Abstract
The use of the filamentous fungus, Ashbya gossypii, to improve riboflavin production at an industrial scale is described in this paper. A riboflavin overproducing strain was isolated by ultraviolet irradiation. Ten minutes after spore suspensions of A. gossypii were irradiated by ultraviolet light, a survival rate of 5.5% spores was observed, with 10% of the surviving spores giving rise to riboflavin-overproducing mutants. At this time point, a stable mutant of the wild strain was isolated. Riboflavin production of the mutant was two fold higher than that of the wild strain in flask culture. When the mutant was growing on the optimized medium, maximum riboflavin production could reach 6.38 g/l. It has even greater promise to increase its riboflavin production through dynamic analysis of its growth phase parameters, and riboflavin production could reach 8.12 g/l with pH was adjusted to the range of 6.0-7.0 using KH2PO4 in the later growth phase. This mutant has the potential to be used for industrial scale riboflavin production.
Riboflavin; Ashbya gossypii; Ultraviolet irradiation; Medium optimization
INDUSTRIAL MICROBIOLOGY
Isolation and characterization of an Ashbya gossypii mutant for improved riboflavin production
Shiping WeiI, * * Corresponding Author. Mailing address: School of Marine Sciences, China University of Geosciences, 29 Xueyuan Rd., Beijing 100083, China.; Tel: 86-10-82334704 Fax: 86-10-82320065.; E-mail: weishiping@cugb.edu.cn ; James HurleyII; Zhenglong JiangI; Siwen WangI; Yuanyuan WangI
ISchool of Marine Sciences, China University of Geosciences, 29 Xueyuan Rd., Beijing 100083, China
IIDepartment of Plant Pathology and Microbiology, Texas A & M University, 2132 TAMU, College Station TX77843, USA
ABSTRACT
The use of the filamentous fungus, Ashbya gossypii, to improve riboflavin production at an industrial scale is described in this paper. A riboflavin overproducing strain was isolated by ultraviolet irradiation. Ten minutes after spore suspensions of A. gossypii were irradiated by ultraviolet light, a survival rate of 5.5% spores was observed, with 10% of the surviving spores giving rise to riboflavin-overproducing mutants. At this time point, a stable mutant of the wild strain was isolated. Riboflavin production of the mutant was two fold higher than that of the wild strain in flask culture. When the mutant was growing on the optimized medium, maximum riboflavin production could reach 6.38 g/l. It has even greater promise to increase its riboflavin production through dynamic analysis of its growth phase parameters, and riboflavin production could reach 8.12 g/l with pH was adjusted to the range of 6.0-7.0 using KH2PO4 in the later growth phase. This mutant has the potential to be used for industrial scale riboflavin production.
Key words: Riboflavin; Ashbya gossypii; Ultraviolet irradiation; Medium optimization
INTRODUCTION
Riboflavin, also called vitamin B2, is an essential compound in living organisms, including microorganisms, plants and mammals. It serves as the precursor of flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), which are required as electron-accepting oxidoreductases (14, 19). It is widely used in the food enrichment, pharmaceutical and feed supplement industries. Riboflavin can be produced by chemical synthesis or by microbial fermentation. Riboflavin for therapeutic purposes is still made by chemical synthesis, but since the market is being driven by heightened interest in utilizing renewable resources and their corresponding cost reductions, riboflavin is increasingly produced by specialized microorganisms (7, 16, 22). Currently, industrial riboflavin production by microbial fermentation generates approximately 6×106 kg annually (20).
Riboflavin is produced by many microorganisms, including bacteria, yeasts and molds (2, 16). Ashbya gossypii, a filamentous fungus, is currently used in industry for the production of riboflavin (16). About 30% of the world industrial riboflavin output, which is greater than 1.25×106 kg, is produced by direct fermentation with this fungus (1, 17). Over the past several decades, great efforts have been made to enhance riboflavin production and develop better production media. Many different methods have been used to improve the productivity of the wild strain, such as UV irradiation (6, 11, 14), chemical-induced mutagenesis (20), antimetabolite mutagenesis (13, 19) and metabolic engineering approaches (5, 18).
