Abstract
Extracellular tannase and gallic acid were produced optimally under submerged fermentation at 37 0C, 72 h, pH 5.0, 10 %(v/v) inoculum and 4 %(w/v) of the agroresidue pomegranate rind (PR) powder by an Aspergillus niger isolate. Tannic acid (1 %) stimulated the enzyme production by 245.9 % while with 0.5 % glucose, increase was marginal. Tannase production was inhibited by gallic acid and nitrogen sources such as NH4NO3, NH4Cl, KNO3, asparatic acid, urea and EDTA. The partially purified enzyme showed temperature and pH optima of 35 0C and 6.2 respectively which shifted to 40 0C and 5.8 on immobilization in alginate beads. Activity of the enzyme was inhibited by Zn+2, Ca+, Mn+2, Mg+2, Ba+2and Ag+. The immobilized enzyme removed 68.8 % tannin from juice of aonla/myrobalan (Phyllanthus emblica), a tropical fruit, rich in vitamin C and other essential nutrients. The enzymatic treatment of the juice with minimum reduction in vitamin C is encouraging as non enzymatic treatments of myrobalan juice results in vitamin C removal.
tannase; pomegranate rind; myrobalan; Aspergillus niger
INDUSTRIAL MICROBIOLOGY
Characterization And Application Of Tannase Produced By Aspergillus Niger ITCC 6514.07 On Pomegranate Rind
Anita Srivastava; Rita Kar* * Corresponding Author. Mailing address: Department of Biochemical Engineering and Food Technology, Harcourt Butler Technological Institute, Kanpur 208002, India.; Tel.: +91-512-253-4001; Fax: 91-512-25900.; Email: rkarhbti@rediffmail.com
Department of Biochemical Engineering and Food Technology, Harcourt Butler Technological Institute, Kanpur 208002, India
ABSTRACT
Extracellular tannase and gallic acid were produced optimally under submerged fermentation at 37 0C, 72 h, pH 5.0, 10 %(v/v) inoculum and 4 %(w/v) of the agroresidue pomegranate rind (PR) powder by an Aspergillus niger isolate. Tannic acid (1 %) stimulated the enzyme production by 245.9 % while with 0.5 % glucose, increase was marginal. Tannase production was inhibited by gallic acid and nitrogen sources such as NH4NO3, NH4Cl, KNO3, asparatic acid, urea and EDTA. The partially purified enzyme showed temperature and pH optima of 35 0C and 6.2 respectively which shifted to 40 0C and 5.8 on immobilization in alginate beads. Activity of the enzyme was inhibited by Zn+2, Ca+, Mn+2, Mg+2, Ba+2and Ag+. The immobilized enzyme removed 68.8 % tannin from juice of aonla/myrobalan (Phyllanthus emblica), a tropical fruit, rich in vitamin C and other essential nutrients. The enzymatic treatment of the juice with minimum reduction in vitamin C is encouraging as non enzymatic treatments of myrobalan juice results in vitamin C removal.
Key words: tannase, pomegranate rind, myrobalan, Aspergillus niger
INTRODUCTION
The enzyme tannase (E.C 3.1.1.20) also known as tannin acyl hydrolase, is a hydrolytic enzyme that acts on tannin. Tannase catalyses the hydrolysis of bonds present in the molecules of hydrolysable tannins and gallic acid esters (8) producing gallic acid. Gallic acid is used in the food industry as a substrate for the chemical synthesis of food preservatives such as gallates and pyrogallol, propyl gallate being a very important food antioxidant, and in the pharmaceutical industry, for the synthesis of antibacterial drugs (16). The main commercial applications of tannase at present are in the preparation of instantaneous tea, action on tea polyphenols, in the production of gallic acid (17), beer chillproofing and wine making. Tannase can also be used to reduce tannin levels wherever desirable such as fruit juices (14). Aonla (Phyllanthus emblica Linn.), a tropical fruit is one of the richest sources of vitamin C and has been recognized since ancient times for its immense medicinal and nutritional properties. This fruit with its high tannin content i.e. gallotannic acid, which upon hydrolysis yields gallic acid, has antioxidant properties and retards the oxidation of vitamin C. The stability of vitamin C in aonla products due to gallic acid makes its processing a matter of great concern.
