Abstracts
Inhibitors of plant proteases can regulate the hydrolysis of proteins inside the cells and also participate in the mechanisms of plant defense against herbivore insects and pathogens. Here, we demonstrated that seeds of Eucalyptus urophylla exhibit activities of trypsin and papain inhibitors, two proteases commonly found in living cells. Low amounts of proteins of the crude protein extract of seeds and fractions partially purified by gel filtration, with inhibitory activity against trypsin, inhibited in vitro the mycelial growth of a compatible isolate of the ectomycorrhizal fungus Pisolithus tinctorius and allowed an unsatisfactory growth of another isolate from Pinus taeda, considered incompatible for this eucalyptus species. The same amounts of inhibitory proteins, when tested in vitro on the pathogen Rhizoctonia solani, did not exhibit any effect on the growth of the pathogen. These results indicate the existence of proteases inhibitors in seeds of E. urophylla which could influence the complex biochemical system that differentiates mechanisms of symbiosis and pathogenicity between plants and microorganisms.
protease; inhibitor; trypsin; Pisolithus tinctorius; Rhizoctonia solani
Os inibidores de proteases de plantas podem regular a hidrólise de proteínas no interior celular e também participar de seus mecanismos de defesa contra insetos herbívoros e patógenos. No presente trabalho, nós demonstramos que sementes de Eucalyptus urophylla apresentam atividades de inibidores de tripsina e papaína, duas proteases comumente encontradas em seres vivos. Pequenas quantidades de proteínas do extrato protéico bruto de sementes e de frações parcialmente purificadas por filtração em gel, com atividade inibitória de tripsina, inibiram o crescimento micelial in vitro de um isolado compatível do fungo ectomicorrízico Pisolithus tinctorius e permitiram um crescimento insatisfatório de outro isolado de Pinus taeda, considerado compatível para esta espécie de eucalipto. As mesmas concentrações de proteínas, quando testadas in vitro sobre o patógeno Rhizoctonia solani, não demonstraram qualquer efeito sobre seu crescimento. Estes resultados indicam a existência de inibidores de proteases em sementes de E. urophylla, os quais poderiam influenciar o complexo sistema bioquímico que diferencia mecanismos de simbiose e patogenicidade entre plantas e microrganismos.
protease; inibidor; tripsina; Pisolithus tinctorius; Rhizoctonia solani
Detection of trypsin inhibitor in seeds of Eucalyptus urophylla and its influence on the in vitro growth of the fungi Pisolithus tinctorius and Rhizoctonia solani
Descoberta de inibidor de tripsina em sementes de Eucalyptus urophylla e sua influência sobre o crescimento in vitro dos fungos Pisolithus tinctorius e Rhizoctonia solani
Célia Regina Tremacoldi; Sérgio Florentino Pascholati
Departamento de Entomologia, Fitopatologia e Zoologia Agrícola, Escola Superior de Agricultura Luiz de Queiroz, Universidade de São Paulo, Piracicaba, SP, Brasil
CorrespondenceCorrespondence to
ABSTRACT
Inhibitors of plant proteases can regulate the hydrolysis of proteins inside the cells and also participate in the mechanisms of plant defense against herbivore insects and pathogens. Here, we demonstrated that seeds of Eucalyptus urophylla exhibit activities of trypsin and papain inhibitors, two proteases commonly found in living cells. Low amounts of proteins of the crude protein extract of seeds and fractions partially purified by gel filtration, with inhibitory activity against trypsin, inhibited in vitro the mycelial growth of a compatible isolate of the ectomycorrhizal fungus Pisolithus tinctorius and allowed an unsatisfactory growth of another isolate from Pinus taeda, considered incompatible for this eucalyptus species. The same amounts of inhibitory proteins, when tested in vitro on the pathogen Rhizoctonia solani, did not exhibit any effect on the growth of the pathogen. These results indicate the existence of proteases inhibitors in seeds of E. urophylla which could influence the complex biochemical system that differentiates mechanisms of symbiosis and pathogenicity between plants and microorganisms.
