Abstracts
Tuberculosis control is a priority for the Ministry of Health policies in Brazil. In the present work, the detection of Mycobacterium tuberculosis by the Polymerase Chain Reaction (PCR) was standardized, and the laboratory diagnosis of pulmonary tuberculosis was evaluated comparing baciloscopy, culture and PCR tests. The study was carried out with 117 sputum samples from different patients suspected of having pulmonary tuberculosis, for whom physicians had ordered a baciloscopy test. Baciloscopy was performed using the Ziehl-Neelsen method, and culture was performed by incubation of treated samples in Lowenstein-Jensen's medium at 37ºC for eight weeks. For PCR, DNA was amplified with a specific pair of primers to the M. tuberculosis complex, with a resulting product of 123 bp from the insertion element IS6110. Three (2.56%) samples presented a positive baciloscopy result and a positive PCR result (100% agreement), and nine (7.69%) presented Mycobacterium sp. growth in culture (P= 0.1384). Among six samples with positive results in culture, one was identified by PCR-RFLP as belonging to the M. tuberculosis complex and one was identified as a non-tuberculosis mycobacteria. Sensitivity and specificity of PCR compared to culture were 33.3% and 100%, respectively.
tuberculosis; baciloscopy; culture; PCR
A tuberculose é um dos agravos prioritários para as políticas do Ministério da Saúde. No presente trabalho, o método de detecção de Mycobacterium tuberculosis pela Reação em Cadeia da Polimerase (PCR) em amostras de escarro foi padronizado e o diagnóstico laboratorial da tuberculose pulmonar foi avaliado, comparando-se as metodologias de baciloscopia, cultura e PCR. Foram analisadas 117 amostras de escarro de diferentes pacientes com suspeita de tuberculose pulmonar, com solicitação de baciloscopia. A baciloscopia foi realizada com a coloração de Ziehl-Neelsen e a cultura pela semeadura das amostras em meio de Lowenstein-Jensen, incubadas a 37ºC por oito semanas. Para realização da PCR, o DNA foi amplificado com um par de oligonucleotídeos específicos para o complexo M. tuberculosis, resultando em um produto de 123 pb do elemento de inserção IS6110. Das 117 amostras analisadas, três (2,56%) apresentaram baciloscopia positiva e PCR positiva para M. tuberculosis (concordância de 100%), e nove (7,69%) tiveram crescimento de Mycobacterium sp. na cultura (P= 0,1384). Das seis amostras que tiveram resultado positivo somente por cultura, uma foi identificada ainda como pertencente ao complexo M. tuberculosis por PCR-RFLP, e outra foi identificada como micobactéria não tuberculosa. A sensibilidade e a especificidade da baciloscopia e da PCR em relação à cultura foram 33,3% e 100%, respectivamente.
tuberculose; baciloscopia; cultura; PCR
MEDICAL MICROBIOLOGY
Evaluation of methods for detection and identification of Mycobacterium species in patients suspected of having pulmonary tuberculosis
Avaliação de métodos para detecção e identificação de espécies de Mycobacterium em pacientes com suspeita de tuberculose pulmonar
Marchi, A. M.I; Juttel, I. D.II; Kawacubo, E. M.II; Dalmarco, E. M.I; Blatt, S. L.I; Cordova, C. M. M.I,* * Corresponding Author. Mailing address: Universidade Regional de Blumenau, FURB, Departamento de Ciências Farmacêuticas, Campus III. Rua São Paulo 2171, Bairro Itoupava Seca, CEP 89030-000, Blumenau, SC. Fone (47) 3321-7328. E-mail: cmcordova@furb.br
IFundação Universidade Regional de Blumenau, Blumenau, SC, Brasil
IILaboratório Municipal de Análises Clínicas de Blumenau, Blumenau, SC, Brasil
ABSTRACT
Tuberculosis control is a priority for the Ministry of Health policies in Brazil. In the present work, the detection of Mycobacterium tuberculosis by the Polymerase Chain Reaction (PCR) was standardized, and the laboratory diagnosis of pulmonary tuberculosis was evaluated comparing baciloscopy, culture and PCR tests. The study was carried out with 117 sputum samples from different patients suspected of having pulmonary tuberculosis, for whom physicians had ordered a baciloscopy test. Baciloscopy was performed using the Ziehl-Neelsen method, and culture was performed by incubation of treated samples in Lowenstein-Jensen's medium at 37ºC for eight weeks. For PCR, DNA was amplified with a specific pair of primers to the M. tuberculosis complex, with a resulting product of 123 bp from the insertion element IS6110. Three (2.56%) samples presented a positive baciloscopy result and a positive PCR result (100% agreement), and nine (7.69%) presented Mycobacterium sp. growth in culture (P= 0.1384). Among six samples with positive results in culture, one was identified by PCR-RFLP as belonging to the M. tuberculosis complex and one was identified as a non-tuberculosis mycobacteria. Sensitivity and specificity of PCR compared to culture were 33.3% and 100%, respectively.