The astringent taste due to tannins of aonla juice on the other hand reduces its acceptance as a beverage. Hence enzymatic hydrolysis of tannin in aonla juice into gallic acid is advantageous since it reduces its astringency with minimum loss of vitamin C.
The novelty of the present work lies in the use of a new agro substrate pomegranate rind (PR) by an A.niger isolate for optimum production of gallic acid and tannase and application of the latter for the first time in tannin removal from juice of aonla, a nutrient rich tropical fruit without loss of its nutritional value.
MATERIALS AND METHODS
Isolation and screening of microorganisms
A fungal strain was isolated on Czapeck-Dox medium containing 1 % (w/v) tannic acid and selected from eight other microbial strains on the basis of zone of lysis and tannase activity (1). The strain was identified as Aspergillus niger by the Indian Type Culture Collection, New Delhi and deposited in their collection unit (A.niger ITCC 6514.07).
Inoculum preparation
The culture was maintained on tannic acid agar slants stored at 4 0C and subcultured at regular intervals of three weeks. For inoculum preparation the culture was grown at 37 0C for 7 days in Czapeck-Dox medium containing(g/l): NaNO3 6.0; KCl 0.52; MgSO4.7H2O 0.52; KH2PO4 1.52; Cu (NO3)2.3H2O traces; ZnSO4.7H2O traces; FeSO4 traces supplemented with 4 % PR and the spores (5.0x107) were scraped into 5 mL of the same medium and incubated at 37 0C for 24 h to inoculate 50 mL of fermentation medium.
Growth conditions
Tannase production was carried out on Czapeck-Dox medium supplemented with 4% PR as the sole carbon source. The pH of the medium was either 4.0 or 5.0 and 50 mL of the medium was sterilized in 250 mL of Erlenmeyer flask at 121 0C for 20 min cooled and inoculated with pre induced inoculum and incubated at 37 0C at 220 rpm, other conditions remaining the same. Extracellular tannase and gallic acid were estimated in the fermented broth at an interval of 24 h. All the experiments were carried out in triplicates and the analyses were done in duplicates. Mean values are shown in the figures and the tables with error bars.
Preparation of substrate
Pomegranate rind was spreaded on trays and oven dried at 70 0C for 24 h. The dried rind was ground and sieved to obtain particle size of 425 mm and stored in polyethylene bags at room temperature (30 ± 5 0C).
Tannase assay
Extracellular tannase is being reported here as intracellular tannase was very negligible (data not shown). For assay of extracellular enzyme the aliquots of the fermented broth were withdrawn at desired time intervals, filtered and centrifuged and the supernatant was used as the extracellular enzyme. Tannase was assayed by the method based on chromogen formation between gallic acid and rhodanine (15). The reaction mixture containing 0.25 mL of 0.01 M methyl gallate in 0.05 M citrate buffer, pH 5.0 and 0.25 mL of extracellular enzyme was incubated at 30 0C for 10 min and 0.3 mL of methanolic rhodanine (0.667 % w/v) was then added. After 5 min. 0.2 mL of 0.5 M KOH was added. A control was run where enzyme was added after the addition of KOH. Finally the reaction mixture was diluted by 4.0 mL distilled water and incubated at 30 0C for 10 min and absorbance was recorded at 520 nm. One unit of tannase is the amount of enzyme which liberated 1 μmol of gallic acid in one minute.
Gallic acid estimation
Gallic acid was estimated in the fermented broth. To 0.5 ml of fermented broth, 0.3 ml of methanolic rhodanine was added followed by 0.5 M KOH and gallic acid content was estimated by the method described above using a calibration graph using (10mg- 50mg) of gallic acid.
Optimization of process parameters for tannase production using PR
Optimum tannase production was determined for incubation period (24 h-96 h), substrate conc. (1 %-5 %), temperature (30 0C-40 0C), pH (3.0-7.0) and inoculum size (5 %-15 %) v/v.
Enzyme Purification
The enzyme was partially purified by acetone precipitation followed by DEAE cellulose column chromatography. Tannase was eluted with 0.02 M acetate buffer of pH 5.0 (13). This enzyme was used for determining the kinetic parameters.
