Abstract
Production of Polyglutamate (PGA) biopolymer by immobilized Bacilluslicheniformis strain-R was intensively investigated. Preliminary experiments were carried out to address the most suitable immobilization methodology. Entrapment of Bacillus cells in alginate-agar led optimal PGA production (36.75 g/l), with 1.32- and 2.18-fold increase in comparison with alginate- or K-carrageenan-immobilized cells, respectively. During semicontinuous cultivation of agar-alginate gel-cell mixture, production of PGA by 10 ml mixture was increased from 2nd to 3rd run whereas, increased till the 4th run using 15ml mixture. Adsorption was the most suitable immobilization technique for production of PGA and the sponge cubes was the preferred matrix recording 43.2 g/l of PGA with the highest cell adsorption. Furthermore, no PGA was detected when B. licheniformis cells were adsorbed on wood and pumice. Although luffa pulp-adsorbed cells recorded the highest PGA production (50.4 g/l), cell adsorption was the lowest. Semicontinuous cultivation of B. licheniformis cells adsorbed on sponge led to increase of PGA production till the 3rd run and reached 55.5 g/l then slightly decreased in the 4th run. The successful use of fixed-bed bioreactor for semicontinuous cultivation of B. licheniformis cells held on sponge cubes (3 runs, 96 hours/run) provides insight for the potential biotechnological production of PGA by immobilized cells.
Poly-g-glutamate; Bacillu slicheniformis; immobilization; bioreactor; semicontinuous production
INDUSTRIAL MICROBIOLOGY
Production of Poly-γ-Glutamate (PGA) Biopolymer by Batch and Semicontinuous Cultures of Immobilized Bacilluslicheniformis strain-R
Mahmoud M. BerekaaI,* * Corresponding Author. Mailing address: Environmental Sciences Department, Faculty of Science (Moharam Bay), Alexandria University, Alexandria, Egypt.; Fax: +203-3911794.; Email: Berekaa2005@yahoo.com ;Samy A. El AassarII; Samia M. El-SayedII; Aliaa M. EL BoraiII
IEnvironmental Sciences Department, Faculty of Science, Alexandria University, Alexandria, Egypt
IIBotany Department, Faculty of Science, Alexandria University, Alexandria, Egypt
ABSTRACT
Production of Polyglutamate (PGA) biopolymer by immobilized Bacilluslicheniformis strain-R was intensively investigated. Preliminary experiments were carried out to address the most suitable immobilization methodology. Entrapment of Bacillus cells in alginateagar led optimal PGA production (36.75 g/l), with 1.32- and 2.18-fold increase in comparison with alginate- or K-carrageenan-immobilized cells, respectively. During semicontinuous cultivation of agar-alginate gel-cell mixture, production of PGA by 10 ml mixture was increased from 2nd to 3rd run whereas, increased till the 4th run using 15ml mixture. Adsorption was the most suitable immobilization technique for production of PGA and the sponge cubes was the preferred matrix recording 43.2 g/l of PGA with the highest cell adsorption. Furthermore, no PGA was detected when B. licheniformis cells were adsorbed on wood and pumice. Although luffa pulp-adsorbed cells recorded the highest PGA production (50.4 g/l), cell adsorption was the lowest. Semicontinuous cultivation of B. licheniformis cells adsorbed on sponge led to increase of PGA production till the 3rd run and reached 55.5 g/l then slightly decreased in the 4th run. The successful use of fixed-bed bioreactor for semicontinuous cultivation of B. licheniformis cells held on sponge cubes (3 runs, 96 hours/run) provides insight for the potential biotechnological production of PGA by immobilized cells.
Key words: Poly-g-glutamate, Bacillu slicheniformis, immobilization, bioreactor, semicontinuous production
INTRODUCTION
Poly glutamic acid (PGA) is one of the few naturally occurring polyamides which are not synthesized by ribosomal proteins (29). It is produced by several Bacillus species as an extracellular polymer (6, 8, 14, 19, 20, 24, 26, 31, 40, 45). It serves as structural component in some bacteria such as Bacillus anthracis and Bacillus megaterium (17, 36, 41). In another bacterial species, e.g. B. subtilis,B. licheniformis and Natrialba aegyptiaca, PGA excreted into the medium to increase the survival of producing strains when exposed to environmental stresses (16, 18). Interestingly, it is completely biodegradable and nontoxic to human (45). Therefore, applications of γ-PGA and its derivatives have been of interest in a broad range of industrial fields such as food, cosmetics, medicine, plastics, oil recovery and water treatment (7, 11, 25, 30, 39).
For a broad application, the cost of bio-products is one of the main factors determining the economics of a process. Reducing costs of biopolymer production by the use of immobilized cells is one of the basic researches for industrial application. Although immobilized cells have received a lot of attention in production of various bio-poroducts, it is not possible to make a general statement about the behavior of microorganisms in gel matrix or adsorbed on support material. The use of immobilized bacillus cells for production of a broad range of bioproducts e.g. amylase, CGTase, prednisolone and applying fluidized and fixed-bed bioreactors, has been reported (10, 13, 22, 32, 38). Interestingly, other researchers have shown that polysaccharide biopolymer can be produced by immobilized bacteria, however production of PGA biopolmer by bacteria was rarely investigated. Anselmo et al., studied the production of xanthan gum in immobilized cultures of Xanthomonas campestris, the immobilization was held using adsorption on three different vegetables (3). West and Strohfus studied the production of pullulan by sponge-immobilized Aureobasidium pullulans ATCC 42023 cells that was used 3 times for pullulan production (44). Aureobasidium pullulans cells were immobilized by adsorption on various solid supports and by entrapement in polyurethane foam for repeated reuse in pullulan biosynthesis (28).
