for ethanol production in a column bioreactor. On the other hand, luffa sponge was demonstrated as an excellent cell carrier for ethanol fermentation by flocculating cells such as S. cerevisiae and non-flocculating cells such as Candida brassicae (30). Further, its strength, abundance, low cost, biodegradability and natural origin of luffa have become the main source of interest for cell immobilization. Also the utility of luffa sponge as an immobilizing matrix has been studied for other fermentative products. Luffa sponge was used as the carrier for yeast cell immobilization for the production of ethanol from sugar beet juice (30) and cane molasses (31). Luffa sponge was also used as an ideal immobilization material for the production of pectinase (36) and α-amylase (18). Likewise, luffa sponge was used as an excellent matrix for removal of heavy metal ions (15).
CONCLUSION
The potential of sugarcane molasses in bio-ethanol production in tropical countries has a big scope in view of the demand of ethanol in the present context. Luffa sponge is found equally good as Ca-alginate matrix as a carrier material for bacterial (Z. mobilis. cell immobilization for ethanol production from cane molasses. Further, it has added advantages such as it is cheap, non corrosive and has no environmental hazard.
ACKNOWLEDGEMENTS
The authors are thankful to UGC [(Letter F. No. 32-573/2006(SR), dated 17.07.07], New Delhi for financial support to carryout this work.
Submitted: July 28, 2010
Returned to authors for corrections: November 25, 2011
Approved: June 07, 2012
- 1. Adinarayana, K.; Jyothi, B.; Ellaiah, P. (2005). Production of alkaline protease with immobilized cells of Bacillus subtilis PE-11 in various matrices by entrapment technique. AAPS Pharm. Sci. Tech 6, 391-397.
- 2. Amerine, M.A.; Ough, C.S. (1984). Wine and Must Analysis. Wiley, New York, USA.
- 3. Amutha, R.; Paramasamy, G. (2001). Production of ethanol from liquefied cassava starch using co-immobilized cells of Zymomonas mobilis and Saccharomyces diastaticus J. Biosci. Bioeng 92, 560-564.
- 4. Bailey, J.E.; Ollis, D.F. (1986). Biochemical engineering fundamentals. McGraw-Hill, New York.
- 5. Bangrak, P.; Limtong, S.; Phisalaphong, M. (2011). Continuous ethanol production using immobilized yeast cells entrapped in loofa-reinforced alginate carriers. Braz. J. Micrbiol. 41, 676-684.
- 6. Behera, S.; Kar, S.; Mohanty, R.C.; Ray, R.C. (2010a). Comparative study of bio-ethanol production from mahula (Madhuca latifolia L.) flowers by Saccharomyces cerevisiae cells immobilized in agar agar and Ca-alginate matrices. Appl. Energy 87, 96-100.
- 7. Behera, S.; Mohanty, R.C.; Ray, R.C. (2010b). Ethanol fermentation of mahula (Madhuca latifolia L.) flowers using free and immobilized bacteria Zymomonas mobilis MTCC 92. Biologia 65, 416-421.
- 8. Behera, S.; Mohanty, R.C.; Ray, R.C. (2011). Ethanol production from mahula (Madhuca latifolia L.) flowers with free and immobilized (in Luffa cylindrica L. sponge discs) cells of Zymomonas mobilis MTCC92. Ann. Microbiol. (DOI: 10.1007/s13213-010-0160-y).
- 9. Berekaa, M.M.; El aassar, S.A.; El-sayed, S.M.; El borai, A.M. (2009). Production of poly-y-glutamate (PGA) biopolymer by batch and semicontinuous cultures of immobilized Bacillus licheniformis strain-R. Braz. J. Microbiol 40, 715-724.
- 10. Busche, R.M.; Scott, C.D.; Davison, B.H.; Lynd, L.R. (1992). Ethanol, the ultimate feedstock. A technoeconomic evaluation of ethanol manufacture in fluidazed bed bioreactors operating with immobilized cells. Appl. Biochem. Biotechnol 34/35, 395-415.
- 11. Cazetta, M.L.; Celligoi, M.A.P.C.; Buzato, J.B.; Scarmino, I.S. (2007). Fermentation of molasses by Zymomonas mobilis Effects of temperature and sugar concentration on ethanol production. Biores. Technol 98, 2824-2828.
- 12. Chandel, A.K.; Chan, E.S.; Rudravaram, R.; Narasu, M.L.; Rao, L.V.; Pogaku, R. (2007). Economics and environmental impact of bio-ethanol production technologies: an appraisal. Biotechnol. Mol. Biol Rev 2, 14-32.
- 13. Davis, L.; Jeon, Y.; Svenson, C.; Rogers, P.; Pearce, J.; Peiris, P. (2005). Evaluation of wheat stillage for ethanol production by recombinant Zymomonas mobilis Biomass Bioener 29, 49-59.
- 14. Davis, L.; Rogers, P.; Pearce, J.; Peiris, P. (2006). Evaluation of Zymomonas -based ethanol production from a hydrolyzed waste starch stream. Biomass Bioener 30, 809-814.
- 15. Iqbal, M.; Edyvean, R.G.J. (2005). Loofa sponge immobilized fungal biosorbent: A robust system for cadmium and other dissolved metal removal from aqueous solution. Chemosphere 61, 510-518.
- 16. Jimenez, A.M.; Borja, R.; Martin, A. (2004). A comparative kinetic evaluation of the anaerobic digestion of untreated molasses and molasses previously fermented with Penicillium decumbens in batch reactors. Biochem. Eng. J 18, 121-132.
