Abstract
GABA (γ-aminobutyric acid) is a four carbon non-protein amino acid that is widely distributed in plants, animals and microorganisms. As a metabolic product of plants and microorganisms produced by the decarboxylation of glutamic acid, GABA functions as an inhibitory neurotransmitter in the brain that directly affects the personality and the stress management. A wide range of traditional foods produced by microbial fermentation contain GABA, in which GABA is safe and eco-friendly, and also has the possibility of providing new health-benefited products enriched with GABA. Synthesis of GABA is catalyzed by glutamate decarboxylase, therefore, the optimal fermentation condition is mainly based on the biochemical properties of the enzyme. Major GABA producing microorganisms are lactic acid bacteria (LAB), which make food spoilage pathogens unable to grow and act as probiotics in the gastrointestinal tract. The major factors affecting the production of GABA by microbial fermentation are temperature, pH, fermentation time and different media additives, therefore, these factors are summarized to provide the most up-dated information for effective GABA synthesis. There has been a huge accumulation of knowledge on GABA application for human health accompanying with a demand on natural GABA supply. Only the GABA production by microorganisms can fulfill the demand with GABA-enriched health beneficial foods.
GABA (γ-aminobutyric acid); microorganisms; optimal conditions; lactic acid bacteria; GABA-enriched food
REVIEW
Production of gaba (γ - aminobutyric acid) by microorganisms: a review
Radhika Dhakal; Vivek K. Bajpai* * Corresponding Author. Mailing address: School of Biotechnology, Yeungnam University, Gyeongsan, Gyeongbuk 712-749, Republic of Korea.; Fax: +82-53-810-4769.; E-mail: khbaek@ynu.ac.kr/ vbiotech04@gmail.com ; Kwang-Hyun Baek* * Corresponding Author. Mailing address: School of Biotechnology, Yeungnam University, Gyeongsan, Gyeongbuk 712-749, Republic of Korea.; Fax: +82-53-810-4769.; E-mail: khbaek@ynu.ac.kr/ vbiotech04@gmail.com
School of Biotechnology, Yeungnam University, Gyeongsan, Gyeongbuk 712-749, Republic of Korea
ABSTRACT
GABA (γ-aminobutyric acid) is a four carbon non-protein amino acid that is widely distributed in plants, animals and microorganisms. As a metabolic product of plants and microorganisms produced by the decarboxylation of glutamic acid, GABA functions as an inhibitory neurotransmitter in the brain that directly affects the personality and the stress management. A wide range of traditional foods produced by microbial fermentation contain GABA, in which GABA is safe and eco-friendly, and also has the possibility of providing new health-benefited products enriched with GABA. Synthesis of GABA is catalyzed by glutamate decarboxylase, therefore, the optimal fermentation condition is mainly based on the biochemical properties of the enzyme. Major GABA producing microorganisms are lactic acid bacteria (LAB), which make food spoilage pathogens unable to grow and act as probiotics in the gastrointestinal tract. The major factors affecting the production of GABA by microbial fermentation are temperature, pH, fermentation time and different media additives, therefore, these factors are summarized to provide the most up-dated information for effective GABA synthesis. There has been a huge accumulation of knowledge on GABA application for human health accompanying with a demand on natural GABA supply. Only the GABA production by microorganisms can fulfill the demand with GABA-enriched health beneficial foods.
Key words: GABA (γ-aminobutyric acid), microorganisms, optimal conditions, lactic acid bacteria, GABA-enriched food.
INTRODUCTION
GABA (γ -aminobutyric acid) is a non-protein amino acid that is widely distributed in nature among microorganisms, plants and animals (85). Natural GABA was first found as a constituent of tuber tissue in potato (80). In microorganisms, GABA is functionally involved in the spore germination of Neurospora crassa and Bacillus megaterium (19, 20, 41). GABA confers resistance to acidic pH in bacteria, including E. coli, Lactococcus lactis, Listeria monocyrogenes, Mycobacterium and Clostridium perfringens (7, 17, 67).
Nowadays, GABA is used considerably in pharmaceuticals, and massively as a major active constitute in foods, such as gammalone, cheese, gabaron tea, and shochu (58, 69, 91). GABA acts as the major inhibitory neurotransmitter in the mammalian central nervous system. GABA improves the plasma concentration, growth hormones and the protein synthesis in the brain (12), but inhibits small airway-derived lung adenocarcinoma (13). In addition, GABA has hypotensive, tranquilizing, diuretic and antidiabetic effects (1, 5). GABA lowers the blood pressure in animals and human subjects. The chronic GABA ingestion ranged from 0.3 to 300 mg/kg decreased systolic blood pressure in spontaneously hypertensive rats (39). The administration of GABA significantly decreased the blood pressure of spontaneously hypertensive rats with a dose-dependent manner (26). The oral administration of GABA of 10 mg daily for 12 weeks was effective for hypertensive patients (34). The daily oral administration of rice germ containing 26.4 mg GABA was effective in treating neurological disorders (59). Furthermore, Hagiwara et al. (22) reported that GABA acted as a strong secretagogue of insulin from the pancrease, therefore, effectively preventing diabetes (1). GABA intake can regulate sensations of pain and anxiety, and lipid levels in serum (40, 55). Furthermore, consumption of GABA-enriched foods can inhibit cancer cell proliferation (61) and improve memory and the learning abilities (55). Therefore, GABA has been classified as a bioactive component in foods and pharmaceuticals (14).
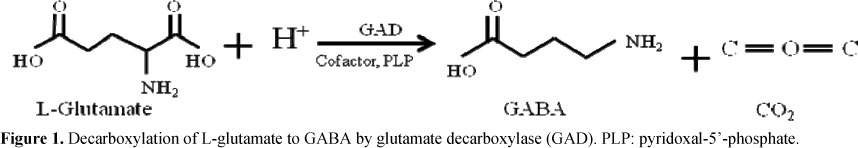
There have been many attempts for synthesizing GABA chemically or biologically (13, 33, 63) because of the beneficial functions of GABA and the increasing commercial demand (85, 66). Biosynthetic methods of GABA may be much more promising than chemical synthesis methods since they have a simple reaction procedure, high catalytic efficiency, mild reaction condition and environmental compatibility (30). The biosynthesis of GABA is one step reaction of decarboxylating glutamate to GABA, catalyzed by glutamate decarboxylase (GAD) (Fig. 1) (85, 48). Various biosynthetic techniques have been developed for the efficient production of GABA, including immobilized cell technology (30), sourdough fermentation (15) and batch fermentation method (13, 40, 43, 63). These techniques can be used for GABA preparations in pharmaceutical, food, and other industries. Although successful replacement of wheat flour with pseudocereals or leguminous flours has been demonstrated for processing bakery products, however, utilization of buckwheat blend, amaranth, chickpea and quinoa flours, subjected to sourdough fermentation by selected GABA-producing strains has provided GABA enriched products to the manufacture, and has been considered a promising possibility in order to increase the nutritional, functional, sensory, and technological properties of food products (15).