Key-words: tuberculosis, Mycobacterium, baciloscopy, culture, PCR.
RESUMO
A tuberculose é um dos agravos prioritários para as políticas do Ministério da Saúde. No presente trabalho, o método de detecção de Mycobacterium tuberculosis pela Reação em Cadeia da Polimerase (PCR) em amostras de escarro foi padronizado e o diagnóstico laboratorial da tuberculose pulmonar foi avaliado, comparando-se as metodologias de baciloscopia, cultura e PCR. Foram analisadas 117 amostras de escarro de diferentes pacientes com suspeita de tuberculose pulmonar, com solicitação de baciloscopia. A baciloscopia foi realizada com a coloração de Ziehl-Neelsen e a cultura pela semeadura das amostras em meio de Lowenstein-Jensen, incubadas a 37ºC por oito semanas. Para realização da PCR, o DNA foi amplificado com um par de oligonucleotídeos específicos para o complexo M. tuberculosis, resultando em um produto de 123 pb do elemento de inserção IS6110. Das 117 amostras analisadas, três (2,56%) apresentaram baciloscopia positiva e PCR positiva para M. tuberculosis (concordância de 100%), e nove (7,69%) tiveram crescimento de Mycobacterium sp. na cultura (P= 0,1384). Das seis amostras que tiveram resultado positivo somente por cultura, uma foi identificada ainda como pertencente ao complexo M. tuberculosis por PCR-RFLP, e outra foi identificada como micobactéria não tuberculosa. A sensibilidade e a especificidade da baciloscopia e da PCR em relação à cultura foram 33,3% e 100%, respectivamente.
Palavras-chave: tuberculose, Mycobacterium, baciloscopia, cultura, PCR.
INTRODUCTION
Mycobacterium tuberculosis is the main causing agent of tuberculosis (TB), an illness responsible for 26% of all possibly preventable deaths in the world (2,14). As a respiratory pathology it is considered a priority of disease control by the Ministry of Health in Brazil. In order to achieve this control, it is imperative to use appropriate diagnosis methods. Baciloscopy is not sensitive enough (5,000 to 10,000 bacilli/mL of sputum), and culture (sensitivity of 10 to 100 viable cells per sample) can take up to eight weeks to provide laboratory evidence towards the diagnosis of tuberculosis (3).
In the Brazilian public health system (SUS), laboratory diagnosis of tuberculosis is routinely made based on baciloscopy, in accordance with the guidelines set in the accepted Consensus in the country (4). This method has a sensitivity of about 60%, and many patients can end up undiagnosed, becoming vectors of the illness before the infection is confirmed and treated. The Consensus recommends culture for Mycobacterium spp. only when there is suspicion of pulmonary tuberculosis but the baciloscopy is negative. The suspicion can be extremely vague, as observed in the routine clinical practice requiring shipment of the sample to the Central Public Health Laboratory, located in the State Capital.
In many situations, definitive diagnosis through identification of M. tuberculosis by culture or baciloscopy is not possible. In the cases, diagnosis is based on clinical and epidemiological criteria, based on histo-pathological examinations and radiological findings, as occurs in extra-pulmonary tuberculosis, tuberculosis in infancy and paucibacillary pulmonary tuberculosis. The histo-pathological findings confirm the diagnosis in the majority of extra-pulmonary tuberculosis, cases except when granulloma with central caseous necrosis is detected. In this case, identification of included bacillus in the granulomatous reaction would be ideal. However, this finding is not common, leaving diagnostic doubts about other granulomatous illnesses.