Numerous studies have been conducted to improve riboflavin productivity by utilizing different substrates (2, 8, 9, 20). Riboflavin overproduction by A. gossypii can be achieved by using glucose, sucrose, fructose, maltose, mannose, or glycerol as the carbon sources; however, oils, such as corn oil and soybean oil, are superior (2). In addition to the carbon source, commercial fermentations employ a crude nitrogen source, with enzymatic digests of collagenous proteins being the most effective. Also, yeast extracts have been shown to be essential in stimulating riboflavin overproduction from A. gossypii (12). Corn steep liquor was found to be a very satisfactory substitute for yeast extract as it is abundantly available at low cost (2). Utilization of waste products to produce riboflavin have been reported (3, 11), with yields as high as 8.7 g/l riboflavin when activated bleaching earth containing rapeseed oil was added to the culture.
The objective of this work was isolation of an UV-induced mutant strain overproducing riboflavin. The UV-induced mutant can synthesize riboflavin both earlier and more prolifically than the wild strain. By dynamic characterization of this mutant in an optimized medium, there is still great potential to increase riboflavin production by adjusting the parameters during fermentation, thus allowing this mutant to serve as an industrial strain for riboflavin production.
MATERIALS AND METHODS
Strain, media and cultivation conditions
Ashbya gossypii ATCC 10895 was used as the wild type strain. Stock culture was grown at 28°C on solid slant medium (SSM) containing (per liter) 10 g glucose, 3 g malt extract, 5 g peptone, 3 g yeast extract and 15 g agar (pH 6.9). After 4 days of cultivation, the agar slants were stored at 4 °C until further analysis. Spore producing liquid medium (SPLM) consisted of (per liter) 10 g glucose, 1 g thiamine, 5 g yeast extract and 5 g Tween 80 (pH 6.8). The mutant screening medium (MSM) consisted of (per liter) 20 g glucose, 5 g yeast extract and 15 g agar (pH 7.2). The preliminary seed medium (PSM) consisted of (per liter) 20 g glucose, 10 g corn steep liquor (North China Pharmaceutical Co. Ltd., China) and 5 g peptone (pH 6.8), and the second seed medium (SSM) consisted of (per liter) 6 g glucose, 10 g corn steep liquor, 5 g gelatin (Beijing Chemical Reagent Co., China) and 10 g soybean oil (Shandong Luhua Co. Ltd., Laiyang, China) (pH 6.8). The riboflavin production medium (RPM) contained (per liter): 30 g corn steep liquor, 15 g osseocolla (Beijing Zhijiao Plant), 40 g soybean oil, 2 g NaCl and 1 g KH2PO4 (pH 6.8).
All the seed cultures were incubated at 28 °C on a rotary shaker (HNY-200B, Honour Instrument Factory, Jiangsu, China) at 150 rpm for 36 h in 250 ml Erlenmeyer flasks containing 15 ml RPM medium. For riboflavin production in flask cultures, 0.3 ml of the preliminary seed culture was inoculated into 15 ml of SSM medium, and 1 ml of the second seed culture was inoculated into 500 ml Erlenmeyer flasks containing 100 ml of RPM medium. The culture was incubated at 28 °C on a rotary shaker at 200 rpm for 7 days.
Spore preparation, UV irradiation and mutant screening
For preparation of spores, following 4 days of cultivation on SSM media, mycelia of A. gossypii were collected by adding 1 ml sterilized water. The suspended mycelia were inoculated into a 250 ml Erlenmeyer flask with 100 ml SPLM medium. This inoculation culture was incubated at 28 °C in a stationary manner. After 5 days of incubation, the spores were collected by filtering through three layers of lens paper and adjusting the concentration to 105-106 spores/ml.