Microorganisms are widely used in biological systems for the production of numerous valuable molecules (10, 27). To improve the quality of diets and enriched-dishes by converting agricultural commodities into fermented foods, microorganisms have been used for commercial and domestic purpose over the centuries for producing fermented foods such as, wine, soy sauce, sufu, vinegar, distilled spirits, rice wine, fermented vegetable, meat products, paocai, pickles and kimchi etc. (23, 47).
A wide range of products can be covered by traditional fermented foods. Table 1 shows the production of GABA synthesized by different species of microorganism isolated from various sources of fermented foods. In particular, we summarize the GABA-producing microorganisms and the optimal fermentation conditions for the GABA production. The information could be applied for isolating high GABA-producing microorganisms as well as for developing efficient processes for maximum production of GABA for pharmaceutical and food industries.
GABA producing microorganisms and their isolation sources
A number of microorganisms of bacteria and fungi have been reported to produce GABA (52, 78, 40). In addition to bacteria, GABA is also found in many molds, fungi and yeast. In microorganisms, GABA was first isolated from acid-treated yeast extracts, and later it was demonstrated to be present in the free state, to the extent of 1 ± 2% by dry weight in untreated yeast (64). GABA was again detected while investigating the amino acid composition of red yeast, Rhodotorula glutinis. (44). An important GABA pool was observed in the early phase of Neurospora crassa. spore germination (70). Other filamentous fungi like Aspergillus nidulans and A. niger. are also known to contain GABA. In A. niger. during acidogenesis, an unusual accumulation of GABAwas recognized (41). The GABA levels increased in parallel with citric acid accumulation in absence of manganese ions by A. niger, while it was virtually undetectable under a normal condition (41).
The most interesting and practical group of bacteria for GABA production is LAB, which produce high levels of GABA. To date, LAB include several strains of Lactobacillus (Lb.) and Lactococcus (Lc.). Lb. brevis. was isolatedfrom many fermented foods, including Korean fermented vegetable kimchi, Chinese traditional paocai, fresh milk, alcohol distillery lees and black raspberry juice (31, 38, 47, 61, 72, 86, 91). Lb. delbrueckii. subsp. bulgaricus, Lb. plantarum and Lb. paracasei. were isolated from cheese (76) and from Japanese traditional fermented fish (43), respectively. The best GABA-producing strains, Lb. paracasei. PF6, Lb. delbrueckii. subsp. bulgaricus PR1, L. lactis. PU1 and Lb. brevis. PM17 have been isolated from Pecorino di Filiano, Pecorino del Reatino, Pecorino Umbro, and Pecorino Marchigiano cheeses, respectively, which had the highest concentrations of GABA (76). Similarly, the strains of Lc. lactis. spp. lactis. 01-4, 01-7, 53-1, and 53-7 were screened and selected with the highest level of GABA production from the cheese starters (58). Ten GABA producing LAB strains were isolated from kimchi and yoghurt. Two of the strains (Y3 and Y4) were bacilliwhile the other eight were known as cocci (52). The strains isolated from kimchi were identified as Lc. lactis. subsp. lactis. based on the 16S rDNA sequences, and the strains produced the highest amount of GABA (3.68 g/l) in de Man, Rogosa and Sharpe (MRS) broth (52). A total of 61 GABA synthesizing isolates were identified in cheese under in vitro. fermentation conditions at 30°C for 24 h at pH 4.7 (74). By partial sequencing of the 16S rRNA genes, twelve species were detected, and the species were composed of many subspecies including Lb. plantarum. (17 isolates), Leuconostocs. (L.) mesenteroides. (2 isolates), Weissella cibaria. (1 isolates), Lb. paracasei. (16 isolates), Lb. brevis. (3 isolates), Lb. casei. (5 isolates), L. pseudomesenteroides. (2 isolates), Lb. lactis. (2 isolates), Lb. delbrueckii. subsp. bulgaricus. (2 isolates), Lb. rhamnosus. (2 isolates), S. thermophilus. (6 isolates) and E. durans. (1 isolate) (74). Although many GABA-producing LAB strains have already been isolated and identified, further research on isolation and characterization of the LAB is needed because various types of GABA-producing LAB are important for the food industry (43).
Due to the potential for producing GABA-enriched foods, the GABA-producing microorganisms, especially LAB are getting a huge attention. The ability of LAB for producing GABA is varied among species and the strains (32, 43, 60, 91). GABA-rich foods fermented with LAB include yogurt and cheese (58, 60, 61, 76), kimchi (52, 61, 74), sourdough (65) and paocai (47). The highest content of GABA was found in Pecorino di Filiano (391 mg/kg) among 22 different varieties of Italian cheeses, in which the responsible microorganisms for the GABA production were Lb. paracasei. PF6, PF8 and PF13, Lb. plantarum. PF14, Lactobacillus. sp. strain PF7, and Enterococcus durans. PF15 (76). In shochu distillery-lees, a Japanese distilled alcoholic beverage, most of the free glutamic acid (10.50 mM) was converted to GABA (10.18 mM) by Lb. brevis. IFO-12005 (91). GABA-enriched grape must beverage, which has a potential anti-hypertensive effect and dermatological protection was manufactured by a fermentation using Lb. plantarum DSM19463 (17, 48).
Other GABA producing bacteria include Lc. lactis. (58, 76), Pediococcus acidilactici, P. pentosaceus, E. durans, E. faecalis, E. faecium and Leuconostocs. (L. )(9). Lc. lactis. PU1 was considered a good GABA producing candidate for the fermentation of reconstituted skim milk (76).Many other species belonging to the genera of Enterococcus, Lactobacillus, Lactococcus, Leuconostoc, Pediococcus and Streptococcus. are often recognized from raw milk (21, 89). In another study, different strains of Rhizopus. species were compared with the GABA production capacity under the best condition of soybean fermentation. High yield of GABA was achieved by anaerobic incubation of R. microsporus var. oligosporus. IFO 32002 and IFO 32003, reaching 1.74 g GABA/100 g and 1.5 g GABA/100 g, respectively (2). A fungus Monascus purpureus. CCRC 31615 produced high amount of GABA when the media was added with sodium nitrate (81, 88). In addition, the seven cultivars of rice grains inoculated with M. purpureus. produced and increased GABA every week whereas the rice grain without the fungus inoculation contained no GABA (36). These results indicated that the glutamic acids in the fermented rice could be converted into GABA by M. purpureus. Ta-Hon-In Pu-erh tea fermented with Streptomyces bacillaris. strain R9 and S. cinereus. strain contained 4- and 8- folds of GABA than the non-inoculated leaves, respectively (37).