Several methods for detection of mycobacteria DNA have been developed. In the United States, the FDA approved two commercial methods for direct detection in baciloscopy positive and negative samples (19). In Brazil, the accepted Consensus indicates the use of the PCR technique in reference laboratories, in cases when a fast diagnostic result is required. Molecular methods of detection of mycobacteria are beneficial for the physicians in the treatment of patients with TB, because they reduce the time for obtaining laboratory results. The group of immunocompromised patients infected with Human Immunodeficiency Virus (HIV) could benefit most, as they frequently present TB caused by species of mycobacteria other than M. tuberculosis, which would be easily identified by molecular methods. Considering that the treatment of infections caused by these atypical mycobacteria is different from that used for M. tuberculosis, these molecular methods would improve the health care for these individuals.
Some studies have used PCR-based methods in biopsy samples presenting granulloma reducing the time of diagnosis and confirming suspicious cases (9,21). The PCR for M. tuberculosis has been successfully applied to bone marrow (7), liver (1) and aspirates of pulmonary nodules (17), with high sensitivity and a negative predictive value of 100% (15).
Even considering that the consensus is that routine application of PCR does not increase the diagnosis efficiency of pulmonary tuberculosis if compared to baciloscopy (4,16), the isolated use of baciloscopy, can fail in detection of a number of clinically significant cases, especially in paucibacillary and immunocompromised individuals. In these cases, molecular methods would be helpful in the identification of atypical mycobacteria (5). The aim of this work was to evaluate the efficiency of the laboratory diagnosis of pulmonary tuberculosis by baciloscopy in comparison to the culture and PCR, using sputum samples. Considering that a significant number of patients with clinical suspicion of pulmonary tuberculosis present negative baciloscopy results, and that patients submitted to treatment of M. tuberculosis infection for more than a year present continuously positive baciloscopy results, the benefits of the standardization of the PCR technique for the users of the system were an additional motivation for the study.
MATERIAL AND METHODS
We analyzed 117 sputum samples sent to the Municipal Public Laboratory for Mycobacterium sp. testing by baciloscopy. After processing, the samples were sent to the Laboratory of Clinical Analyses of Fundação Universidade Regional de Blumenau (FURB), SC, Brazil, for culture and PCR. This project was approved by the Committee of Ethics in Research with Human Beings of FURB (Protocol n. 032/03).
Sputum samples were obtained in the morning, immediately after patients wake up (3,20). The samples were handled in Class II biological safety cabinets. For culture and PCR, samples were treated with NaOH and SDS, followed by neutralization with phosphoric acid. For each 2 mL of sputum, 3 mL of Solution A (SDS 3%, NaOH 1%) were added. After incubation at ambient temperature for 30 min, Solution B (phosphoric acid 0.567%, bromothymol blue 0.4%) was added slowly, until the a light green color appeared, indicating the neutralization of pH. The samples were centrifuged for 30 min at 3,000 x g and the sediment used for culture and the PCR.
The baciloscopy was carried out directly on sputum samples smears, after the coloration of Ziehl-Neelsen, in accordance with the procedures established in the Municipal Public Laboratory (13).
Culture of the treated samples was carried out in Lowenstein-Jensen - MTBAC culture media (Probac, Brazil, São Paulo, SP), incubated at 37ºC for eight weeks (13).
For PCR, the remaining sediment (about 1 mL) of the samples treated as described above was added to 1 mL of two fold concentrated lysis buffer (final concentration: Tris 10 mM, Triton X-100 0.1%, proteinase K 400 ug/mL) and incubated at 56ºC for 16 hs. DNA was extracted with phenol/chloroform followed by precipitation with ethanol overnight (12). The purified DNA was dissolved in 100 uL of Grade I DNase/RNase free water, and amplified with a pair of primers specific to the M. tuberculosis complex strains (MT1: 5'-CCT.GCG.AGC.GTA. GGC.GTC.GG-3' and MT2: 5'-CTC.GTC.CAG.CGC.CGC. TTC. GG-3'), resulting in a 123 bp product of the insertion element IS6110 (6). For the test, 10 uL of sample were added to 50 uL of reaction solution containing 1 pmol of each primer, 2.5 mM MgCl2, 400 mM dNTPs, and 1.0 U of Taq DNA Polymerase (Invitrogen Brasil Ltda., São Paulo).