Key words: protease, inhibitor, trypsin, Pisolithus tinctorius, Rhizoctonia solani
RESUMO
Os inibidores de proteases de plantas podem regular a hidrólise de proteínas no interior celular e também participar de seus mecanismos de defesa contra insetos herbívoros e patógenos. No presente trabalho, nós demonstramos que sementes de Eucalyptus urophylla apresentam atividades de inibidores de tripsina e papaína, duas proteases comumente encontradas em seres vivos. Pequenas quantidades de proteínas do extrato protéico bruto de sementes e de frações parcialmente purificadas por filtração em gel, com atividade inibitória de tripsina, inibiram o crescimento micelial in vitro de um isolado compatível do fungo ectomicorrízico Pisolithus tinctorius e permitiram um crescimento insatisfatório de outro isolado de Pinus taeda, considerado compatível para esta espécie de eucalipto. As mesmas concentrações de proteínas, quando testadas in vitro sobre o patógeno Rhizoctonia solani, não demonstraram qualquer efeito sobre seu crescimento. Estes resultados indicam a existência de inibidores de proteases em sementes de E. urophylla, os quais poderiam influenciar o complexo sistema bioquímico que diferencia mecanismos de simbiose e patogenicidade entre plantas e microrganismos.
Palavras-chave: protease, inibidor, tripsina, Pisolithus tinctorius, Rhizoctonia solani
INTRODUCTION
Plants, microorganisms and animals contain proteins that exhibit the peculiar property of forming complexes with proteolytic enzymes, promoting the inhibition of their activity by competing for the catalytic site. These proteins are called protease inhibitors. Their molecular mass varies from 10 to 90 kDa, and they exhibit high or no specificity to the target enzyme. Most of the protease inhibitors known and characterized in plants belong to the group of the serine-protease inhibitors, which includes trypsin (9).
The physiological role of plant protease inhibitors is not very clear. Besides regulating the hydrolysis of proteins inside the cell, they can participate in defense mechanisms of plants against herbivore insects and/or pathogens. For example, in interactions between plants and fungi, during the penetration and colonization processes, extracellular proteases of a pathogen would promote the hydrolysis of proteins of the cellular wall. Thus, protease inhibitors of the host plant could act directly against those enzymes, delaying the proteolysis of cellular walls and membranes, and reducing the cellular desorganization (10).
Differences in the accumulation of protease inhibitors and proteases in compatible and incompatible interactions between plants and microorganisms (4,8) let us associate these groups of proteins to the resistance and susceptibility concepts, generating perspectives of a better understanding of the biochemical bases that govern the plant-pathogen interactions. However, there are not many studies showing the role of protease inhibitors in the mechanisms of plant defense against pathogens. For trees, even those cultivated in commercial scale, as Eucalyptus spp. and Pinus spp., there are no reports on the occurrence of protease inhibitors and, consequently, on the possible role of the inhibitors on pathogenic or symbiont fungi, as the ectomycorrhiza, present in all species of those plants.
In the present work, we studied the activity of protease inhibitors in seeds of E. urophylla and the influence of the trypsin inhibitor on the in vitro growth of compatible and incompatible isolates of the ectomycorrhizal fungus Pisolithus tinctorius. We also studied its influence on the growth of the pathogen Rhizoctonia solani, agent of damping-off in nursery plants.
MATERIALS AND METHODS
Biological materials and chemical reagents
The ectomycorrhizal fungus Pisolithus tinctorius, isolate 1604, obtained from Eucalyptus grandis (Rio Claro/SP/Brazil) and isolate 185, obtained from Pinus taeda (USA) were used in the biological assays. The pathogen Rhizoctonia solani was isolated from seedlings of Eucalyptus urophylla (SP/Brazil). Seeds of E. urophylla were obtained from the Institute of Forest Research at ESALQ/USP - Piracicaba, SP, Brazil. The chemicals bovine pancreas trypsin, chymotrypsin, Carica papaya papain, BAPNA (a-benzoyl-arginyl-p-nitroanilide), BTPNA (a-benzoyl-tyrosine-p-nitroanilide), Triton X-100, Dimethyl sulfoxide (DMSO), dithiothreitol (DTT) and Sephacryl S-100-HR were obtained from Sigma (USA).