Factors affecting GABA synthesis
Different fermentation factors affect the rate of GABA production by microorganisms. Among them the most common and essential factors are pH, temperature, cultivation time and media additives of culture. The fermentation conditions can be optimized based on the biochemical characteristics of GAD of the fermenting microorganisms. Decarboxylation of glutamate occurred in LAB results in the stoichiometric release of the end product GABA and the consumption of a proton (Fig. 1). The net effect of this reaction increases the alkalinity of the cytosol and environment. To maintain the optimum pH 5.0 at which the highest GABA production was obtained by Lb. brevis, H2SO4 was therefore supplemented into the fermenting broth in order to offset pH increase, arisen from the decarboxylation (49). Similarly, the glutamate content 500 mM in the culture medium was converted to 302 mM GABA by optimizing the fermentation condition of Lb. paracasei. NFRI 7415 at pH 5.0 with the addition of pyridoxal-5'-phosphate (PLP) (43). The GABA production by Streptococcus salivarius. subsp. thermophilus. Y2 was also enhanced by optimizing fermentation condition at pH 4.5 and by the addition of PLP (90).
The optimum conditions vary among the fermenting microorganisms due to the different properties of the GADs, therefore, characterization of the biochemical properties of the GADs will be required in the interested microorganisms to achieve the highest GABA production. Optimal conditions for GABA-producing microorganisms are summarized below, especially on the effects of pH, temperature, cultivation time and media additives.
Effect of pH
The biosynthesis of GABA in microorganisms is mainly regulated by pH, which usually has the most pronounced effect for a fermentation process (43, 83, 90). The biochemical characteristics of GAD vary among different microorganisms, therefore, the effective pH value for the maximum GABA production is species-dependent (49, 90). Lb. plantarum. DSM19463 synthesized the maximum GABA (59 µM/h) at the pH 6.0 (17), however, Lb. paracasei. NFRI 7415 produced the highest GABA (210 mM) at pH 5.0 (42). In S. salivarius. subsp. thermophilus,. GABA production was highest (7984 mg/l) at pH 4.5 (90). Lb. brevis. GABA 057 converted total 10% of monosodium glutamate (MSG) to GABA at pH 4.2 (79). In cheese Lactobacillus, Lb. paracasei. PF6, PF8, PF13, Lb. plantarum. PF14, Lb. sp. strain PF7 and E. durans. PF15 produced high amounts of GABA (289 mg/kg - 391 mg/kg) under the pH range of 4.68 - 5.70 (76). When GABA-producing Lb. paracasei. was compared the GABA production capacity under different pHs (4 - 6), GABA was produced significantly high (210 mM) at pH 5.0 (43). Lb. lactis. produced highest amount of GABA (7.2 g/l) at pH ranged from 7.5 to 8.0, however, reduced GABA production pH above 8.0, indicating that Lb. lactis. has the optimum GABA production at weak alkaline pH, ranged from 7.5 to 8.0 (53).
The pH in fermentation medium changes with time during fermentation, therefore, the initial pH affects final GABA yield and the pH of the medium should be adjusted timely to maintain the optimum pH (53, 38, 49). The black raspberry juice fermented with Lb. brevis. GABA 100 and monosodium glutamate (MSG) changed the initial pH of juice from 4, 4.5, 5, 5.5 and 6 to 3.9, 4.2, 4.4, 4.5 after 48 h, respectively (31). The MRS medium inoculated with Lb. paracasei. changed the pH from 6.5 to about 4.5 within 50 h of fermentation (37).
High production of GABA by fermentation is not only dependent on activating the activities of GAD but also on inhibiting the activities of GABA-decomposing enzymes. One pathway for GABA decomposition exists in a large variety of plants and microorganisms. GABA transaminase catalyses the reversible conversion of GABA to succinic semi-aldehyde using either pyruvate or α-ketoglutarate as the amino acceptor, and succinic semi-aldehyde dehydrogenase catalyzes the reversible conversion of succinic semi-aldehyde to succinate (75, 42). GABA transaminase isolated from Pseudomonas aeruginosa. transaminased GABA most actively at pH 8.5 (87). Other organisms, including pseudomonads as well as higher organisms also showed the highest activity of GABA transaminase approximately at pH 8.5 (4, 6, 56, 71). Succinic semi-aldehyde dehydrogenase isolated from Saccharomyces. had the optimum pH 8.4 for the highest enzymatic activity (35). Therefore, the activities of the two enzymes should be obstructed from pH by adjusting the pH of the buffer to achieve maximum production of GABA (42, 75).
Effect of temperature
The incubation temperature is also a major factor affecting maximum GABA yield by fermentation. In addition to an effect on biocatalyst activity and stability, temperature has an effect on the thermodynamic equilibrium of a reaction. The high efficient conversion of glutamate to GABA needs the high cell density and also the appropriate culture temperature (38). GABA production in Lb. brevis. NCL912 had a positive correlation with the cell density, which was dependent on the culture temperature (49). Lb. brevis. NCL912 growth increased with higher temperature and peaked at 35°C, then decreased over the temperature (49). Cultured Lb. plantarum. DSM19463 synthesized the highest amount of GABA (59 µM/h) at temperatures between 30°C and 35°C (17). The optimum temperatures for Lb. brevis. GAD and Lb. brevis. CGMCC 1306 were found to be as 30°C and 37°C, respectively (85, 86). Lb. brevis. GABA 100 fermenting black raspberry juice produced maximum GABA (27.6 mg/ml) at pH 3.5 and 30°C on 12th day of fermentation (38). Lb. buchneri. in MRS broth medium also had the optimum temperature for GABA production as 30°C (12). Immobilized whole cells of Lb. brevis. at 40°C produced 92% of GABA after 8 h of fermentation (30). The maximum GABA yields by Lc. lactis. at the optimum temperature of 33°C and 34°C were found to be 310 mg/ml and 439 mg/ml, respectively (50, 52). S. salivarius. subsp. thermophilus. had the optimum temperature for GABA production as 34°C, at which, 12% of the total MSG was completely converted into GABA (79). Lb. paracasei. NFRI 7415 produced the highest GABA (302 mM) at 37°C, however drastically decreased the GABA production and cell growth at 43°C (43). Generally, fermenting temperatures ranged from 25°C to 40°C result in a high GABA yield within the temperatures.