The objective of this study was to investigate the possible production of PGA biopolymer by immobilized Bacillus licheniformis strain-R. Preliminary experiments have been conducted to address the most suitable immobilization methodology that can be used for PGA production. The choice of the most suitable supporting matrix used for immobilization was also investigated. Special emphasis was given to the semicontinuous production of PGA in fixed-bed bioreactor using Bacillus licheniformis cells adsorbed on sponge. To our knowledge, this is the first report provides insight for possible production of PGA biopolymer by application of different immobilization techniques.
MATERIALS AND METHODS
Microorganism
The Bacillus strain used throughout this study was isolated from an agriculture farm in ABIS rectorate in Egypt, characterized by previous history of fertilizer's use, as previously described (6). Further morphologically and physiologically characterization by the help of Fermentation Biotechnology and Applied Microbiology (FERM-BAM) Center, Azhar University revealed that the bacterium was closely related to Bacillus licheniformis.
Fermentation and PGA production
The bacterium was allowed to grow in 50ml aliquot of nutrient broth medium composed of (g/l): Peptone, 5 and beef extract, 3 and NaCl, 3, dispensed in 250ml Erlenmeyer flask and incubated at 37°C for 12h at 120 rpm. 1% inoculum of the overnight culture was used to inoculate the basal salt production medium of the following composition (g/l): K2HPO4; 14, KH2PO4; 6, MgSO4; 0.2, ammonium sulfate; 4, glucose; 20 and 2 ml of trace element solution (FeSO4.4H2O, CaCl2.2H2O, MnSO4.4H2O, ZnCl2 1 mM each) at 37°C. For semicontinuous production of PGA in flasks or trickle flow bioreactor, the fermentation medium was decanted at the end of four days incubation period, and fresh medium was added under aseptic conditions.
Growth and PGA determination
During fermentation process growth was estimated as OD at 600nm and PGA was determined in culture supernatants after clarifying cultures by centrifugation. For recovery, PGA biopolymer was precipitated by the addition of 2 volumes of ice-cold ethanol 95% and separated by centrifugation at 5,000 rpm for 20 min in a cooling centrifuge then dried at 60ºC to constant weight in a weighed vials (4, 5, 40).
Measurement of relative viscosity
Polyglutamic acid (PGA) is a water soluble highly hygroscopic white powder, leading to highly viscous solutions even at low concentrations (33). In this study, the relative viscosity of the culture filtrate, after cell removal, was measured using a conventional Ostwald viscometer at 30ºC (37, 42). The apparent relative viscosity d (app) was determined as follows:
Where ts is the falling time of the sample at 30ºC and to is that of water under the same conditions.
Immobilization of B. licheniformis cells by entrapment
Entrapment in Ca-alginate and agar-alginate
Cells were entrapped in 4% calcium alginate gel beads as described by Eikmeier et al. (12), four percent gel solution (w/v) was prepared by dissolving 4 g Na-alginate in 90 ml distilled water (for preparation of alginate beads) or 2g agar and 2g alginate (for agar-alginate beads) in 90 ml distilled water, then autoclaved at 108ºC for 10 min. Ten ml cell suspension was added to the sterile gel solution. Ten ml of the gel-bacterial cell mixture were drawn with the aid of a sterile syringe, and allowed to drop through a hypodermic needle into a cross-linking solution (100 ml of 2% CaCl2 solution in 250 ml Erlenmeyer flask) to obtain spherical beads (about 3mm in diameter). The beads were left in calcium chloride solution for one hour and then washed several times with sterile distilled water. Beads resulted from 10ml alginate were added to 50 ml sterile medium in a 250 ml Erlenmeyer flask. The flasks were incubated on a rotary shaker (120 rpm) at 37ºC.
Entrapment in K-Carrageenan
Cells were entrapped in K-carrageenan under sterile conditions as described by Wada etal. (43). About twenty ml cell suspension containing an equal cell inoculum as that used for the preparation of alginate beads were added to 80 ml K-carrageenan solution (3 g/80 ml of the desired concentration) previously autoclaved and kept in water bath at 40ºC. The solution was well mixed and 10ml fractions were introduced dropwise with sterile syringe into 100 ml of well-stirred 2% KCl solution. The hardened gel particles (about 3mm in diameter) were isolated from the KCl solution and washed several times with a sterile physiologic solution (0.85% NaCl), then transferred aseptically to 50 ml of the cultivation medium.
Entrapment in agar
The gel was prepared by dissolving 2g agar in 80 ml water. After sterilization, about 20 ml cell suspension was added and mixed well (at about 45.50ºC). 10 ml of this mixture were aseptically poured into a Petri-dish. After solidification, the gel was cut with a sterile cutter into small cubes of about 0.5 cm in length that was subsequently transferred to 50 ml of the cultivation medium.
Immobilization of B. licheniformis cells by adsorption
Two ml cell suspension was added to the Erlenmeyer flasks containing 50 ml sterilized culture medium and sponge cubes (about 20 cubes), luffa pulp, pumice and wood (about 20 particles). The flasks were incubated in a rotary shaker (120 rpm) at 37ºC.