Estimation of tannin content
The tannin content in the aonla juice was measured following the protein precipitation method by tannins (5).
Immobilization of tannase
A 10 mL suspension containing 488 units of tannase and 4 % sodium alginate was extruded dropwise through a 2 mL syringe into a 0.2 M CaCl2 solution at 4 0C to form beads of 0.4 mm diameter. After 2 h the beads were washed with water and either preserved in 0.2 M CaCl2 solution at a temperature of 6 0C or used (6).
Juice Preparation
5.0 g of aonla fruit was taken, cut into small pieces and ground in a mortar pestle. Juice was then extracted by adding 100 mL of distilled water and homogenizing in a blender followed by filtration through muslin cloth. The extracted juice was stored at 4 0C for further use.
Use of immobilized enzyme for tannin removal in aonla juice
15 mL of aonla juice was treated in a 100 mL conical flask with 15 beads of alginate entrapped enzyme (36.6 units) at 37 0C with stirring. Aliquots of 0.2 mL from the treated juice were taken out at 60 min intervals for analysis of the tannin content. The treatment was carried out for 180 min as the optimum enzyme concentration and incubation period were found to be 36.6 units and 180 min for tannin removal (data not shown). After completion of the process, the beads were taken out from the juice and washed with distilled water. These beads were reused with fresh juice for two more cycles and then washed and stored in CaCl2 solution at 6 0C for further use.
Statistical analysis
A completely randomized design was used throughout this study. Data were subjected to analysis of variance (ANOVA) and mean comparison was carried using student't' test. Statistical analysis was performed using the statistical package for social sciences (SPSS for windows; SPSS Inc., Chicago, IL
RESULTS AND DISCUSSION
Optimization of process parameters using PR as substrate
Optimum tannase production (28.72 U/mL) and gallic acid (52 μg/mL) were achieved after 72 h with 4 % (w/v) substrate (PR), at 37 0C, pH 5.0 with an inoculum size of 10 % (v/v). The decrease in enzyme activity after 72 h may be due to reduced nutrient level of medium affecting the metabolic activity and enzyme synthesis, inhibition and denaturation of the enzyme (4). The isolated A.niger reported here produced maximum tannase of 28.72 U/mL and gallic acid 52 μg/mL at 37 0C whereas most other A.niger strains reported have an optimum temperature of 30 0C (7,12,15).
Effect of tannic acid on tannase production
Tannase is an inducible enzyme produced only in the presence of tannic acid or its end product. The effect of tannic acid concentrations ranging from 0.2 %-1.5 % in the fermentation medium was studied. The tannase activity was stimulated by 245.9 % with 1 % tannic acid (101.2 U/mL) whereas the tannase activity with 1.5 % tannic acid was also significantly higher (45.1 U/mL) compared to control value (29.25 U/mL) shown in Fig. 1. This suggests that pure tannic acid induced and stimulated tannase production while tannin present in PR is in complex form and not as readily available as tannic acid.
Effect of glucose on tannase production
The effect of glucose concentrations ranging from 0.25-1.0 % was studied which showed a marginal increase in tannase activity (30.8 U/mL) with 0.5 % of glucose as compared to control (29.25 U/mL) whereas the activity decreased with 0.75 % of glucose (Fig. 2). Similar observations were made where enzyme activity increased with 0.2 % of glucose while decreased with 0.05 and 0.5 % concentrations as compared to the control value (1). Glucose is one of the products of tannin degradation hence in higher concentrations it perhaps shows feed back inhibition.
Effect of gallic acid on tannase production
Different concentrations of gallic acid ranging from 0.2-1 % when added to the production medium containing 4 % PR, showed inhibition (Fig. 3) which could be due to competitive inhibition. It has been reported that gallic acid, pyrogallol and gallaldehyde competitively inhibited the tannase of A.niger (9).
Effect of nitrogen sources and EDTA on tannase production
The effects of NH4NO3, NH4Cl, aspartic acid, urea, KNO3 and EDTA on tannase production were studied. 0.2 % of these nitrogen sources were added to the production medium containing PR as substrate. All the nitrogen sources and EDTA acted as inhibitors for tannase production as compared to the control value where NaNO3 was used as a nitrogen source (Fig.4).