Effect of the fermentation time
The time factor plays an important role in the fermentation and the production of GABA as temperature and pH do. Lb. plantarum DSM19463 and Lb. paracasei NFRI 7415 required 72 h and 144 h of fermentation to reach the highest production of GABA at 4.83 mM and 60 mM, respectively (17, 28). Black raspberry juice fermented with Lb. brevis GABA 100 reached the highest production of GABA at 25.4 mg/ml and 26.5 mg/ml at the 15th day of the fermentation at (25°C, pH 4.0) and (37°C, pH 5.5), respectively, whereas it reached the highest level of GABA at the 12th day when the samples were fermented at pH 3.5 and 30°C (38).
The addition time for the GABA substrate also affects the final GABA yield as well as the concentration of the substrate in the medium. A significant differences in GABA yield among various times of MSG addition was shown in the fermentation of Lc. lactis, as the highest GABA yield was obtained when MSG was added at the beginning of fermentation (0 h) however, the GABA yield lowered when MSG was added during 6 to 96 h of fermentation at 6 h interval of time (53). The addition of PLP in different time intervals also affected the production of GABA (90). The GABA production at 72 h reached 6272, 6570 and 7333 mg/l when PLP was added at 0, 24 and 48 h, respectively. The higher amount of GABA was produced by the addition of PLP at 48 h than at 0 and 24 h suggested that PLP could easily lose the role as coenzyme due to the denaturalization in the culture broth during the fermentation, however, addition of PLP at 48 h could partly recover GAD activity (90). These results indicate that the highest GABA production by microorganisms can depend on the addition of appropriate medium additives and optimum additional time for the additives.
Effect of media additives
Nutrient composition and culture conditions affect the GABA production by microbe fermentation (88). Also, media additives including glutamate and PLP as the coenzymes of GAD are the major factors affecting the production of GABA during the fermentation (12, 43, 50, 52, 90). The medium composition, especially carbon and nitrogen sources and other components can influence the amount of GABA production (3, 62, 88). Furthermore, the concentrations of substrates are important for achieving high GABA yield (90). Lb. plantarum. DSM19463 produced 0.9 mM GABA by the fermentation of grape must diluted to 4% (w/v) of total carbohydrates (17). Among different carbohydrates tested such as L-arabinose, ribose, D-xylose, galactose, glucose, fructose, maltose, melibiose, a-methyl D-glucoside, N-acetyl D-glucosamine and gluconate as carbon source, 1.25% glucose was the best carbon source for high production of GABA (48). The mixed ratio (33:58:9) of brown rice juice, germinated soybean juice and enzymolyzed skim milk, a milk having deteriorated properties by the means of enzymatic action, as a source of carbon and nitrogen produced the highest GABA (6.41 g/l) by Lc. lactis. B (52). Addition of 0.5% ethanol as a carbon source in a fermentation using M. purpureus. NTU 601and M. pilosus. increased the production of GABA, and reached to 7453 and 385 mg GABA/kg, respectively (82, 88). The addition of each 2.5% of yeast extract, soya peptone and beef extract as a nitrogen source produced approximately 200 mM GABA (47). Compared with the fermentation of M. pilosuss. without MSG addition as a nitrogen source, the addition of 1.0% MSG into the basal medium of 60 g sterilized rice with 1.0% (w/w) peptone produced approximately 2.8 times higher GABA (502.39 mg/kg) (82). Even 0.5% urea as a source of nitrogen enhanced the production of GABA in fermentation with M. purpureus. NTU 601 (88).
Glutamate addition increased GABA production in Lb. paracasei and Lb. brevis. (25, 30, 43, 49). GABA concentration reached 161 mM after cultivation of 144 h in the medium containing 500 mM of glutamate by Lb. paracasei. NFRI 7415 (43). Lb brevis. NCL912 and Lb. brevis. also increased GABA production by the addition of glutamate (25, 30, 49). However, S. salivarius. subsp. thermophilus. Y2 did not increase GABA production significantly when glutamate was added 10 - 20 g per liter of media, suggesting that these concentrations of glutamate are not appropriate for the synthesis of GABA in this species (90). The production of GABA by using glutamate as a substrate still remains with several problems, such as the high cost of the culture medium.
PLP is used as a coenzyme of GAD for enhancing GAD activity (43, 68). By the addition of PLP, GABA production increased and reached to 7333 mg/l, 200 mM and 504 mg/kg during the fermentation with S. salivarius subsp. thermophilus. Y2, Lb. paracasei. NFRI 74150 and Lb.plantrum. C48, respectively (15, 43, 90). The addition of 0.1 mM PLP to the diluted grape must, however, did not enhance the synthesis of GABA (17), which may be due to the presence of endogenous PLP in grape must (8). The addition of PLP in the culture medium for the production of GABA by Lb. brevis. NCL912 did not increased the amount of GABA, indicating that Lb.brevis. NCL912 could synthesize the PLP by itself necessarily (48).
The addition of sulfate ions increased the GAD activity of Lb. brevis. IFO 12005 in a dose-dependent manner, suggesting that the increased GAD activity is due to an increased hydrophobic interaction between the subunits (86). Total 5% of the MSG was converted into GABA within 48 h when 10 mM ammonium sulfate was added to the reaction medium of Lb. brevis. GABA 057 (79). In glucose-yeast peptone medium, 7% of MSG as glucose concentration with 10 mM ammonium sulfate was the best combination for GABA production (86). The addition of over 0.6% glucose without ammonium sulfate, however, did not increase the GABA conversion rate (81).
The cell viability and stability in the beads can be improved for the higher rate of GABA conversion by adjusting the concentrations of media additives, including skim milk, isomalto-oligosaccharide, erythritol, and pectin in an optimum concentration (79). The beads with 0.6% isomalto-oligosaccharide were the most effective combination for GABA production and also improved probiotic survival in fermented milk (79, 11).
The addition of other substrates such as the wholemeal wheat sourdough and 50% of tomo koji enhanced the GABA production using Lb. plantarum. C48 and M. pilosus. IFO 4520, respectively (65, 40). GABA could be produced by LAB using shochu kasu. as a growth medium without addition of glutamate. The GABA concentration reached 10.05 mM or 10.18 mM after one or two day cultivation in kome shochu kusu, respectively (91). Similarly, the addition of buckwheat and quinoa sourdough with Lb. plantarum. C48 and amaranth and chickpea sourdoughs with Lc. Lactis. subsp.lactis. PU1 enhanced the GABA production and reached to 643 ±13, 415 ±10, 816 ± and 1031 ± 9 mg/kg, respectively (15). These processes have advantages over other fermentation processes due to the simplicity and low operation price.