Trickle Flow Column bioreactor
The trickle flow column used in the present work consisted of a glass tubing (75 cm long and 3 cm inner diameter). The column was connected by a quick fit to a reservoir (250 ml conical flask). The column was filled with adsorbent granules for adsorption and 4% cell solution was used to inoculate the support. After adsorption of cells on the packed support-matrix approximately 200 ml of the mineral medium was recycled through the support by the aid of a peristaltic pump at a flow rate of about 120 ml/h or 240 ml/h. The column was also aerated with a sterile air pump from the upper part of the column at a flow rate of about 0.21l/min.
RESULTS
Production of PGA biopolymer by entrapped B. licheniformis strain-R cells
Different gel materials namely; Na-alginate, agar-alginate, k-carrageenan and agar were used for immobilization of Bacillus licheniformis cells by entrapmet. The gel-cell beads (or cubes) were used to inoculate 50 ml production medium. Results shown in Table 1 indicated that immobilization by entrapment using alginate-agar as the gel material show the highest polygutamate production (36.75 g/l), and relative viscosity (2.2) but lower than those of free cultures (45.8 g/l and 2.4, respectively). Furthermore, application of different levels of gel-cell mixture showed that the optimal PGA production (36.75 g/l) and relative viscosity (2.2) were obtained by the use of 10 ml gel-cell mixture, higher or lower number of beads decreased polyglutamate production and relative viscosity (Figure 1).
Semicontinuous production of PGA by entrapped B. licheniformis strain-R cells on alginate-agar beads
The production of PGA by the use of B. licheniformis cells entrapped in alginate-agar was investigated using different number of immobilized beads (10 and 15 ml gel-cell mixture). The gel-cell mixture was incubated for 4 days in the production medium. At the end of each reuse, medium was decanted and fresh medium was added. Results in Figure 2 indicated that the reuse of 10 ml gel-cell mixture led to increase in PGA production from the 2nd till the 3rd run and then decreased at the 4th run. On the other hand, PGA production continued to increase till the 4th run by the use of 15 ml gel-cell mixture, however it was still lower than the batch fermentation by free cells.
Production of PGA by B. licheniformis strain-R cells adsorbed on different solid supports
In a trial to investigate the possible production of PGA by adsorption technique B. licheniformis cells were adsorbed on different solid porous supports such as luffa pulp, sponge cubes, wood pieces and pumice particles. Production medium containing porous supports were inoculated with B.licheniformis strain-R cell suspension, at the end of incubation period PGA was determined. Results recorded in Table 2 indicated that cultures containing adsorbed cells on sponge cubes and luffa pulp showed an optimal cell adsorption as well as PGA production. However, PGA was not detected in cultures containing adsorbed cells on wood pieces and pumice particles. Although, cultures containing luffa pulp as solid support exhibited the highest PGA production (50.4 g/l), even higher than that of free cultures, however cultures containing sponge cubes as solid supports exhibited the highest cell adsorption.
Semicontinuous production of PGA by adsorbed B. licheniformis strain-R cells
The effect of reusing adsorbed B. licheniformis cells on the production of PGA using different solid supports as sponge cubes and luffa pulp was investigated. For this purpose, cultures containing different solid supports were used and after time interval of 4 days for repeated reuse, PGA was determined. Results in Figure 3 show that by reusing cells adsorbed on sponge cubes, a slow cell leakage was observed up to the 4th run (data not shown), while PGA production increased till the 3rd run and reached 55.5 g/l then slightly decreased in the 4th run, however it was still higher than that of batch fermentation cultures by free cells. On the other hand, by reusing cells adsorbed on luffa pulp PGA production decreased in the cultures of the 2nd run and continued to decrease up to the 4th run till it was nearly the same as that of batch fermentation cultures via free cells. The results collectively indicated the possible semicontinuous production of PGA by B. licheniformis cells adsorbe on sponge.
Cultivation of adsorbed B. licheniformis cells on sponge in a trickle flow bioreactor
In a trial to test the possible production of PGA in bioreactor, a trickle-flow column was used in cultivation of B. licheniformis cells adsorbed on sponge. About 200ml of the production medium was recycled through the support at a flow rate of about 120ml/h or 240ml/h and the column was incubated at 37ºC for 5 days. Results in Figure 4 showed that the optical density decreased gradually during incubation indicating a good adsorption of the cells to the sponge. Also, results showed an increase in PGA production from 24 to 48 h incubation, then slowly increased till 72h followed by subsequent increase with maximum PGA production (31.25 g/l) after 96h of incubation. Subsequent decrease in PGA and relative viscosity was finally recorded. Interestingly, similar growth and PGA production were recorded by increase of the medium flow rate during feeding process to 240 ml/h. Polyglutamate production reached 45 g/l after 96h incubation i.e.1.44-fold as compared with PGA produced applying flow rate of 120ml/h at the same incubation period.
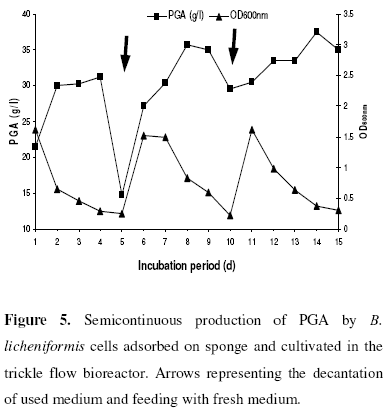
Semicontinuous production of PGA in the trickle flow bioreactor
In order to investigate the possible reduction in costs of PGA production, B. licheniformis cells adsorbed on sponge cubes were cultivated in a trickle flow column in semicontinuous manner. For this purpose, cells were cultivated in the production medium and reused after a time interval of 5 days. Samples were taken every 24h to trace the amount of PGA produced. Results shown in Figure 5 indicate that the optical density showed a gradual decrease during the incubation period of each run reflecting adsorption of B. licheniformis cells on the support. On the other hand, PGA production showed a gradual increase for each run till 96h of incubation, and decreased after wards (data not shown). Also, the results clarified that there was an increase in polyglutamate production and relative viscosity (data not shown) till the 3rd run, in which the polyglutamate production and relative viscosity reached 37.5 g/l and 2.4, respectively.