Determination of kinetic parameters of tannase
The partially purified (soluble) enzyme was found to have an optimum temperature and pH of 35 0C and 6.2 respectively (Fig. 5 and 6). The Km and Vmax values of the latter were found to 0.012 mM and 33.3 µmole/min from the LineWeaver Burk plot. The enzyme in immobilized state however had an optimum temperature of 40 0C and optimum pH of 5.8 (Fig. 5 and 6). The elevation of temperature in case of immobilized enzyme is due to the entrapment of the enzyme which protects it from adverse heat effects and makes it thermally stable. A shift in the optimum temperature of immobilized enzyme has been reported in other cases also (11).
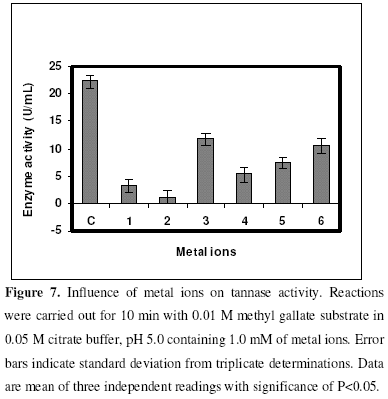
Effect of metal ions and nitrogen sources on tannase activity
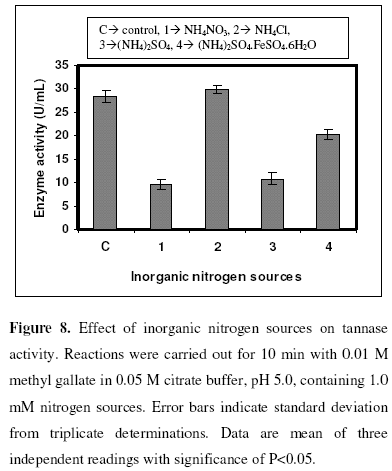
The effect of metal ions such as ZnCl2, CaCl2, MnCl2, AgNO3, MgCl2 and BaCl2 on tannase activiy were studied. A volume of 0.25 mL of partially purified enzyme was added to 0.25 mL of substrate (methyl gallate) containing 1.0 mM concentration of metal ions and incubated at 35 0C for 10 min. All the metal ions studied acted as inhibitors in which MnCl2 showed minimum inhibition whereas CaCl2 showed maximum inhibition (Fig. 7). The influence of different inorganic nitrogen sources like NH4NO3, NH4Cl, (NH4)2SO4, (NH4)2SO4.FeSO4.6H2O in the reaction mixture for enzyme assay were also studied. NH4Cl was found to stimulate the activity marginally while all the other nitrogen sources exerted inhibitory effects (Fig. 8).
Application of immobilized tannase for tannin removal from fresh aonla juice
Tannin removal by immobilized tannase in fresh aonla juice containing 1125 µg/ml of tannin having pH of 2.5 was tested at 37 0C for 180 min. It can be seen that maximum hydrolysis of 68.8 % took place after 3 h in the first run (Table 1 & Figure 9). The beads were used for three successive runs resulting in 37.7 % and 24.4 % tannin hydrolysis after 2nd and 3rd run respectively. The results were encouraging considering the beads could be used successively for three runs (9 h) with 24.4 % tannin removal. Enzymatic treatments of fruit juices to reduce haze and bitterness have been reported by various authors (2, 3, 10). Reports on application of tannase in fruit juice processing are rare and there is no report of tannin removal from aonla juice which has a very strong astringency due to tannins. However a 25 % reduction in tannin content from pomegranate juice by soluble tannase has been reported (14). Better performance of the immobilized enzyme used in this work is shown with 68.8 % tannin removal. After 3 h treatment of aonla juice with tannase there was a 2 % reduction in vitamin C content (Table 1) which was considerably low compared to other conventional processing methods which involve non- enzymatic heat treatments and results in removal of vitamin C. Based on these findings further studies are being carried out as enzyme catalyzed detannification of aonla juice has not been reported so far and can be nutritionally beneficial.