Extraction
The crude protein extract was obtained from 10 g of E. urophylla seeds, disrupted in liquid nitrogen. A saline solution (NaCl 15%; 1:10 v/v) was added to the powder and the suspension was kept under agitation during 1 h, followed by centrifugation at 12,300 g (4ºC) for 20 min. The supernatant was collected and four volumes of cold acetone was added to it and centrifuged at 12,300 g (4ºC) for 20 min. After centrifugation, the pellet was mixed with distilled water (1:10 w/v), maintained under agitation for 15 min and centrifuged at 12,300 g for 20 min. The supernatant obtained represented the crude protein extract, from which the tests for protease inhibitory activity were accomplished. To eliminate the risk of a possible inhibition of the protease inhibitor activity because of the presence of lectins (proteins that can associate to carbohydrates), before the assay of inhibitory activity for any protease, the crude extract was heated at 70ºC for 20 min (9), temperature that is supported by the protease inhibitors but not by the lectins (3).
Measurement of trypsin, chymotrypsin and papain inhibitory activities
To study the trypsin activity, volumes of 30, 50, 70, 85, 100 and 150 µL of the crude extract were incubated with 10 mg of trypsin (diluted in 1mM HCl) and with variable volumes of the 0.1 M Tris-HCl buffer, pH 8.0, containing 0.01M CaCl2, to make a final volume of 500 µL in each test tube. This reaction mixture was pre-incubated by 10 min at 37ºC, and 500 µL of the cromogenic substrate BAPNA (1 mM in DMSO) were added to each tube, which was incubated for 40 min at 37ºC. The reaction was stopped by the addition of 500 µL of acetic acid (30% v/v) to each test tube. An enzyme activity of 100% was obtained by not adding the protein extract to the reaction mixtures. The controls for each volume of the extract tested were obtained by the addition of acetic acid just before the incubation with the substrate BAPNA (7). The experiment was repeated 4 times with 2 replicates for each volume of the extract tested. An average of the absorbance readings at 405 nm for the replicates was calculated and it produced a value that was the difference between the absorbance in the reaction tubes and their respective control test tubes. This value, for each volume of the extract, was subtracted of the value that represented 100% of enzyme activity, allowing the calculation of the residual activity of trypsin based upon µL of crude extract. The activity evaluation for the chymotrypsin inhibitor of the protein crude extract was accomplished in a similar way to that described for trypsin having BTPNA (1mM in DMSO) as the substrate for this protease. For the evaluation of the papain activity volumes of 100, 150 and 200 µL of the protein extract reacted with the substrate BAPNA, in presence of 0.2 M sodium acetate buffer, pH 5.5, added of 0.01% Triton X-100 and of 1mM DTT. Before the addition of papain to the test tubes containing the reaction mixture, the papain was pre-incubated with the buffer during 10 min at 37ºC.
Gel filtration
The crude extract was applied to a chromatography column (60 cm x 1.5 cm) filled out with Sephacryl S-100-HR, equilibrated and eluted with 0.1 M Tris-HCl buffer, pH 8.0. Samples of the extract (1.5 mL) were applied to the column and were eluted in a flow rate of 18 mL/h, with fractions of 1 mL collected every 3.3 min. The void volume (Vo) of the column was determined by using 1 mL of blue dextran (1mg/mL) under the same flow conditions.
Activity of trypsin inhibitor in the fractions
All the fractions obtained by gel filtration had the absorbance read at 280 nm in a spectrophotometer, and an aliquot of 50 µL of each one was tested for trypsin inhibitory activity. These aliquots reacted with 300 µL of 0.1 M Tris-HCl buffer, pH 8.0, and 10 mg of trypsin diluted in 1mM HCl. The incubation was carried out at 37ºC for 10 min, followed by the adition of 500 µL of BAPNA for a new incubation in the same temperature during 40 min. The reaction was stopped by the addition of 500 µL of acetic acid (30% v/v). The inhibitory activity was calculated as the difference between the trypsin activity in the absence and in the presence of the fractions. The fractions with trypsin inhibitory activity superior to 50% were pooled. The concentration of proteins in the crude extract of seeds and in the several fractions was estimated by the method of Bradford (1), using bovine serum albumin as standard.
Estimate of the molecular masses
The standard curve for the calculation of the molecular masses of the fractions with inhibitory activity was obtained by using the kit MW-GF-70 (Sigma), with samples of 1.5 mL of bovine serum albumin (5 mg/mL), aprotinin (3 mg/mL), carbonic anidrase (2 mg/mL) and cytochrome c (2 mg/mL), eluted in the 0.1 M Tris-HCl buffer, pH 8.0, in a flow rate of 0.3 mL/min, with fractions of 1 mL collected every 3.3 min.