DISCUSSION
In this study, the production of a useful biodegradable polyamide biopolymer, polyglutamate (PGA), by immobilized Bacillus licheniformis strainR was closely investigated. In practical utilization of bacterial cells entrapped in gel matrix, diffusion of essential nutrients, oxygen transfer, physical and chemical properties of the gel and immobilization procedure are the important factors affecting microbial metabolism and effeciency of the system. It was found that PGA production reached its maximun yield (36.75 g/l) after 96 h when using 10 ml agar-alginat gel beads mixture. This result is in concordance with the finding of Shih et al. (39) and Berekaa et al. (6), where the maximum PGA production during batch fermentation using free cells was obtained after 96h. The results also revealed the possible production PGA biopolymer by entrapped cells. However, the fluctuation in the amount of PGA produced by Bacillus cells entrapped in various gel matrices as well as during the use of different gel beads concentrations attributed to variation in aeration and diffusion of nutrient in the immobilized cell system (9, 27, 34, 35). Interestingly, polysacchrides biopolymers were successfully produced by entrapped microbial cells. Indeed, immobilization of A. pullulans by entrapement in polyurethane foam was investigated and immobilized cells could be repeatedly used for pullulan biosynthesis (28). Measurement of relative viscosity during biopolymer production can be an important factor of a successful bioprocess. Furthermore, the change in medium viscosity during fermentation by B. licheniformis cells is attributed to the production of PGA of different molecular weights. Berekaa et al. (6) reported that the produced PGA polymer by B. licheniformis strain-R during batch fermentation by free cells is in the range of 80 to 180 Kda. The result is supported by the finding that the PGA from bacilli are usually produced as mixture with molecular weights ranging from 10 to 1000 Kda, due to PGA depolymerases activity (2). Generally, the final molecular weight of PGA is dependent on many factors, and can decrease as fermentation time increases, owing to enzyme that catalizes the hydrolytic breakdown of PGA (15, 23).
Given the industrial importance of Bacilli, we examined semicontinuous production of PGA by adsorbed B. licheniformis strain-R cells in more details. Adsorption was the most suitable immobilization technique for production of PGA biopolymer and the sponge cubes was the preferred matrix recording 43.2 g/l of PGA with the highest cell adsorption. Moreover, no PGA was detected when B. licheniformis cells were adsorbed on wood and pumice. Although luffa pulp-adsorbed cells recorded the highest PGA production (50.4 g/l), cell adsorption was the lowest. The use of adsorbed cells in production of biopolymer was investigated by several scientists. Aureobasidium pullulans was immobilized on various solid supports in a liquid medium as diatomaceous earth, fiber glass mat, cellulose fiber mat, ceramic, polyurethane foam for polysaccharide production. The immobilized cells could be repeatedly used for pullulan biosynthesis (28). Furthermore, application of fed-batch cultivation of Bacillus cells adsorbed on sponge led to increase of production till the 3rd run and reached 55.5 g/l then slightly decreased in the 4th run. In concordance with these results, B. licheniformis ATCC 9945a produced 35g/l PGA during fed-batch cultivation of free cells in 2.5L bioreactor with pulsed feeding of citric acid and glutamic acid (45). Similarly, remarkable increase in PGA production was recorded by Abdel-Fattah and his co-workers (1). They managed to optimize PGA production by free cells of B. licheniformis SAB-26 strain from 33.5 g/l to 89 g/l by fed-batch and pulsed feeding experiments. Interestingly, West and Strohfus (44) studied the production of pullulan by sponge-immobilized Aureobasidium pullulans ATCC 42023 cells, the immobilization was held on sponge cubes in media containing either corn syrup, sucrose or glucose as a carbon source, the cells could be used 3 times for pullulan production. Immobilized cells revealed the highest polysaccaride levels in the culture medium after 5-7 days of growth at 30°C. Indeed, Anselmo et al. (3) studied the production of xanthan gum in immobilized cultures of Xanthomonas campestris, the immobilization was held using adsorption on three different vegetables.
The practical use of trickle flow bioreactor for semicontinuous cultivation of Bacillus cells held on sponge cubes (3 runs for 96 hours/run) for PGA poduction was successfully established in this work. There was an increase in polyglutamate production and relative viscosity till the 3rd run, in which the polyglutamate production and relative viscosity reached 37.5 g/l and 2.4, respectively. The successful use of immobilized B. cereus cells employing packed- or fixed bed bioreactor to continuously synthesize several bioproducts of industrial interest was reported by many working groups (22, 32, 38). Finally, results obtained in this study provide insight for the successful cultivation of B. licheniformis cells in trickle flow bioreactor and reflects the potential biotechnological production of PGA by immobilized cells.
REFERENCES
1. Abdel-Fattah, Y.; Soliman, N.; Berekaa, M. (2007). Application of Box-Behnken Design for Optimization of Poly-γ-Glutamic Acid Production by Bacillus licheniformis SAB-26. Res. J. Microbiol. 2(9): 664-670.