- 17. Kar, S.; Ray, R.C. (2008). Statistical optimization of α-amylase production by Streptomyces erumpens MTCC 7317 cells in calcium alginate beads using response surface methodology. Pol. J. Microbiol 57, 49-57.
- 18. Kar, S.; Swain, M.R.; Ray, R.C. (2009). Statistical optimization of alpha-amylase production with immobilized cells of Streptomyces erumpens MTCC 7317 in Luffa cylindrica L. sponge discs. Appl. Biochem. Biotechnol 152, 177-188.
- 19. Lee, E.C.; Huang, C.T. (2000). Modeling of ethanol fermentation using Zymomonas mobilis ATCC 10988 grown on the media containing glucose and fructose. Biochem. Eng. J 4, 217-227.
- 20. Liu, Y.K.; Seki, M.; Tanaka, H.; Furusaki, S. (1998). Characteristics of loofa (Luffa cylindrica sponge as a carrier for plant cell immobilization. J. Ferment. Bioeng 85, 416-421.
- 21. Mahadevan, A.; Sridhar, R. (1999). Methods in Physiological Plant Pathology, 5th edition, Sivakami Publication, Madras, India.
- 22. Mariam, I.; Manzoor, K.; Ali, S.; Ulhaq, I. (2009). Enhanced production of ethanol from free and immobilized Saccharomyces cerevisiae under stationary culture. Pak. J. Bot 41, 821-833.
- 23. Marques, L.L.M.; Buzato, J.B.; Celligoi, M.A.P.C. (2006). Effect of raffinose and ultrasound pulses on invertase release by free and immobilized Saccharomyces cerevisiae in loofa (Luffa cylindrica sponge. Braz. Arch. Biol. Technol 49, 873-880.
- 24. Monte, R.A.; Maurico, R.; Ines, J. (2003). Ethanol fermentation of a diluted molasses medium by Saccharomyces cerevisiae immobilized on crysilite. Braz. J. Microbiol 46, 751-757.
- 25. Nigam, J.N. (2000). Continuous ethanol production from pineapple cannery waste using immobilized yeast cells. J. Biotechnol 80, 189-193.
- 26. Nowak, J.; Roszyk, H. (1997). Co-immobilization of Aspergillus niger and Zymomonas mobilis for ethanol production from starch. Pol. J. Food Nutr. Sci 6, 65-70.
- 27. Ogbonna, J.C.; Liu, Y.C.; Liu, Y.K.; Tanaka, H. (1994). Loofa (Luffa cylindrical sponge as a carrier for microbial cell immobilization. J. Ferment. Bioeng 78, 437-442.
- 28. Ogbonna, J.C.; Tomiyama, S.; Tanaka, H. (1996). Development of a method for immobilization of non-flocculating cells in loofa (Luffa cylindrica sponge. Process Biochem 31, 737-744.
- 29. Ogbonna, J.C.; Tomiyama, S.; Liu, Y.C.; Tanaka, H. (1997). Efficient production of ethanol by cells immobilized in loofa (Luffa cylindrica sponge. J. Ferment. Bioeng 84, 271-274.
- 30. Ogbonna, J.C.; Mashima, J.; Tanaka, H. (2001). Scale up of fuel ethanol production from sugar beet juice using luffa sponge immobilized bioreactor. Biores. Technol 76, 1-8.
- 31. Phisalaphong, M.; Budiraharj, R.; Bangrak, P.; Mongkolkajit, J.; Limtong, S. (2007). Alginate-loofa as carrier matrix for ethanol production. J. Biosci. Bioeng 104, 214-217.
- 32. Queresi N.; Manderson, G.J. (1995). Bioconversion of renewable resources into ethanol: an economic evaluation of selected hydrolysis, fermentation, and membrane technologies. Energy Sources 17, 241 - 265.
- 33. Rogers, P.; Joachimsthal, E.; Haggett, K. (1997). Ethanol from lignocellulosics: potential for Zymomonas -based process. Austral. Biotechnol. 7, 305-309.
- 34. Roukas, T. (1996). Ethanol production from non-sterilized beet molasses by free and immobilized Saccharomyces cerevisiae cells using fed-batch culture. J. Food Eng. 27, 87-98.
- 35. Ruanglek, V.; Maneewatthana, D.; Tripetchkul, S. (2006). Evaluation of Thai agro-industrial wastes for bio-ethanol production by Zymomonas mobilis Process Biochem 41, 1432-1437.
- 36. Slokoska, L.S.; Angelova, M.B. (1998). Immobilization of polymethylgalacturonase producing Aspergillus niger on Luffa sponge material. J. Biosci 53, 968-972.
- 37. Tano, M.S.; Buzato, J.B. (2003). Effect of presence of initial ethanol on ethanol production in sugar cane juice fermented by Zymomonas mobilis Braz. J. Microbiol. (DOI: 10.1590/S1517-83822003000300012).
- 38. Usha, M.S.; Sanjay, M.K.; Gaddad, S.M.; Shivannavar, C.T. (2010). Degradation of h-acid by free and immobilized cells of Alcaligenes latus Braz. J. Micrbiol 41, 931-945.
- 39. Yansong, G.U.; Min, Q.; Quan, Z.; Zhengmao, Z.; Guoquiang, C. (2001). Hyperproduction of alcohol using yeast fermentation in highly concentration molasses medium. Tsinghua Sci. Technol 6, 225-230.
Publication Dates
-
Publication in this collection
19 Feb 2013 -
Date of issue
Dec 2012
History
-
Received
28 July 2010 -
Accepted
07 June 2012 -
Reviewed
25 Nov 2011