CONCLUSIONS
Tannase produced by A.niger under optimized conditions on a novel agroresidue was partially purified and immobilized in alginate beads. The immobilized enzyme showed a higher temperature and lower pH optima than the soluble enzyme and could remove 68.8% tannin from myrobalan juice in 180 min at 370C, which has not been reported earlier.
Submitted: August 06, 2008; Returned to authors for corrections: February 10, 2009; Approved: June 28, 2009.
- 1. Bradoo, S.; Gupta, R.; Saxena, R.K. (1997). Parametric optimization and biochemical regulation of extracellular tannase from Aspergillus japonicus. Proc. Biochem,32(2),135- 139.
- 2. Chatterjee, S.; Chatterjee, S.; Chatterjee, B.P.; Guha, A.K.(2004). Clarification of fruit juice with chitosan. Proc. Biochem., 39, 2229-2232.
- 3. Floribeth, V.; Lastreto, C.A. (2004). A study of the production of clarified banana juice using pectinolyutic enzymes. J. Food. Technol., 16, 115-125.
- 4. Gautam, P.; Sabu, A.; Pandey, A.; Szakacs, G.; Saccol, C.R. (2002). Microbial production of extracellular phytase using polystyrene as inert solid support. Bioresour. Technol., 83, 229-233.
- 5. Haggerman, A.E.; Butler, L.G. (1978). Protein precipitation method for determination of tannins. J. Agric. Food Chem., 26, 809- 812.
- 6. Kierstan, M.; Bucke, C. (1977). The immobilization of microbial cells, subcellular organelles and enzymes in calcium alginate gels. Biotech.Bioeng., 19, 387-397.
- 7. Lekha, P.K.; Lonsane, B.K. (1994). Comparative titres, location and properties of tannin acyl hydrolase produced by Aspergillus niger PKL 104 in solid state, liquid surface and submerged fermentation. Proc.Biochem., 29, 497-503.
- 8. Lekha, P.K.; Lonsane, B.K. (1997). Production and application of tannin acyl hydrolase; state of the art. Adv. Appl. Microbiol., 44,: 215-260.
- 9. Mahadevan, A.; Sivaswamy, S.N. (1985). Tannins and microorganisms. In Frontiers of Appl. Microbiol. eds K.G Mukherji, N.C. Pathak and V.P. Singh. Rastogi and company, Meerut., India 327-347.
- 10. Mishra, P.; Kar, R. (2003). Treatment of grapefruit juice for bitterness removal by amberlite IR 120 and amberlite IR 400 and alginate entrapped naringinase enzyme. J. Food Sci., 68 (4), 1229-1233.
- 11. Park, N.H.; Chang, H.N. (1979). Preparation and characterization of immobilized naringinase on porous glass beads. J. Ferm. Technol., 57, 310-306.
- 12. Pourrat, H.; Regerat, F. Pourrat, A.; Jean D. (1982). Production of tannase (Tannin acyl hydrolase E.C. 3.1.1.20) by a strain of Aspergillus niger Biotechnol. Lett., 4(9), 583- 588.
- 13. Rajakumar, G.S.; Nandy, S.C. (1983). Isolation, purification and some properties of Penicillium chrysogenum tannase. Appl. Environ. Microbiol., 46(2), 525- 527.
- 14. Rout, S.; Banerjee, R. (2006). Production of tannase under mSSF and its application in fruit juice debittering. Ind. J. Biotechnol., 5, 346-350.
- 15. Sharma, S.; Bhat, T.K.; Dawra, R.K. (2000). A spectrophotometric method for assay of tannase using rhodanine. Anal. Biochem. 279 (1), 85- 89.
- 16. Sharma, S.; Gupta, M.N. (2003). Synthesis of antioxidant propyl gallate using tannase from Aspergillus nigervan Tiegham in non aqueous media, Bioorg.Med.Chem. Lett., 13(3), 395-397.
- 17. Vardin, H.; Fenercioglu, H. (2003). Study on the development of pomegranate juice processing technology: clarification of pomegranate juice. Nahrung., 42, 300-303.
Publication Dates
-
Publication in this collection
06 Oct 2009 -
Date of issue
Dec 2009
History
-
Accepted
28 June 2009 -
Received
06 Aug 2008