2. Abe, K.; Ito,Y.; Ohmachi, T.; Asada, Y. (1997). Purification and properties of two isozymes of γ-glutamyltranspeptidase from Bacillus subtilis TAM-4. Biosci. Biotechnol. Biochem. 61, 16211625.
3. Anselmo, R.J.; Viora, S.; Carletti, S. (1992). Production of xanthan gum in immobilized cultures of xanthomonas campestris. Rev. Argent. Microbiol. 24(2), 86-90.
4. Ashiuchi, M.; Soda, K.; Misono, H. (1999). A poly-δ-glutamate Synthetic System of IFO 3336: Gene Cloning and Biochemical Analysis of Poly-δ-glutamate Produced by Escherichia coli Clone Cells. Biochem. Biophys.Res. Com. 263, 6-12.
5. Ashiuchi, M.; Tani, K.; Soda, K.; Misono, H. (1998). Properties of glutamate racemase from Bacillus subtilis IFO 3336 producing poly-γ-glutamate. J. Biochem. 123, 11561163.
6. Berekaa, M.M.; Abdel-Fattah, Y.R.; El-Sayed, S.M.; El-Borai, A.M.; El Aassar, S.A. (2006). Optimization of culture conditions for production of polyamide biopolymer (Polyglutamate) by Bacillus sp. Strain-R. J. Biological Sciences 6(4), 687-694.
7. Cell Therapeutics Inc., (2000). Company press release available at: http://www.cticseatle.com. Cell Therapeutics Inc., Seattle, WA, 98119.
8. Cromwick, A.M.; Birrer, G.; Gross, R. (1996). Effect of pH and Aeration on δ-Poly (glutamic acid) Formation by Bacillus licheniformis in Controlled Batch Fermentor Cultures. Biotechnol. Bioeng. 50, 222-227.
9. Dias, J.C.T.; Rezende, R.P.; Linardi, V.R. (2001). Effects of immobilization in Ba-alginate on nitrile-dependent oxygen uptake rates of Candida guilliermondii. Brazilian J. Microbiol. 32(3), 221-224.
10. Dobreva, E.; Tonkova, A.; Ivanova, V.; Stefanova, M.; Kabaivanova, L.; Sapasova, D. (2004). Immobilization of Bacillus licheniformis cells, producers of thermostable α-amylase, on polymer membranes. J. Industr. Microbiol. Biotechnol. 20 (3-4), 166-170.
11. Du, G.; Yang, G.; Qu, Y.; Chen J.; Lun, S. (2004). Effect of glycerol on the production of poly (δ-glutamic acid) by Bacillus licheniformis. Proc. Biochem. In Press, Available online 20 October 2004.
12. Eikmeier, H.; Westmeier F.; Rehm, H.J. (1984). Morphological development of Aspergillus niger immobilized in Ca-alginate and K-carrageenan. Appl. Microbiol. Biotechnol. 19, 53-57.
13. El-Hadi, A.A. (2003). Factors affecting the production of prednisolone by immobilization of Bacillus pumilus E601 cells in poly(vinyl alchol) cryogels produced by radiation polymerization. Process. Biochem. 38 (12), 1659-1664.
14. Giannos, S., Gross, A.; Kaplan, D.; Mayer, J. (1990). Poly(glutamic acid) Produced by Bacterial Fermentation. In: Noval Biodegradable Microbial Polymers. Kluwer Academic Publishers, Dordrecht, Netherlands, p457-460.
15. Goto, A.; Kunioka, M. (1992). Biosynthesis and hydrolysis of Poly(δ-glutamic acid) from Bacillus subtilis IFO3335. Biosci. Biotech. Biochem. 56, 1031-1035.
16. Gross, A. (1998). Bacterial δ-Poly(glutamic acid). In: Kaplan, D.L. (eds.) Biopolymers from Renewable Resources, Springer-Verlag, New York, p195-219.
17. Hanby, W.; Rydon, H. (1946). The capsular substance of Bacillus anthracis. Biochem. 40, 297-307.
18. Hezayen, F.F.; Rehm, B.R.; Eberhardt, R.; Steinbüchel, A. (2000). Polymer production by two newly isolated extremely halophilic Archaea: Application of a novel corrosion-resistant bioreactor. Appl. Microbiol. Biotechnol. 54, 319-325.
19. Hoppensack, A.; Oppermann-Sanio, F.B.; Steinbüchel, A. (2003). Conversion of the nitrogen content in liquid manure into biomass and polyglutamic acid by a newly isolated strain of Bacillus licheniformis. FEMS. Mocrobiol. Lett. 218, 39-45.
20. Ito, Y.; Tanaka, T.; Ohmachi, T.; Asada, Y. (1996). Glutamic acid independent production of poly(γ-glutamic acid) formation by Bacillus subtilis TAM-4. Biosci. Biotechnol. Biochem. 60, 1239-42.
21. Iwata, H.; Matsuda, S.; Mitsuhashi, K.; Kenji, I.; Ioh, E.; Ikada, Y. (1998). A novel surgical glue composed of gelatin and N-hydroxysuccinimide activated poly (L-glutamic acid). Biomaterials 19, 1869-1876.
22. Jamuna, R.; Saswathi, N.; Sheela, R.; Ramakrichna, S.V. (1993). Synthesis of cyclodextrin glucosyle transferase by Bacillus cereus for the production of cyclodextrin. Appli. Biochem. Biotechnol. 43, 163-176.
23. King, E.; Blacker, A.; Bugg, T. (2000). Enzymatic Breakdown of Poly-δ-D-glutamic acid in Bacillus licheniformis: identification of a polyglutamyl-δ-hydrolase enzyme. Biomacromolecules 1, 75-83.