A Petri dish containing 10 ml of spore suspension was stirred at 60 rpm and exposed to UV light (254 nm) at a distance of 30 cm from the UV lamp (15 W, Brightek Optoelectronic Co., Ltd., Shenzhen, China). 100 µl of spore suspension was removed at every 5 or 10 min and plated on the MSM media. The colony-forming units were counted after 5 days incubation at 28 °C to determine the percentage of survival of A. gossypii spores at each time point. 20 colonies were randomly chosen at each time point and their riboflavin producing potential was compared with A. gossypii ATCC 10895 using the RPM media to determine the ratio of positive mutants. The optimized UV irradiation dose was used to generate a mutant library, and the preliminary screening of candidate positive mutants was based on the intensity of yellow color produced by colonies on the MSM medium. The second screening was performed using 500 ml Erlenmeyer flasks containing 100 ml RPM medium and incubated with shaking at 28 °C for 7 days.
Optimization of riboflavin production medium
The riboflavin production medium was optimized using a three factor and three level L9(33) orthogonal design (Table. 1). Levels of factor A (corn steep liquor) were 20 g/l, 30 g/l and 40 g/l; levels of factors B (osseocolla) were 15 g/l, 25 g/l and 30 g/l; and levels of factor C (soybean oil) were 4 g/l, 4.5 g/l and 5 g/l. A total of 9 experimental runs were performed in 500 ml Erlenmeyer flasks containing 100 ml medium incubated with shaking at 28 °C for 7 days, with three replicates for every experimental run. The more important factors and most effective levels were determined based on the orthogonal analysis and subjected to further tests of riboflavin production.
The optimized concentrations of the three aforementioned components were combined and subjected to flask tests of riboflavin production. In order to further increase the riboflavin production, fed-batch fermentation was employed by adding 10 g soybean oil on the fifth and the sixth day based on the optimized medium.
Analytical methods
The riboflavin concentration was measured according to the method described by Tanner et al (21) with modifications. Before sampling, the culture broth was mixed thoroughly and 2 ml of culture broth was drawn and mixed well with 1 ml of HCl buffer (pH 2.0). The mixture was treated at 100 °C for 30 min, and then centrifuged at 10000 g for 5 min. A 0.5ml aliquot of the supernatant was removed and diluted to 100 ml with sterilized water. The diluted supernatant was subjected to riboflavin analysis. The absorbance of the diluted supernatant was monitored by a Photofluorometer 930 (Kexiao Co., Ltd., Hangzhou, China), with riboflavin concentration being calculated from the calibration curve. Fluorometric analyses matched well with those values obtained microbiologically and spectrophotometrically (10, 20, 21).
In order to determine the correlation of riboflavin production to the parametric changes during the growth phase of A. gossypii, parameters such as biomass, pH, free amino nitrogen and reduced sugar were monitored at constant time intervals. Biomass was determined gravimetrically by obtaining 10 ml aliquots of culture broth at each time point. The culture was centrifuged at 10000 g for 5 min, the pellet mycelia was weighed to determine the ratio of mycelia to volume of the culture, and the supernatant was used to analyze free amino nitrogen and reduced sugar. Free amino nitrogen was determined by the formaldehyde method (4), and reduced sugar was determined by the Fehling method (15).
RESULTS AND DISCUSSION
Effect of UV irradiation on survival of A. gossypii spores and riboflavin-overproducing mutants
UV irradiation on the spore suspension of A. gossypii lasted a total of 60 min. The survival of colonies was ascertained after 5 days incubation at 28°C. Fig. 1 shows the effect of UV irradiation time on the survival of A. gossypii spores. At the 10 minute time point, spore survival had already decreased dramatically with only 5.5% surviving the UV treatment. After 10 minutes, only a few colonies appeared on the MSM media plates with these colonies being more resistant to the UV radiation.
The percentage of riboflavin-overproducing mutants increased with the time of UV irradiation up to 10 min, with the percentage as high as 10%. After this time point, the percentage of riboflavin-overproducing mutants tended to decline with increasing UV irradiation time (Fig. 1). For every time point, most mutants were negative riboflavin producing when compared with the wild type strain. We chose the UV irradiation time of 10 min to generate a mutant library containing 2996 mutants, which was screened to identify riboflavin-overproducing mutants.