Potential application of GABA producing microorganisms
Microorganisms can be harmless, beneficial or pathogenic. The beneficial microorganisms reduce the risk of the growth and survival of food borne pathogens and food spoilage organisms (29). The consumer attention towards the selection of health-beneficial foods makes the significant growth of GABA-enriched foods. Natural addition of GABA is demanding over the addition of chemical nutrient GABA since consumers prefer naturally-occurring substances, and the fermentation helps to reduce the cost of the foods due to the omission of chemical addition of GABA and also provides attractive foods with better taste and at the same time replaces the chemical GABA by natural GABA (48). Therefore, GABA production by naturally-occurring microorganisms during fermentation is getting higher request.
Especially, the production of GABA by LAB has been extensively explored during the manufacture of black raspberry juice, kimchi, soymilk, cheese and other dairy products (58, 38, 75, 83, 26, 33, 77). For the manufacture of functional foods and beverages, LAB also have the advantage of the production of GABA using cheap ingredients, such as by-products in food industry (48). The GABA producing LAB act as probiotics but are only effective if they remain viable as they pass through stomach and intestine (14, 57). Three Lactobacillus. strains, Lb. paracasei. PF6, Lb. delbrueckii. subsp. bulgaricus. PR1, and Lb. plantarum. C48 isolated from cheese were subjected to pepsin and pancreatin digestion, but they survived and synthesized GABA, suggesting that these bacteria survive and synthesize GABA under simulated gastrointestinal conditions (76). In fact, GABA-producing LAB as probiotics could inhabit in the gastrointestinal tract and produce GABA in situ. (48). There have been long and safe histories of the production of fermented foods and beverages by LAB, which can accumulate high amounts of GABA (46). These GABA producing LAB accumulate high amount of GABA and also protect foods by controlling the food spoilage pathogens by secreting bacteriocins (18).
Conclusion and future prospects
Processes for high GABA production by microorganisms are summarized here to develop functional foods and to provide natural GABA. Supply of natural GABA and the enriched food is a big challenge for the growing global demand. Therefore, the production of GABA enriched foods by fermentation using beneficial microorganisms is an indispensable process. For food and medicinal industries, further studies will be required to screen various types of GABA-producing microorganisms from as many as possible fermented foods. There is a positive relation between the optimal fermentation condition and GABA synthesis by microorganisms. Since the production of GABA is totally dependent on the biochemical properties of GAD, clarification of biochemical properties of the GAD for the fermenting microorganism facilitates the optimization of fermentation processes. Many factors including pH, temperature, culture time and media additives can be optimized to achieve the maximum GABA production in various microorganisms. These all processes will be directed to higher flexibility of the microbe cultures for a wider application of GABA.
ACKNOWLEDGEMENTS
This work was supported by a grant from Systems and Synthetic Agro-biotech Center through Next-Generation BioGreen 21 Program (PJ008034), Rural Development Administration, Republic of Korea.
Submitted: August 25, 2011
Approved: June 07, 2012
- 1. Adeghate, E.; Ponery, A.S. (2002). GABA in the endocrine pancreas: cellular localization and function in normal and diabetic rats. Tissue Cell 34, 1-6.
- 2. Aoki, H.; Uda, I.; Tagami, K.; Furuya, Y.; Endo, Y.; Fujimoto, K. (2003). The production of a new tempeh like fermented soybean containing a high level of γ- aminobutyric acid by anaerobic incubation with Rhizopus. Biocsi. Biotechnol. Biochem 67(5), 1018-1023.
- 3. Blanc, P.J.; Loret, M.O.; Santerre, A.T.; Pareilleux, A.; Prome, D.; Prome, J.C.; Laussac, J.P.; Goma, G. (1994). Pigment of Monascus J. Food Sci. 59, 862-865.
- 4. Bloch-Tardy, M.; Roland, B.; Gonnard, P. (1974). Pig brain 4-aminobutyrate 2-ketoglutarate transaminase. Purification, kinetics and physical properties. Biochimie 56, 823-832.
- 5. Capitani, G.D.E.; Biase, D.; Aurizi, C.; Gut, H.; Bossa, F.; Grutter, M.G. (2003). Crystal structure and functional analysis of Escherichia coli glutamate decarboxylase. EMBO J. 22, 4027-4037.
- 6. Cash, C.; Maitre,M.; Ciesielski, L.; Mandel, P. (1974). Purification and partial characterisation of 4- aminobutyrate 2-ketoglutarate transaminase from human brain. FEBS Lett. 47, 199-203.
- 7. Castanie-Cornet, M. P.; Smith, T. A. D.; Elliott, J. F.; Foster, J. W. (1999). Control of acid resistance in Escherichia coli J. Bacteriol 181, 3525-3535.
- 8. Castor, J.G.B. (1953). The B-complex vitamins of musts and wines as microbial growth factors. Appl. Microbiol. 1, 97-102.
- 9. Chamba, J.F.; Irlinger, F. (2004). Secondary and adjunct cultures. In: Fox, P.F., McSweeney, P.L.H., Cogan, T.M., Guinee, T.P. (Eds.), Cheese: Chem. Phy. Microbiol. Elsevier, London, UK, p. 191-206.
- 10. Chembler, J.A.; Koffas, M.A.G. (2008). Metabolic engeneering for plant natural product biosynthesis in microbes. Curr. Opin. Biotech. 19, 597-605.
- 11. Chen, K. N.; Chen, M. J.; Liu, J. R.; Lin, C. W.; Chiu, H. Y. (2004). Optimization of incorporated probiotics as coating materials for probiotic microencapsulation. J. Food Sci. 70, 260-266.
- 12. Cho, Y.R., Chang, J.Y., Chang, H.C. (2007). Production of gamma-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from kimchi and its neuroprotective effect on neuronal cells. J. Microbiol. Biotechnol. 17, 104-109.
- 13. Choi, S.I.; Lee, J.W.; Park, S.M.; Lee, M.Y.; Ge J.I.; Park, M.S.; Heo, T.R. (2006). Improvement of gamma-aminobutyric acid (GABA) production using cell entrapment of Lactobacillus brevis GABA 057. J. Microbiol. Biotechnol. 16, 562-568.