24. Ko, Y.; Gross, R. (1998). Effect of glucose and glycerol on poly (glutamic acid). Formation by Bacillus licheniformis ATCC9945a. Biotechnol. Bioeng. 57, 430-437.
25. Konno, A.; Taguchi, T.; Yamaguchi, T. (1988). Bakery products and noodles containing polyglutamic acid. US Pat. 4,888,193.
26. Kunioka, M.; Goto, A. (1994). Biosynthesis of poly (δ-glutamic acid) from L-glutamic acid, citric acid, and ammonium sulfate in Bacillus subtilis IFO3335. Appl. Microbiol. Biotechnol. 40, 867-872.
27. Mignot, L.; Junter, G. (1990). Diffusion in immobilized-cell agar layers: influence of bacterial growth on the diffusivity of potassium chloride. Appl. Microbiol. Biotechnol. 33(2), 167-171.
28. Mulchandani, A.; Luong, J.H.T.; LeDuy, A. (1989). Production of pullulan by surface adsorbed and foam entarapped cells. Abstr. Pap. Am. Chem. Soc., 196 Meet., MBTD p 93.
29. Oppermann-Sanio, F.B.; Steinbüchel, A. (2002). Occurrence, functions and biosynthesis of polyamides in microorganisms and biotechnological production. Naturwissenschaften 89, 11-22.
30. Otani, Y.; Tabata, Y.; Ikada, Y. (1999). Sealing effect of rapidly curable gelatin-poly (L-glutamic acid) hydrogel glue on lung air leak. Ann. Thorac. Surg. 67, 922-926.
31. Pötter, M.; Oppermann-Sanio, F.B.; Steinbüchel, A. (2001). Cultivation of bacteria producing polyamino acids with liquid manure as carbon and nitrogen source. Appl. Environ. Microbiol. 67, 617-622.
32. Ramakrishna, S.V.; Saswathi, N.; Sheela, R.; Jamuna, R. (1994). Evaluation of solid, slurry, and submerged fermentations for the production of cyclodextrin glycosyltransferase by Bacillus cereus. Enzy. Microb. Technol. 16, 441-444.
33. Richard, A.; Margaritis, A. (2001). Polyglutamic acid for biomedical applications. Crit. Rev. Biotechnol. 21 (4), 219 - 232.
34. Riley, M.R.; Muzzio, F.J.; Reyes, S.C. (1999). Experiemental and modeling studies of diffusion in immobilized cell systems. Appl. Microbiol. Biotechnol. 80(2), 151-188.
35. Riley, M.R.; Muzzio, F.J.; Buettner, H.M.; Reyes, S.C. (2004). Diffusion in heterogeneous media: Application to immobilized cell systems. AlChE J. 41(3), 691-700.
36. Roelants, G.; Goodman, J. (1968). Immunochemical studies on the poly-δ-d-glutamyl capsule of Bacillus anthracis. IV. The association peritoneal exudates cell ribonucleic acid of the polypeptide in immunogenic and non-immunogenic forms. Biochem. 7, 1432-1440.
37. Sakai, K.; Hamada N.; Watanabe, Y. (1984). Non-enzymatic degradation of secondary alcohol oxidaseoxidized poly(vinylalcohol). Agri. Biol. Chem. 48(4), 1093-1974.
38. Saswathi, N.; Sheela, R.; Jamuna, R.; Ramakrishna, S.V. (1995). Synthesis of cyclodextrin glycosyltransferase by immobilized cells of Bacillus circulans. Bioprocess Biosys. Engi. 12, 283-286.
39. Shih, I.L.; Van, T.Y.; Yeh, C.L.; Lin, G.H.; Chang, N.Y. (2001). Production of a biopolymer flocculant from Bacillus licheniformis and its flocculation properties. Bioresour Technol. 78, 267-72.
40. Soliman, N.A.; Berekaa, M.M.; Abdel-Fattah, Y.R. (2005). Poly glutamic acid (PGA) production by Bacillus sp. SAB-26: application of PlackettBurman experimental design to evaluate culture requirements. Appl. Microbiol. Biotechnol. 69, 259-267.
41. Thorne, C.B. (1956). Capsule formation and glutamyl polypeptide synthesis by Bacillusanthracis and Bacillus subtilis. In: Symposia of the Society for General Microbiology, no.VI, Bacterial Anatomy, Cambridge University Press, New York, p 68-80.
42. Vemulapalli, V.; Hoseney, R.C. (1998). Glucose oxidase effects on gluten and water solubles. Cereal Chem. 75(6), 859-862.
43. Wada, M.; Kato, J.; Chibata, I. (1980). Continous production of ethanol using immobilized growing yeast cells. Eur. J. Appl. Microbiol. Biotechnol. 10, 275-287.
44. West, T.P.; Strohfus, B.R.H. (1996). Polysaccaraide production by sponge-immobilized cells of the fungus Aureobasidium pullulans. Appl. Microbiol. 22( 2), 162-164.
45. Yoon, S.; Do, J.; Lee S.; Chag, H. (2000). Production of poly-δ-glutamic acid by fed-batch culture of Bacillus licheniformis. Biotechnol. Lett. 22, 585-588.
Submitted: March 10, 2008; Returned to authors for corrections: August 26, 2008; Approved: May 19, 2009.