Effect of the crude extract of seeds on the mycelial growth of P. tinctorius and R. solani isolates
To test the in vitro effect of the protein crude extract of E. urophylla seeds on the growth of the symbiont P. tinctorius and on the pathogen R. solani both fungi were grown inside Petri dishes of 5 cm diameter. The isolate 1604 of P. tinctorius is considered highly compatible for ectomycorrhiza formation in E. urophylla (2) while the isolate 185 is seen as incompatible because it does not produce typical ectomycorrhiza in the same eucalyptus species.
A disk of mycelia of each fungus was placed onto PDA (potato dextrose agar) medium, pH 6.7, previously flooded with 21 and 47 µg of protein, after sterilization through Millipore filter 0.2 µm. Each concentration and the control (to which the protein extract was not increased) had 3 replicates. The volume of the extract corresponding to the wanted protein concentration was adjusted so that each plate contained 7 mL of final volume (PDA+ protein extract added). In each plate, after the hardening of the growth medium, was placed a disk of 4 mm diameter of P. tinctorius or R. solani from colonies 30 and 40 days old, respectively. The incubation was carried out at 28ºC in the darkness. Fungal growth was evaluated through the measurement of colony diameter daily until the mycelial growth in the control plates for each species had covered all the medium. The experiment was repeated once.
Effect of fractions with trypsin inhibitory activity on the mycelial growth of P. tinctorius and R. solani isolates
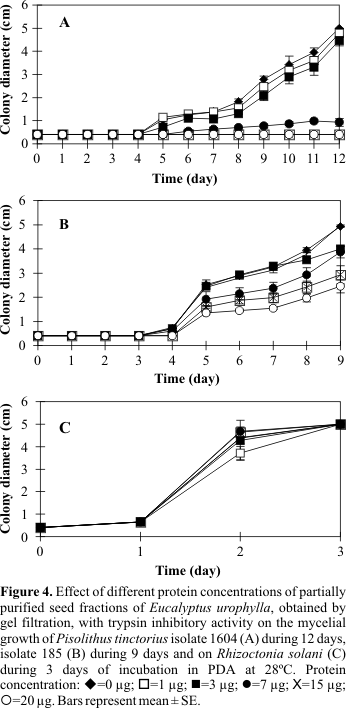
The fractions obtained by gel filtration chromatography of the crude seed extract that exhibited inhibition of the trypsin activity superior to 50% were pooled and tested against the mycelial growth of P. tinctorius isolates 1604 and 185 and R. solani. The procedure used for growth, incubation time and incorporation of the protein fractions into the medium were identical to those carried out with the crude extract. Control treatment was represented by growth medium without adding the fractions, while treatments included media with aliquots from the fractions having 1, 3, 7, 15 and 20 µg of protein for each plate of 5 cm diameter with a 7.0 mL final volume. The experiment was repeated once.
RESULTS
The crude extract from E. urophylla seeds showed the existance of trypsin and papain inhibitors (
Fig. 1Gel chromatography of the crude seed extract showed that peak I, based upon the absorbance at 280 nm (
Fig. 2The crude extract of E. urophylla seeds, based upon two protein concentrations (21 and 47 µg), when incorporated into the growth medium, caused complete growth inhibition of P. tinctorius compatible isolate 1604 (
Fig. 3 - AFig. 3 - BR. solaniFig. 3 - C Fig. 4 Fig. 2DISCUSSION
The low residual activity of trypsin (around 25%) in the presence of the seed extract of E. urophylla made it possible to check for chymotrypsin inhibitory activity, since these two groups of protease inhibitors are not commonly associated (6). Thus, it can be assumed that chymotripsin inhibitors do not occur in seeds of E. urophylla. Although the inhibition of the papain activity was around 90%, we decided to test initially the effects of the trypsin inhibitor, since it is the most studied among the inhibitors of plant proteases. The studies were carried out using extracts from E. urophylla seeds due to the difficulty to obtain from the roots the adequate amount of protein for the biological and biochemical assays. In addition, seeds are easier to be obtained compared to roots and most of the studies already accomplished on inhibitors of plant proteases were centered around seeds, especially from leguminous plants (5).