- 14. Chou, L.S., Weimer, B. (1999). Isolation and characterization of acid and bile-tolerant isolates from strains of Lactobacillus acidophilus J. Dairy Sci 82, 23-31.
- 15. Coda, R.; Rizzello, C.G.; Gobbetti, M. (2010). Use of sourdough fermentation and pseudo-cereals and leguminous flours for the making of a functional bread enriched of γ-aminobutyric acid (GABA). Int. J. Food Microbiol. 137, 236-245.
- 16. Cotter, P. D.; C. Hill. (2003). Surviving the acid text: responses of grampositive bacteria to low pH. Microbiol. Mol. Biol. Rev. 67, 429-453.
- 17. Di Cagno, R.; Mazzacane, F.; Rizzello, C.G.; Angelis, M.D.E.; Giuliani, G.; Meloni, M.; Servi, B.D.E.; Marco, G. (2010). Synthesis of γ -aminobutyric acid (GABA) by Lactobacillus plantarum DSM19463: functional grape must beverage and dermatological applications. Appl. Microbiol. Biotechnol 86, 731-741.
- 18. Djenane, D.; Martínez, L.; Blanco, D.; Yanguela, J., Yanguela, J.A.; Roncales, P. (2005). Effect of lactic acid bacteria on extention of shelf life and growth of listeria monocytogenes in beef steaks stored in co2-rich atmosphere. Braz. J. Microboil. 36, 405-412.
- 19. Foester, C. W.; Foester, H. F. (1973). Glutamic acid decarboxylase in spores of Bacillusmegaterium and its possible involvement in spore germination. J. Bacteriol 114, 1090-1098.
- 20. Foester, H.F. (1971). G-aminobutyric acid as a required germinant for mutant spores of Bacillus megaterium. J. Bacteriol. 817-823.
- 21. Franciosi E.; Settanni L.; Cavazza A.; Poznanski E. (2009a). Biodiversity and technological potential of wild lactic acid bacteria from raw cows' milk. Int. Dairy J 19, 3-11.
- 22. Hagiwara, H.; Seki, T.; Ariga, T. (2004). The effect of pre-germinated brown rice intake on blood glucose and PAI-1 levels in streptozotocin-induced diabetic rats. Biosci. Biotechnol. Biochem. 68, 444-447.
- 23. Han B.Z.; Rombouts, F.M.; Robert Nout, M.J. (2001). A Chinese fermented soybean food. Int. J. Food Microbiol 65, 1-10.
- 24. Hao R.; Schmit J.C. (1993). Cloning of the gene for glutamate decarboxylase and its expression during conidiation in Neurospora crassa.Biochem. J 293, 735-738.
- 25. Hayakawa K.; Ueno Y.; Kawamura S.; Taniguchi R.; Oda K. (1997). Production of y-aminobutyric acid by lactic acid bacteria. Seibutsu Kogaku 75, 239-244.
- 26. Hayakawa, K.; Kimura, M.; Kasaha, K.; Matsumoto, K.; Sansawa, H.; Yamori, Y. (2004). Effect of a γ-aminobutyric acid-enriched dairy product on the blood pressure of spontaneously hypertensive and normotensive Wistar - Kyoto rats. Bri. J. Nutr. 92, 411-417.
- 27. Heck; J.X.; Hertz; P.F.; Ayub; M.A.Z. (2002). Cellulase and xylanase production by isolated amazon Bacillus strains using soybean industrial residue based solid-state cultivation. Braz. J. Microbiol 33, 213-218.
- 28. Higuchi, T.; Hayashi, H.; Abe, K. (1997). Exchange of glutamate and g-aminobutyrate in a Lactobacillus strain. J. Bacteriol. 179, 3362-3364.
- 29. Holzapfel, W.H.; Geisen, R.; Schillinger, U. (1995). Biological preservation of foods with reference to protective cultures, bacteriocins and food-grade enzymes. Int. J. Food Microbiol 24, 343-362.
- 30. Huang, J.; Mei, L.; Wu, H.; Lin, D. (2007). Biosynthesis of c-aminobutyric acid (GABA) using immobilized whole cells of Lactobacillus brevis World J. Microbiol. Biotechnol 23, 865-871.
- 31. Huang, J.; Mei, L.; Sheng, Q.; Yao, S.; Lin, D. (2007). Purification and characterization of glutamate decarboxylase of lactobacillus brevis CGMCC 1306 isolated from fresh milk. Chinese J. Chem. Eng. 15, 157-161.
- 32. Huang, J.; Mei, L.; Xia, J. (2007). Application of artificial neural network coupling particle swarm optimization algorithm to biocatalytic production of GABA. Biotechnol. Bioeng 96, 924-931.
- 33. Inoue, K.; Shirai, T.; Ochiai, H.; Kasao, M.; Hayakawa, K.; Kimura, M.; Sansawa, H. (2003). Blood-pressure-lowering effect of a novel fermented milk containing -aminobutyric acid (GABA) in mild hypertensives. Eur. J. Clin. Nutr. 57. 490-495.
- 34. Izquierdo, E.; Marchioni, E.; Aoude-Werner, D.; Hasselmann, C.; Ennahar, S. (2009). Smearing of soft cheese with Enterococcus faecium WHE 81, a multi-bacteriocin producer, against Listeria monocytogenes Food Microbiol. 26, 16-20.
- 35. Jakoby, W.B.; Scott, E.M. (1959). Aldehyde oxidation. III. Succinic semialdehyde dehydrogenase. J. Biol. Chem. 234(4), 937-940.
- 36. Jannoey, P.; Niamsup, H.; Lumyong, S.; Suzuki, T.; Katayama, T.; Chairote, G. (2010). Comparison of gamma-aminobutyric acid production in Thai rice grains. World J. Microbiol. Biotechnol 26, 257-263.
- 37. Jeng, K.C.; Chen, C.S.; Fang, Y.P.; Hou, R.C.W.; Chen, Y.S. (2007). Effect of microbial fermentation on content of statin, GABA, and polyphenols in Pu-Erh tea. J. Agric. Food Chem. 55, 8787-8792.
- 38. Kim, J.Y.; Lee, M.Y.; Ji, G.E.; Lee, Y.S.; Hwang, K.T. (2009). Production of γ-aminobutyric acid in black raspberry juice during fermentation by Lactobacillus brevis GABA100. Int. J. Food Microbiol. 130, 12-16.
- 39. Kimura, M.; Hayakawa, K.; Sansawa, H. (2002). Involvement of γ-aminobutyric acid (GABA) B receptors in the hypotensive effect of systemically administered GABA in spontaneously hypertensive rats. Jpn. J. Pharmacol. 89, 388-394.