Isolation and screening of riboflavin-overproducing mutants
A total of 2996 mutants were grown on MSM media and compared with respect to their yellow coloration. 125 of them had intense yellow colors and were chosen as the candidates for riboflavin-overproducing mutants. Following the second screening on RPM media, a mutant designated as ATCC 10895-32 was identified as the top riboflavin producer. Riboflavin production of ATCC 10895-32 was dynamically compared with that of the wild strain at different time points (Fig. 2). The mutant strain synthesized riboflavin at an earlier stage than the wild strain, and riboflavin production of the mutant strain was also higher than that of the wild strain at all sampling times. Riboflavin production by ATCC 10895-32 at 8 d was 4.48 g/l, a 42% increase compared to the wild strain on the RPM media.
UV irradiation usually generates point mutations, which are easily recovered in natural conditions. To determine whether or not ATCC 10895-32 was a stable mutant, the mutant strain was subjected to twenty successive transfers, and 50 colonies were randomly isolated to compare their riboflavin production. There was no significant change in the riboflavin production among them, suggesting that the isolated mutant strain can be used as a candidate potential riboflavin overproducer.
Optimization of the riboflavin production medium for the A. gossypii 10895-32 strain
Out of all the components in the riboflavin production medium, corn steep liquor, osseocolla and soybean oil were the most crucial factors for riboflavin production (19). L9(33) orthogonal design experiments were performed to determine the factors' optimal concentrations. The combined effects of the three components on the riboflavin concentration are shown in Table 1. The results were analyzed by the standard orthogonal design analysis. From the data of R (R=Max ti - Min ti.), it shows that corn steep liquor has the largest effect and soybean oil has the smallest effect on riboflavin production. This means riboflavin production was greatest when level 1 of corn steep liquor, level 3 of osseocolla and level 3 of soybean oil were used (Fig. 3). The optimal combination of media for riboflavin production is corn steep liquor 20 g/l, osseocolla 25 g/l and soybean oil 50 g/l, with the other parts comprising the media being NaCl 2 g/l and KH2PO4 1 g/l (pH 6.8). This indicated that a higher riboflavin production could be expected when the optimized medium is used.
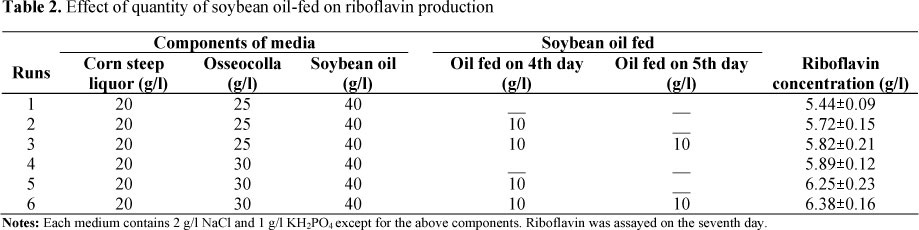
Based on the optimized medium, we made a slight change in the components of this medium for consideration in industrial utilization. Özbas and Kutsal (5) reported that oxygen was a very important factor for riboflavin overproduction. More oil being added to the medium in the beginning may affect the oxygen uptake of A. gossypii, so the initial amount of oil in optimized medium was decreased by 10 g/l, with this omitted amount being later added to the media by feeder. Osseocolla was used at 25 g/l and 30 g/l (Table 2).
Fed-batch culture is often used in industrialized fermentation to increase production. We observed that little soybean oil was left during the latter growth phage of ATCC 1895-32. Soybean oil was putatively the limiting factor for further riboflavin synthesis. For the purpose of further increasing riboflavin, 10 g soybean oil was fed on the fourth or the fifth day based on the optimized medium. The result shows that the riboflavin production of fed batch culture was increased by 5%-8% in comparison with that of non-fed batch culture (Table 2). The riboflavin concentration could reach 6.25 g/l when 10 g soybean oil was added on the fourth day. However, riboflavin concentration did not increase significantly when another 10 g soybean oil was fed on the fifth day, probably because the other components of the medium had become depleted, or non-appropriate environmental factors (pH, etc.) restricted riboflavin overproduction.