The mycobacteria species were identified using the PCR-RFLP developed by Wong et al., 2001 (23). Briefly, a 294 bp fragment of the hsp65 gene was amplified directly from the purified DNA of clinical samples with primers HSP1 (5'-CC.AAG.AAG.ACC.GAY.GAC.GT-3') and HSP2 (5'-GT.GAT.GAC.GCC.CTC.GTTT.GC-3'). Reactions were performed with 2 uL of sample in a final volume of 50 uL, containing 1 pmol of each primer, 0.5 mM MgCl2, 400 mM dNTPs, and 1.0 U of Taq DNA Polymerase (Invitrogen). PCR products (10 uL) were digested using the enzymes CfoI and Sau96I (Promega Corporation, Madison, USA), according to the manufacturer's instructions. For identification of the species The restriction pattern was evaluated according to the protocol established by Wong et al., 2001.
The results of positivity for Mycobacterium spp. by the evaluated methods were analyzed by the Chi Square Test (22), with the aid of GraphPad InstatTM software (San Diego, CA, USA).
RESULTS
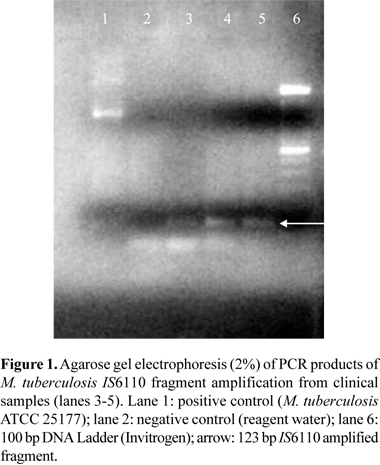
The baciloscopy after the coloration of Ziehl-Neelsen revealed that three (2.56%) samples were positive for M. tubertulosis. After amplification of the DNA of these samples through PCR using specific primers to the M. tuberculosis complex, the same three samples presented positive results, as indicated by the presence of the 123 bp fragment in the 2% agarose gel electrophoresis (Fig. 1).
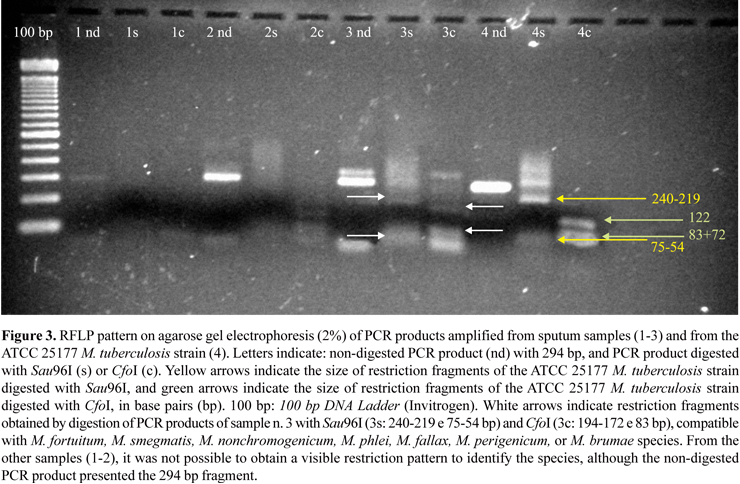
After incubation of the samples in Lowenstein-Jensen media at 37ºC for eight weeks, growth of Mycobacterium spp. was observed in 7.69% of the samples (9/117; P = 0.1384). These results are presented in Fig. 2. Among six samples positive only by culture, PCR-RFLP indicated that one belonged to the M. tuberculosis and another one to the group that includes the species M. fortuitum, M. smegmatis, M. nonchromogenicum, M. phlei, M. fallax, M. perigenicum, and M. brumae (Fig. 3).