- 40. Kono, I.; Himeno, K. (2000). Changes in g-aminobutyric acid content during beni-koji making. Biosci. Biotechnol. Biochem 64, 617-619.
- 41. Kubicek, C. P.; Hampel W.; Rohr M. (1979). Manganese deficiency leads to elevated amino acid pools in critic acid accumulating Aspergillus niger Arch. Microbiol. 123, 73-79.
- 42. Kumar, S.; Punekar, N.S.; Satyanarayan, V.; Venkatesh, K.V. (2000). Metabolic fate of glutamate and evaluation of flux through the 4-aminobutyrate (GABA) shunt in Aspergillus niger Biotechnol. Bioeng. 67, 575-584.
- 43. Komatsuzaki, N.; Shima, J.; Kawamotoa, S.; Momosed, H.; Kimurab, T. (2005). Production of g-aminobutyric acid (GABA) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiol 22, 497-504.
- 44. Krishnaswamy, P. R.; Giri, K. V. (1953). The occurrence of 4-aminobutyric acid and glutamic acid decarboxylase in red yeast (Rhodotorula glutinis). Curr. Sci. 22, 143-144.
- 45. Lee, B.J.; Kim, J.S.; Kang, Y.M.; Lim, J.H.; Kim, Y.M.; Lee, M.S.; Jeong, M.H.; Ahn, C.B.; Je, J.Y. (2010). Antioxidant activity and γ-aminobutyric acid (GABA) content in sea tangle fermented by Lactobacillus brevis BJ20 isolated from traditional fermented foods. Food Chem. 122 (1), 271-276.
- 46. Leroy, F.; Vuyst, L.D. (2004). Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 15, 67-78.
- 47. Li, H.; Gao, D.; Cao, Y.; Xu, H. (2008). A high γ-aminobutyric acid-producing Lactobacillus brevis isolated from Chinese traditional paocai. Ann. Microbiol. 58 (4), 649-653.
- 48. Li, H.; Cao, Y. (2010). Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids 39, 1107-1116.
- 49. Li, H.; Qiu, T.; Huang, G.; Cao, Y. (2010). Production of gamma-aminobutyric acid by Lactobacillus brevis NCL912 using fed-batch fermentation. Microb. Cell Fact. 9, 85.
- 50. Li, H.; Qiu, T.; Gao, D.; Cao, Y. (2010). Medium optimization for production of gamma-aminobutyric acid by Lactobacillus brevis NCL912. Amino Acids 38, 1439-1445.
- 51. Li, Q.; Yao, H.Y.; Zhang, H. (2004). Studies on Screening for γ -aminobutyric acid producer and optimization of fermentation conditions. Chin. J. Amino Acids Biotic Res. 26(1), 40-43.
- 52. Lu, X.; Xie, C.; Gu, Z. (2008). Isolation of γ-aminobutyric acid-producing bacteria and optimization of fermentative medium. Biochem. Eng. J. 41, 48-52.
- 53. Lu, X.; Xie, C.; Gu, Z. (2009). Optimisation of fermentative parameters for GABA enrichment by Lactococcus lactis.Czech. J. Food Sci. 27(6), 433-442.
- 54. Maras, B.; Sweeney, G.; Barra, D.; Bossa, F.; John, R.A. (1992). The amino acid sequence of glutamate decarboxylase from Escherichia coli Eur. J. Biochem 204, 93-98.
- 55. Miura, D.; Ito, Y.; Mizukuchi, A.; Kise, M.; Aoto, H.; Yagasaki, K. (2006). Hypercholesterolemic action of pre-germinated brown rice in hepatoma-bearing rats. Life Sci. 79, 259-264.
- 56. Nikolaeva, Z. K.; Yu, V.V. (1972). Mechanism of action of y-aminobutyrate-glutamate transaminase from pig kidney. Biokhimiya 37, 572-578.
- 57. Nishida, S.; Michinaka, A.; Nakashima, K.; Iino, H.; Fujii, T. (2008). Evaluation of the probiotic potential of Lactobacillus paracasei KW3110 based on in vitro tests and oral administration tests in healthy adults. J. Gen. Appl. Microbiol. 54, 267-276.
- 58. Nomura, M.; Kimoto, H.; Someya, Y.; Furukawa, S.; Suzuki, I. (1998). Production of gamma-aminobutyric acid by cheese starters during cheese ripening. J. Dairy Sci 81, 1486-1491.
- 59. Okada, T.; Sugishita, T.; Murakami, T.; Murai, H.; Saikusa, T.; Horino, T.; Onoda, A.; Kajimoto, O.; Takahashi, R.; Takahashi, T. (2000). Effect of the defatted rice germ enriched with GABA for sleeplessness, depression, autonomic disorder by oral administration. J. Jap. Soc. Food Sci 47, 596-603.
- 60. Park, K.B.; Oh, S.H. (2006b). Isolation and characterization of Lactobacillus buchneri strains with high gamma-aminobutyric acid producing capacity from naturally aged cheese. Food Sci. Biotechnol. 15, 86-90.
- 61. Park, K.B.; Oh, S.H. (2007). Production of yogurt with enhanced levels of gamma-aminobutyric acid and valuable nutrients using lactic acid bacteria and germinated soybean extract. Biores. Technol. 98, 1675-1679.
- 62. Pimentel, M.C.B.; Melo, E.H.M.; Filho, J.L.L. (1996). DURAN N. Production of lipase free of citrinin by Penicillium citrinum Mycopathologia 133, 119-121.
- 63. Plokhov, A.Y.; Gusyatiner, M.M.; Yampolskaya, T.A.; Kaluzhsky, V.E.; Sukhareva, B.S.; Schulga, A.A. (2000). Preparation of gamma-aminobutyric acid using E. coli cells with high activity of glutamate decarboxylase. Appl. Biochem. Biotechnol. 88, 257-265.
- 64. Reed, L. J. (1950). The occurrence of c-aminobutyric acid in yeast extract; its isolation and identification. J. Biol. Chem. 183, 451-458.
- 65. Rizzello, C.G.; Cassone, A.; Cagno, R. DI.; Gobbetti, M. (2008). Synthesis of Angiotensin I-Converting Enzyme (ACE)-Inhibitory peptides and γ -aminobutyric acid (GABA) during sourdough fermentation by selected lactic acid bacteria. J. Agric. Food Chem. 56, 6936-6943.