The observation of the growth pattern of P. tinctorius and R. solani isolates in the presence of the crude extract from seeds and partially purified fractions with trypsin inhibitory activity allowed important considerations. For both, the symbiont and the pathogenic fungus, the growth curves exhibited the same patterns when the crude extract or the partially purified fractions were used, demonstrating that there is a correlation between the inhibition of the fungal growth and the presence of the trypsin inhibitor. The differencial effect of the trypsin inhibitor from E. urophylla seeds on P. tinctorius and R. solani growth is another interesting aspect, since this protein group can participate in the complex biochemical mechanism of recognition of a symbiont or a pathogen microorganism by the host plant. However, future studies are necessary to verify the activity of trypsin inhibitor in roots of E. urophylla infected or not with symbionts and pathogens to reach specific conclusions regarding the involvement of the inhibitor in the recognition process. Another aspect to be considered is the effect of the trypsin inhibitor on the growth of the two isolates of the ectomycorrhizal fungus, where the isolate 185 (incompatible) was able to grow even in an unsatisfactory way in presence of the crude extract or partially purified fractions (protein concentrations equal to 21 e 47 µg in
Fig. 3The results presented open new perspectives for the study of proteins related to the recognition processes between pathogens and symbionts for eucalyptus species, since for these interactions protease inhibitors and proteases were not previously reported in the literature.
REFERENCES
Departamento de Entomologia, Fitopatologia e Zoologia Agrícola - ESALQ-USP
Caixa Postal 9. 13418-900, Piracicaba, SP, Brasil
Tel.: (+5519) 3429-4124. Fax: (+5519) 3434-4839
E-mail: sfpascho@esalq.usp.br
Submited: June 26, 2001; Returned to authors for corrections: October 18, 2002; Approved: December 05, 2002.
- 1. Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. An. Biochem, 72: 248-254, 1976.
- 2. Galli, M.A. Influence of organic matter sources on formation of ectomycorrhizas in Eucalyptus citriodora seedlings inoculated with Pisolithus tinctorius. Piracicaba, Brazil, 1996, 90p. (Ph.D. thesis. ESALQ/São Paulo State University).
- 3. Gatehouse, A.M.R.; Powell, K.S.; Peumans, W.J.; Van Damme, E.J.M.; Gatehouse, J.A. Insecticidal properties of plant lectins: their potential in plant protection. In: Pusztai, A.; Bardocz, S. (eds). Lectins: biomedical perspectives, Taylor and Francis, London, 1995, p.35-57.
- 4. Giri, A.P.; Harsulkar, A.M.; Patankar, A.G.; Gupta, V.S.; Sainani, M.N.; Deshpande, V.V.; Ranjekar, P.K. Association of induction of protease and chitinase in chickpea roots with resistance to Fusarium oxysporum f. sp. ciceri. Plant Pathol., 47: 693-699, 1998.
- 5. Kalume, D.E.; Sousa, M.V.; Morthy, L. Purification, characterization, sequence determination and mass spectrometric analysis of a trypsin inhibitor from seeds of the brazilian tree Dipteryx alata (Leguminosae). J. Prot. Chem., 14: 685-693, 1995.
- 6. Laskowski, M.; Kato, I. Protein inhibitors of proteinases. Annu. Rev. Biochem, 49: 593-626, 1980.
- 7. Lin, J.Y.; Chu, S.C.; Wu, H.C.; Hsieh, Y.S. Trypsin inhibitor from the seeds of Acacia confusa J. Biochem, 110: 879-883, 1991.
- 8. Peng, J.H.; Black, L.L. Increased proteinase inhibitor activity in response to infection of resistant tomato plants by Phytophthora infestans Phytopathology, 66: 958-963, 1976.
- 9. Richardson, M. Seed storage proteins: The enzyme inhibitors. In: Dey, P.M.; Harborne, J.B. (eds). Methods in plant biochemistry, Vol. 5, Academic Press, New York, 1991. p.259-306.
- 10. Ryan, C.A. Proteinase inhibitors in plants: genes for improving defenses against insects and pathogens. Annu. Rev. Phytopathol., 28: 425-449, 1990.
Publication Dates
-
Publication in this collection
18 June 2003 -
Date of issue
Dec 2002
History
-
Reviewed
18 Oct 2002 -
Received
26 June 2001 -
Accepted
05 Dec 2002