- 1. Abdel-Fattah, Y.; Soliman, N.; Berekaa, M. (2007). Application of Box-Behnken Design for Optimization of Poly-γ-Glutamic Acid Production by Bacillus licheniformis SAB-26. Res. J. Microbiol. 2(9): 664-670.
- 2. Abe, K.; Ito,Y.; Ohmachi, T.; Asada, Y. (1997). Purification and properties of two isozymes of γ-glutamyltranspeptidase from Bacillus subtilis TAM-4. Biosci. Biotechnol. Biochem. 61, 16211625.
- 3. Anselmo, R.J.; Viora, S.; Carletti, S. (1992). Production of xanthan gum in immobilized cultures of xanthomonas campestris Rev. Argent. Microbiol. 24(2), 86-90.
- 4. Ashiuchi, M.; Soda, K.; Misono, H. (1999). A poly-δ-glutamate Synthetic System of IFO 3336: Gene Cloning and Biochemical Analysis of Poly-δ-glutamate Produced by Escherichia coli Clone Cells. Biochem. Biophys.Res. Com. 263, 6-12.
- 5. Ashiuchi, M.; Tani, K.; Soda, K.; Misono, H. (1998). Properties of glutamate racemase from Bacillus subtilis IFO 3336 producing poly-γ-glutamate. J. Biochem. 123, 11561163.
- 7. Cell Therapeutics Inc., (2000). Company press release available at: http://www.cticseatle.com Cell Therapeutics Inc., Seattle, WA, 98119.
- 8. Cromwick, A.M.; Birrer, G.; Gross, R. (1996). Effect of pH and Aeration on δ-Poly (glutamic acid) Formation by Bacillus licheniformis in Controlled Batch Fermentor Cultures. Biotechnol. Bioeng. 50, 222-227.
- 9. Dias, J.C.T.; Rezende, R.P.; Linardi, V.R. (2001). Effects of immobilization in Ba-alginate on nitrile-dependent oxygen uptake rates of Candida guilliermondii Brazilian J. Microbiol. 32(3), 221-224.
- 10. Dobreva, E.; Tonkova, A.; Ivanova, V.; Stefanova, M.; Kabaivanova, L.; Sapasova, D. (2004). Immobilization of Bacillus licheniformis cells, producers of thermostable α-amylase, on polymer membranes. J. Industr. Microbiol. Biotechnol. 20 (3-4), 166-170.
- 11. Du, G.; Yang, G.; Qu, Y.; Chen J.; Lun, S. (2004). Effect of glycerol on the production of poly (δ-glutamic acid) by Bacillus licheniformis Proc. Biochem. In Press, Available online 20 October 2004.
- 12. Eikmeier, H.; Westmeier F.; Rehm, H.J. (1984). Morphological development of Aspergillus niger immobilized in Ca-alginate and K-carrageenan. Appl. Microbiol. Biotechnol. 19, 53-57.
- 13. El-Hadi, A.A. (2003). Factors affecting the production of prednisolone by immobilization of Bacillus pumilus E601 cells in poly(vinyl alchol) cryogels produced by radiation polymerization. Process. Biochem. 38 (12), 1659-1664.
- 14. Giannos, S., Gross, A.; Kaplan, D.; Mayer, J. (1990). Poly(glutamic acid) Produced by Bacterial Fermentation. In: Noval Biodegradable Microbial Polymers Kluwer Academic Publishers, Dordrecht, Netherlands, p457-460.
- 15. Goto, A.; Kunioka, M. (1992). Biosynthesis and hydrolysis of Poly(δ-glutamic acid) from Bacillus subtilis IFO3335. Biosci. Biotech. Biochem. 56, 1031-1035.
- 16. Gross, A. (1998). Bacterial δ-Poly(glutamic acid). In: Kaplan, D.L. (eds.) Biopolymers from Renewable Resources, Springer-Verlag, New York, p195-219.
- 17. Hanby, W.; Rydon, H. (1946). The capsular substance of Bacillus anthracis Biochem. 40, 297-307.
- 18. Hezayen, F.F.; Rehm, B.R.; Eberhardt, R.; Steinbüchel, A. (2000). Polymer production by two newly isolated extremely halophilic Archaea: Application of a novel corrosion-resistant bioreactor. Appl. Microbiol. Biotechnol. 54, 319-325.
- 19. Hoppensack, A.; Oppermann-Sanio, F.B.; Steinbüchel, A. (2003). Conversion of the nitrogen content in liquid manure into biomass and polyglutamic acid by a newly isolated strain of Bacillus licheniformis FEMS. Mocrobiol. Lett. 218, 39-45.
- 20. Ito, Y.; Tanaka, T.; Ohmachi, T.; Asada, Y. (1996). Glutamic acid independent production of poly(γ-glutamic acid) formation by Bacillus subtilis TAM-4. Biosci. Biotechnol. Biochem. 60, 1239-42.
- 21. Iwata, H.; Matsuda, S.; Mitsuhashi, K.; Kenji, I.; Ioh, E.; Ikada, Y. (1998). A novel surgical glue composed of gelatin and N-hydroxysuccinimide activated poly (L-glutamic acid). Biomaterials 19, 1869-1876.
- 22. Jamuna, R.; Saswathi, N.; Sheela, R.; Ramakrichna, S.V. (1993). Synthesis of cyclodextrin glucosyle transferase by Bacillus cereus for the production of cyclodextrin. Appli. Biochem. Biotechnol. 43, 163-176.
- 23. King, E.; Blacker, A.; Bugg, T. (2000). Enzymatic Breakdown of Poly-δ-D-glutamic acid in Bacillus licheniformis: identification of a polyglutamyl-δ-hydrolase enzyme. Biomacromolecules 1, 75-83.