- 66. Saikusa, T.; Horino, T.; Mori Y. (1994). Accumulation of g-aminobutyric acid (Gaba) in the rice germ during water soaking. Biosci. Biotech. Biochem 58, 2291-2292.
- 67. Sanders, J. W.; Leenhouts, K.; Burghoorn,J.; Brands, J. R.; Venema, G.; Kok, J. (1998.) A chloride-inducible acid resistance mechanism in Lactococcus lactis and its regulation. Mol. Microbiol 27, 299-310.
- 68. Sandmeier, E.; Hale, T.I.; Christen, P. (1994). Multiple evolutionary origin of pyridoxal 50-phosphate-dependent amino acid decarboxylase. Eur. J. Biochem. 221, 997-1002.
- 69. Sawai, Y.; Yamaguchi, Y.; Miyana, D.; Yoshitomi, H. (2001). Cycling treatment of anaerobic and aerobic incubation increases the content of g-aminobutyric acid in tea shoots. Amino Acids 20, 331-334.
- 70. Schmit, J. C.; Brody, S. (1975). Neurospora crassa conidial germination: role of endogenous amino acid pools. J. Bacteriol. 124, 232-242.
- 71. Scott, E. M.; Jakoby, W. B. (1959). Soluble y-aminobutyric- glutamic transaminase from Pseudomonas fluorescens J. Biol. Chem 234, 932-936.
- 72. Servili, M.; Rizzello, C.G.; Taticchi, A.; Esposto, S.; Urbani, S.; Mazzacane, F.; Di Maio, I.; Selvaggini, R.; Gobbetti, M.; Di Cagno, R. (2011). Functional milk beverage fortified with phenolic compounds extracted from olive vegetation water, and fermented with functional lactic acid bacteria. Int. J. Food Microbiol. 147(1), 45-52.
- 73. Settanni, L.; Corsetti, A. (2008). Application of bacteriocins in vegetable food biopreservation. Int. J. Food Microbiol 121, 123-138.
- 74. Seok, J.H.; Park, K.B.; Kim, Y.H.; Bae, M.O.; Lee, M.K.; Oh, S.H. (2008). Production and characterization of kimchi with enhanced levels of gamma-aminobutyric acid. Food Sci. Biotechnol. 17, 940-946.
- 75. Shelp, B. J.; Bown, A. W.; Mclean, M. D. (1999). Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci. 4, 446-452.
- 76. Siragusa, S.; Angelis, M. De.; Cagno, R. Di.; Rizzello, C. G.; Coda, R.; Gobbetti, M. (2007). Synthesis of γ -aminobutyric acid by lactic acid bacteria isolated from a variety of Italian cheeses. Appl. Environ. Microbiol. 7283-7290.
- 77. Skeie, S.; Lindberg, C.; Narvhus, J. (2001). Development of amino acids and organic acids in Norvegia, influence of milk treatment and adjunct Lactobacillus Int. Dairy J. 11, 399-411.
- 78. Smith, D.K.; Kassam, T.; Singh, B.; Elliott, J.F. (1992). Escherichia coli has two homologousglutamate decarboxylase genes that map to distinct loci. J. Bacteriol. 174, 5820-5826.
- 79. Soo, I.M.C.; Lee, J.W.; Park, S.M.; Lee, M.Y.; Ji, G.E.; Park, M.S.; Heo, T.R. (2006). Improvement of γ-aminobutyric acid (GABA) production using cell entrapment of Lactobacillus brevis GABA 057. J. Microbiol. Biotechnol. 16(4), 562-568.
- 80. Steward, F.C.; Thompson, J. F.; Dent, C. E. (1949). γ- aminobutyric acid: a constituent of the potato tuber? Science 110, 439-440.
- 81. Su, Y.C.; Wang, J.J.; Lin, T.T.; Pan, T.M. (2003). Production of the secondary metabolites gamma-aminobutyric acid and monacolin K by Monascus.J. Ind. Microbiol. Biotechnol 30, 41-46.
- 82. Sun, B.S.; Zhou, L.P.; Jia, X.Q.; Sung, C.K. (2008). Response surface modeling for g-aminobutyric acid production by Monascus pilosus GM100 under solid-state fermentation. Afr. J. Biotechnol 7(24), 4544-4550.
- 83. Tsai, J.S.; Lin, Y.S.; Pan, B.S.; Chen, T.J. (2006). Antihypertensive peptides and gamma-aminobutyric acid from prozyme 6 facilitated lactic acid bacteria fermentation of soymilk. Process Biochem 41, 1282-1288.
- 84. Tsushida, T.; Murai, T. (1987). Conversion of glutamic acid to g-aminobutyric acid in tea leaves under anaerobic conditions. Agric. Biol. Chem. 51, 2865-2871.
- 85. Ueno, H. (2000). Enzymatic and structural aspects on glutamate decarboxylase. J. Mol. Catal. B: Enzym. 10, 67-79.
- 86. Ueno, Y.; Hayakawa, K.; Takahashi, S.; Oda, K. (1997). Purification and characterization of glutamate decarboxylase from Lactobacillus brevis IFO 12005. Biosci. Biotech. Biochem 61, 1168-1171.
- 87. Voellmy, R.; Leisinger, T. (1976). Role of 4-aminobutyrate aminotransferase in the arginine metabolism of Pseudomonas aeruginosa J. Bacteriol 128(3), 722-729.
- 88. Wang, J.J.; Lee, C.L.; Pan, T.M. (2003). Improvement of monacolin K, c-aminobutyric acid and citrinin production ratio as a function of environmental conditions of Monascus purpureus NTU 601. J. Ind. Microbiol. Biotechnol. 30, 669-676.
- 89. Wouters, J.T.M.; Ayad, E.H.; Hugenholtz, J.; Smit, G. (2002). Microbes from raw milk for fermented dairy products. Int. Dairy J. 12, 91-109.
- 90. Yang, S.Y.; Lu, F.X.; Lu, Z.X.; Bie, X.M.; Jiao, Y.; Sun, L.J.; Yu, B. (2008). Production of gamma-aminobutyric acid by Streptococcus salivariu subsp thermophilus Y2 under submerged fermentation. Amino Acids 34, 473-478.
- 91. Yokoyama, S.; Hiramatsu, J.; Hayakawa, K. (2002). Production of y-aminobutyric acid from alcohol distillery lees by Lactobacilus brevis IFO- 12005. J. Biosci. Bioeng 93(1), 95-97.
Publication Dates
-
Publication in this collection
19 Feb 2013 -
Date of issue
Dec 2012
History
-
Received
25 Aug 2011 -
Accepted
07 June 2012