Dynamic characterization of A. gossypii 10895-32 in the optimized medium
To determine the correlation of riboflavin production with the metabolic parameter changes during the growth phase of A. gossypii ATCC 10895-32, the parameters, pH, biomass, free amino nitrogen and reduced sugar, were monitored daily. The pH quickly dropped from the initial pH of 6.8 to 5.9 on the first day after inoculation. Relatively less riboflavin was synthesized during this period. The growth of A. gossypii was beginning to reach the log phase after pH reached 6.47 on the third day (Fig. 4A). The rise in pH signals the initial phase of riboflavin production (2). When the pH was rising in the range of pH 6.47 to 7, A. gossypii was in the stationary phase and the riboflavin was oversynthesized. When the pH climbed from 7.0 to 7.9, the mycelia were lysed and the biomass of A. gossypii declined, which caused the riboflavin synthesis to stop (Fig. 4A and Fig. 4B). Tanner et al (3) and Özbas et al (5) reported that the pH was as low as 4.5 in the first 24 to 36 hours, and that the best riboflavin yields was obtained when the pH was in the range of 6.0 to 7.0. With the continuous consumption of nutrients in the medium, the pH can reach as high as 8.5, and subsequent riboflavin synthesis is negligible or absent.
The metabolic parameters, free amino nitrogen and reduced sugar, were assayed during the growth phase of A. gossypii. As free amino nitrogen dramatically decreased in the first two days, we presumed that A. gossypii quickly used the free amino nitrogen to synthesize its own structure because of sustained growth of A. gossypii. Subsequently, the released free amino nitrogen levels rose along with the reduced sugar levels. Especially in the later growth phase, the released free amino nitrogen increased rapidly, which was believed to have contributed to the rise in pH (2). Since both free amino nitrogen and reduced sugar were continuously released during the growth phase, they were available for riboflavin overproduction. However, the alkaline pH caused mycelial lysis and the riboflavin synthesis was terminated. Based on the analysis of dynamic characterization of A. gossypii 10895-32 in the optimized medium, we performed a new experiment with the pH adjusted to the range of 6.0-7.0 using KH2PO4 in the later growth phase, riboflavin production was increased to 8.12 g/l.
In summary, we used the most effective method of UV irradiation to generate a stable mutant strain. Through the optimization of its medium and characterization of its growth, enhanced riboflavin production was observed. This strain constitutes an excellent candidate for use in industrialized fermentation for riboflavin production.
ACKNOWLEDGEMENTS
The authors acknowledge Professor Paul deFigueiredo of the Department of Plant Pathology and Microbiology at Texas A&M University for making a critical reading and revision of this paper. This research was supported by "the Fundamental Research Funds for the Central Universities".
Submitted: November 27, 2010;
Returned to authors for corrections: August 06, 2011;
Approved: January 16, 2012.
- 1. Bigelis, R. (1989). BiotechnologyIndustrial products of biotechnology: application of gene technology, VCH, Weinheim, p243.
- 2. Demain, A.L. (1972). Riboflavin oversynthesis. Annu. Rev. Microbiol. 26 369-388.
- 3. Ertrk E.; Erkmen O.; Öner, M.D. (1998). Effects of various supplements on riboflavin production by Ashbya gossypii in whey. J. Environ. Eng. Sci 22 (2) 371-376.
- 4. European Brewing Commission. (1972). Method for the determination of free amino nitrogen. Brauwissenschaft. 8 (1) 250.
- 5. Jiménez, A.; Santos, M. A.; Pompejus, M.; Revuelta, J.L. (2005). Metabolic Engineering of the Purine Pathway for Riboflavin Production in Ashbya gossypii Appl. Environ. Microbiol 71 (10) 5743-5751.
- 6. Maresma, B.G.; Castillo, B.G.; Fernández, R.C.; da Silva, E.S.; Maiorano, A.E.; de Andrade Rodrigues M.F. (2010). Mutagenesis of Aspergillus oryzae IPT-301 to improve the production of □-fructofuranosidase. Braz. J. Microbiol. 41 (1) 186-195.
- 7. Martens, J.H.; Barg, H.; Warren, M.J.; Jahn, D. (2002). Microbial production of vitamin B2 Appl. Microbiol. Biotechnol. 58 (3) 275-285.