For the samples analyzed in this work, the sensitivity and specificity of the PCR in relation to the culture were 33.3% and 100%, respectively.
DISCUSSION
The culture method resulted in a larger positivity rate for Mycobacterium spp. than the baciloscopy and the PCR. However, this difference was not statistically significant. The samples presenting a positive baciloscopy also presented a positive PCR for the M. tuberculosis complex, indicating that the PCR did not present a higher sensitivity than the baciloscopy. The agreement between the two methods was 100%. Samples presenting negative baciloscopy also presented negative PCR results, although some had a positive culture. It can be hypothesized that these findings occurred because these patients were paucibacillary, and neither method is capable of detecting small amounts of bacilli in the samples (4).
The sensitivity of PCR in relation to the culture, which is considered the method of reference for the diagnosis of TB, was 33.3%, or rather, 9/117 cultures presented growth of Mycobacterium spp. while 3/117 samples were PCR positive. These values are in accordance with other studies that indicate that the sensitivity of the PCR can vary from 9 to 100% (16).
The occurrence of samples that presented a negative result by PCR and a positive result by culture suggests that the growth observed in the cultures may not be M. tuberculosis, but other mycobacteria. Infection of immunocompromised individuals is generally caused by bacilli of the Mycobacterium avium-intracellulare complex, which resembles TB in these patients. The PCR technique used in this study for the amplification of the insertion element IS6110 is not capable of detecting these atypical mycobacteria, since the primers are specific for the M. tuberculosis complex. Among the samples with positive results only by culture, one was identified by PCR-RFLP as containing non-tuberculosis mycobacteria, and the other belonged to the M. tuberculosis complex. These results suggest that the two methods have different sensitivity. Considering the other culture positive samples, it was not possible to obtain an acceptable restriction pattern for the identification of the species directly from sputum purified DNA, despite the presence of the 294 bp PCR product with primers HSP1 and HSP2 in all samples.
Studies carried out by other authors over the last few decades demonstrate that the incidence of infection by mycobacteria not belonging to the M. tuberculosis complex has increased. In the United States, isolation of non-tuberculosis species is more common than M. tuberculosis (18). As not all immunocompromised patients suspected of having TB are infected by M. tuberculosis, identification tests must be used to differentiate the species of mycobacteria (8). Early identification of the mycobacterium species causing the disease would have a significant clinical impact, since the treatment of the infection caused by M. tuberculosis is different from that of non-tuberculosis species (18). In this sense, molecular methods such as the PCR can be used as a faster way to differentiate and identify the various species of mycobacteria, thus assisting in the effective treatment of infections through the use of antimicrobial therapy adjusted to the specific agent, besides preventing the transmission of TB as well.
In the Public Health System (SUS) in Brazil, diagnosis of TB is routinely based on baciloscopy results (4), but in specific cases, cultures are performed at some of the few Central Laboratories of Public Health, leading to long delays in the confirmation of a diagnosis. Cases of patients with infection by mycobacteria are not diagnosed as soon as needed so that non-treated infected individuals continue to spread the bacilli . The importance of molecular methods is evident, as tools for increased diagnostic efficiency in health services. For those immunocompromised individuals where the species of mycobacterium causing the infection is not clear, the PCR identifies the agent (18) and a faster response than a conventional culture, preventing the need for additional investigations (10). This early diagnosis with prompt initiation of treatment, which is different for the species of the M. tuberculosis complex and atypical mycobacteria, can prevent infected patients from continuing to spread the bacillus, contributing to the control of the infection and stemming the development of serious forms of the disease reaching the bone marrow, liver, spleen and other sites, especially in infection by M. avium (11).
In conclusion, our evaluation revealed that the laboratorial diagnosis of pulmonary tuberculosis in Blumenau is satisfactory, without a significant difference between results obtained by baciloscopy and by the culture method. Furthermore, the evaluated PCR method presented a specificity of 100%, indicating that molecular methods can improve the diagnosis of the infection in cases where they are indicated.
ACKNOWLEDGEMENTS
A.M.M. received a fellowship from the Pibic/CNPq program. This work was supported by a grant from FAPESC/CNPq/MS. We thank the head of the Municipal Public Laboratory for authorizing the accomplishment of this work at that facility.