- 24. Ko, Y.; Gross, R. (1998). Effect of glucose and glycerol on poly (glutamic acid). Formation by Bacillus licheniformis ATCC9945a. Biotechnol. Bioeng. 57, 430-437.
- 25. Konno, A.; Taguchi, T.; Yamaguchi, T. (1988). Bakery products and noodles containing polyglutamic acid. US Pat. 4,888,193.
- 26. Kunioka, M.; Goto, A. (1994). Biosynthesis of poly (δ-glutamic acid) from L-glutamic acid, citric acid, and ammonium sulfate in Bacillus subtilis IFO3335. Appl. Microbiol. Biotechnol. 40, 867-872.
- 27. Mignot, L.; Junter, G. (1990). Diffusion in immobilized-cell agar layers: influence of bacterial growth on the diffusivity of potassium chloride. Appl. Microbiol. Biotechnol. 33(2), 167-171.
- 28. Mulchandani, A.; Luong, J.H.T.; LeDuy, A. (1989). Production of pullulan by surface adsorbed and foam entarapped cells. Abstr. Pap. Am. Chem. Soc., 196 Meet., MBTD p 93.
- 29. Oppermann-Sanio, F.B.; Steinbüchel, A. (2002). Occurrence, functions and biosynthesis of polyamides in microorganisms and biotechnological production. Naturwissenschaften 89, 11-22.
- 30. Otani, Y.; Tabata, Y.; Ikada, Y. (1999). Sealing effect of rapidly curable gelatin-poly (L-glutamic acid) hydrogel glue on lung air leak. Ann. Thorac. Surg. 67, 922-926.
- 31. Pötter, M.; Oppermann-Sanio, F.B.; Steinbüchel, A. (2001). Cultivation of bacteria producing polyamino acids with liquid manure as carbon and nitrogen source. Appl. Environ. Microbiol. 67, 617-622.
- 32. Ramakrishna, S.V.; Saswathi, N.; Sheela, R.; Jamuna, R. (1994). Evaluation of solid, slurry, and submerged fermentations for the production of cyclodextrin glycosyltransferase by Bacillus cereus Enzy. Microb. Technol. 16, 441-444.
- 33. Richard, A.; Margaritis, A. (2001). Polyglutamic acid for biomedical applications. Crit. Rev. Biotechnol. 21 (4), 219 - 232.
- 34. Riley, M.R.; Muzzio, F.J.; Reyes, S.C. (1999). Experiemental and modeling studies of diffusion in immobilized cell systems. Appl. Microbiol. Biotechnol. 80(2), 151-188.
- 35. Riley, M.R.; Muzzio, F.J.; Buettner, H.M.; Reyes, S.C. (2004). Diffusion in heterogeneous media: Application to immobilized cell systems. AlChE J. 41(3), 691-700.
- 36. Roelants, G.; Goodman, J. (1968). Immunochemical studies on the poly-δ-d-glutamyl capsule of Bacillus anthracis IV. The association peritoneal exudates cell ribonucleic acid of the polypeptide in immunogenic and non-immunogenic forms. Biochem. 7, 1432-1440.
- 37. Sakai, K.; Hamada N.; Watanabe, Y. (1984). Non-enzymatic degradation of secondary alcohol oxidaseoxidized poly(vinylalcohol). Agri. Biol. Chem. 48(4), 1093-1974.
- 38. Saswathi, N.; Sheela, R.; Jamuna, R.; Ramakrishna, S.V. (1995). Synthesis of cyclodextrin glycosyltransferase by immobilized cells of Bacillus circulans Bioprocess Biosys. Engi. 12, 283-286.
- 39. Shih, I.L.; Van, T.Y.; Yeh, C.L.; Lin, G.H.; Chang, N.Y. (2001). Production of a biopolymer flocculant from Bacillus licheniformis and its flocculation properties. Bioresour Technol. 78, 267-72.
- 40. Soliman, N.A.; Berekaa, M.M.; Abdel-Fattah, Y.R. (2005). Poly glutamic acid (PGA) production by Bacillus sp. SAB-26: application of PlackettBurman experimental design to evaluate culture requirements. Appl. Microbiol. Biotechnol. 69, 259-267.
- 41. Thorne, C.B. (1956). Capsule formation and glutamyl polypeptide synthesis by Bacillusanthracis and Bacillus subtilis In: Symposia of the Society for General Microbiology, no.VI, Bacterial Anatomy, Cambridge University Press, New York, p 68-80.
- 42. Vemulapalli, V.; Hoseney, R.C. (1998). Glucose oxidase effects on gluten and water solubles. Cereal Chem. 75(6), 859-862.
- 43. Wada, M.; Kato, J.; Chibata, I. (1980). Continous production of ethanol using immobilized growing yeast cells. Eur. J. Appl. Microbiol. Biotechnol. 10, 275-287.
- 44. West, T.P.; Strohfus, B.R.H. (1996). Polysaccaraide production by sponge-immobilized cells of the fungus Aureobasidium pullulans Appl. Microbiol. 22( 2), 162-164.
- 45. Yoon, S.; Do, J.; Lee S.; Chag, H. (2000). Production of poly-δ-glutamic acid by fed-batch culture of Bacillus licheniformis Biotechnol. Lett. 22, 585-588.
Publication Dates
-
Publication in this collection
08 Oct 2009 -
Date of issue
Dec 2009
History
-
Received
10 Mar 2008 -
Accepted
26 Aug 2008