- 8. Özbas, T.; Kutsal, T. (1986). Comparative study of riboflavin production from two microorganisms: Eremothecium ashbyii and Ashbya gossypii Enzyme. Microb. Technol 8 (10) 593-596.
- 9. Özbas, T.; Kutsal, T. (1991). Effects of growth factors on riboflavin production by Ashbya gossypii Enzyme. Microb. Technol 13 (7) 594-596.
- 10. Park, E.Y.; Ming, H. (2004). Oxidation of rapeseed oil in waste activated bleaching earth and its effect on riboflavin production in culture of Ashbya gossypii J. Biosci. Bioeng 97 (1) 59-64.
- 11. Park, E.Y.; Zhang, J.H.; Tajima, S.; Dwiarti, L. (2007). Isolation of Asybya gossypii mutant for an improved riboflavin production targeting for biorefinery technology. J. Appl. Microbiol 103 (2) 468-476.
- 12. Robbins, W.J.; Schmidt, M.B. (1939). Preliminary experiments on biotin. Bull. Torrey. Botan. Club. 66 (3) 139-150.
- 13. Schmidt, G.; Stahmann, K.P.; Sahm, H. (1996). Inhibition of purified isocitrate lyase identified itaconate and oxalate as potential antimetabolites for the riboflavin overproducer Ashbya gossypii Microbiology 142 (2) 411-417.
- 14. Stahmann, K.P.; Jr, H.N.A.; Althöfer, H.; Revuelta, J.L.; Monschau, N.; Schlüpen C.; Gätagens, C.; Wiesenburg A.; Schlösser, T. (2001). Riboflavin overproduced during sporulation of Ashbya gossypii, protects its hyaline spores against ultraviolet light. Environ. Microbiol. 3 (9), 545-550.
- 15. Shi, J.; Yang J.; Meng, Q.; Yang, Y.; Ma, Y.; Zhang, L. (2006). Evaluation of reducing sugar and glucose fermentation process control. Food and Fermentation Industries 32 (10) 165-166.
- 16. Stahmann K.P.; Revuelta J.L.; Seulberger H. (2000). Three biotechnical processes using Ashbya gossypii, Candida famata, or Bacillus subtilis compete with chemical riboflavin production. Appl. Microbiol. Biotechnol. 53 (5) 509-516.
- 17. Stahmann, K.P.; Kupp, C.; Feldmann, S.D.; Sahm, H. (1994). Formation and degradation of lipid bodies found in the riboflavin-producing fungus Ashbya gossypii Appl. Microbiol. Biotechnol. 42 (1) 121-127.
- 18. Sugimoto T.; Kanamasa, S.; Kato, T.; Park, E. Y. (2009). Importance of malate synthase in the glyoxylate cycle of Ashbya gossypii for the efficient production of riboflavin. Appl. Microbiol. Biotechnol 83 (3) 529-539.
- 19. Sugimoto T.; Morimoto, A.; Nariyama, M.; Kato, T.; Park, E.R. (2009). Isolation of an oxalate-resistant Ashybya gossypii strain and its improved riboflavin production. J. Ind. Microbiol. Biotechnol. 37 (1) 57-64.
- 20. Tajima S.; Itoh, Y.; Sugimoto, T.; Kato, T.; Park, E. Y. (2009). Increases in riboflavin production from activated bleaching earth by a mutant strain of Ashbya gossypii J. Biosci. and Bioeng. 108 (4) 325-329.
- 21. Tanner, F.W.; Vojnovich, C.; Lanen, J.M.V. (1949). Factors affecting riboflavin production by Ashbya gossypii J. Bacteriol 58 (6) 737-745.
- 22. Vandamme, E.J. (1992). Production of vitamins, coenzymes and related biochemicals by biotechnological processes. J. Chem. Technol. Biotechnol. 53 (4) 313-327.
Publication Dates
-
Publication in this collection
07 Aug 2012 -
Date of issue
June 2012
History
-
Received
27 Nov 2010 -
Accepted
16 Jan 2012 -
Reviewed
06 Aug 2011