Submitted: May 16, 2007; Returned to authors for corrections: November 21, 2007; Approved: June 24, 2008
- 1. Akcan, Y.; Tuncer, S.; Hayran, M.; Sungur, A.; Unal, S. (1997). PCR on disseminated tuberculosis in bone marrow and liver biopsy specimens: correlation to histopathological and clinical diagnosis. Scand. J. Infect. Dis., 29(3): 271-274.
- 2. Bloom, B.R.; Murray, C.J.L. (1992). Tuberculosis: commentary on a reemergent killer. Science, 257(3): 1055-1064.
- 3. Campinas, L.L.S.L.; Ferrazoli, L.; Telles, M.A.S.; Matsumoto, N.F.; Biagolini, R.A.M.; Ferraz, S.M.P.; O., A.S. (2002). Tuberculose, manual de orientação São Paulo: Secretaria da Saúde, Divisão de Tuberculose. p. 15.
- 4. Castelo Filho, A.; Kritski, A.L.; Barreto, Â.W.; Lemos, A.C.M.; Netto, A.R.; Guimarães, C.A.; Silva, C.L.; Sant'anna, C.C.; Haddad, D.J.; Lima, D.S.; Matos, E.D.; Melo, F.C.Q.; Melo, F.A.F.; Gerhardt Filho, G.; Marsico, G.A.; Silva, G.; Siqueira, H.R.; Campos, H.; Saconato, H.; Dourado, I.; Rosemberg, J.; Braga, J.U.; Santos, J.R.; Seiscento, M.; Conde, M.B.; Dalcolmo, M.P.; Almeida, M.M.B.; Penna, M.L.F.; Barreto, M.L.; Hijjar, M.A.; Andrade, M.K.N.; Cardoso, N.C.; Pineda, N.I.S.; Leite, O.H.M.; Picon, P.; Silva, R.F.; Cavalcanti, S.; Pereira, S.M.; Augusto, V.M.; Galesi, V.; Pinto, W.P. (2004). II Consenso Brasileiro de Tuberculose: Diretrizes Brasileiras para Tuberculose 2004. J. Bras. Pneumol., 30(Supl 1): S57-S86.
- 5. Cheng, V.C.; Yew, W.W.; Yuen, K.Y. (2005). Molecular diagnostics in tuberculosis. Eur. J. Clin. Microbiol. Infect. Dis., 24(11): 711-720.
- 6. Eisennach, K.D.; Cave, M.D.; Crawford, J.T. (1993). PCR detection of Mycobacterium tuberculosis In: Pershing, D.H.; Smith, T.F.; Tenover, F.C.; White, T.J.; Pershing, D.H.; Smith, T.F.; Tenover, F.C.; White, T.J.S. Diagnostic Molecular Microbiology, Principles and Applications. Rochester: Mayo Foundation. p. 191-196.
- 7. Escobedo-Jaimes, L.; Cicero-Sabido, R.; Criales-Cortez, J.L.; Ramirez, E.; Romero, M.; Rivero, V.; Islas, F.; Olivera, H.; Gonzalez, S.; Escobar-Gutierrez, A. (2003). Evaluation of the polymerase chain reaction in the diagnosis of miliary tuberculosis in bone marrow smear. Int. J. Tuberc. Lung Dis., 7(6): 580-586.
- 8. Fukushima, M.; Kakinuma, K.; Hayashi, H.; Nagai, H.; Ito, K.; Kawaguchi, R. (2003). Detection and identification of Mycobacterium species isolates by DNA microarray. J. Clin. Microbiol., 41(6): 2605-2615.
- 9. Hofman, V.; Selva, E.; Landraud, L.; Sicard, D.; Venissac, N.; Castillo, L.; Kermarec, A.; Mouroux, J.; Dellamonica, P.; Hofman, P. (2003). Value of PCR amplification from formalin-fixed paraffin-embedded tissues in the diagnosis of Mycobacterium tuberculosis infection. Ann. Pathol., 23(3): 206-215.
- 10. Honore-Bouakline, S.; Vincensini, J.P.; Giacuzzo, V.; Lagrange, P.H.; Herrmann, J.L. (2003). Rapid diagnosis of extrapulmonary tuberculosis by PCR: impact of sample preparation and DNA extraction. J. Clin. Microbiol., 41(6): 2323-2329.
- 11. Inderlied, C.B.; Kemper, C.A.; Bermudez, L.E. (1993). The Mycobacterium avium complex. Clin. Microbiol. Rev., 6(3): 266-310.
- 12. Kirshner, P.; Meier, A.; Bottger, E.C. (1993). Genotypic identification and detection of Mycobacteria - Facing novel and uncultured pathogens In: Pershing, D.H.; Smith, T.F.; Tenover, F.C.; White, T.J.; Pershing, D.H.; Smith, T.F.; Tenover, F.C.; White, T.J. Diagnostic Molecular Microbiology, Principles and Applications. Rochester: Mayo Foundation. p. 173-190.
- 13. Koneman, E.W.; Allen, S.D.; Dowell Jr, V.R.D.; Sommers, H.M. (1993). Micobactérias In: Koneman, E.W.; Allen, S.D.; Dowell Jr, V.R.D.; Sommers, H.M.; Koneman, E.W.; Allen, S.D.; Dowell Jr, V.R.D.; Sommers, H.M. Diagnóstico Microbiológico. 2 ed. São Paulo: Ed. Médica Panamericana. p. 487-536.
- 14. Raviglione, M.C.; Snider, D.E.J.; Kochi, A. (1995). Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA, 273(3): 220-226.
- 15. Salian, N.V.; Rish, J.A.; Eisenach, K.D.; Cave, M.D.; Bates, J.H. (1998). Polymerase chain reaction to detect Mycobacterium tuberculosis in histologic specimens. Am. J. Respir. Crit. Care Med., 158(4): 1150-1155.
- 16. Sarmiento, O.L.; Weigle, K.A.; Alexander, J.; Weber, D.J.; Miller, W.C. (2003). Assessment by meta-analysis of PCR for diagnosis of smear-negative pulmonary tuberculosis. J. Clin. Microbiol., 41(7): 3233-3240.
- 17. Shim, J.J.; Cheong, H.J.; Kang, E.Y.; In, K.H.; Yoo, S.H.; Kang, K.H. (1998). Nested polymerase chain reaction for detection of Mycobacterium tuberculosis in solitary pulmonary nodules. Chest, 113(1): 20-24.
- 18. Shrestha, N.K.; Tuohy, M.J.; Hall, G.S.; Reischl, U.; Gordon, S.M.; Procop, G.W. (2003). Detection and differentiation of Mycobacterium tuberculosis and nontuberculous mycobacterial isolates by real-time PCR. J. Clin. Microbiol., 41(11): 5121-5126.
- 19. Soini, H.; Musser, J.M. (2001). Molecular diagnosis of mycobacteria. Clin. Chem., 47(5): 809-814.
- 20. Tisiologia, S.S.B.P. (1997). I Consenso Brasileiro de TB. J. Pneumol., 23(6): 294-296.
- 21. Vago, L.; Barberis, M.; Gori, A.; Scarpellini, P.; Sala, E.; Nebuloni, M.; Bonetto, S.; Cannone, M.; Marchetti, G.; Franzetti, F.; Costanzi, G. (1998). Nested polymerase chain reaction for Mycobacterium tuberculosis IS6110 sequence on formalin-fixed paraffin-embedded tissues with granulomatous diseases for rapid diagnosis of tuberculosis. Am. J. Clin. Pathol., 109(4): 411-415.
- 22. Vieira, S. (2003). Bioestatística - tópicos avançados, testes não-paramétricos, tabelas de contingências e análise de regressão. Rio de Janeiro: Campus, 212 p.
- 23. Wong, D.A.; Yip, P.C.; Cheung, D.T.; Kam, K.M. (2001). Simple and rational approach to the identification of Mycobacterium tuberculosis, Mycobacterium avium complex species, and other commonly isolated mycobacteria. J. Clin. Microbiol., 39(10): 3768-3771.
Publication Dates
-
Publication in this collection
06 Apr 2009 -
Date of issue
Dec 2008
History
-
Received
16 May 2007 -
Accepted
21 Nov 2007