Abstract
In addition to findings from conventional magnetic resonance imaging, modern magnetic resonance imaging techniques have provided important information about tumor metabolism, in vivo metabolite formation, water molecule diffusion, microvascular density, and blood-brain barrier permeability, all of which have improved the in vivo diagnostic accuracy of this method in the evaluation of primary central nervous system lymphoma. These nonconventional magnetic resonance techniques are useful in the clinical practice because they enhance conventional magnetic resonance imaging by reinforcing the possibility of a diagnosis and by allowing the early detection of disease recurrence.
This report is a review of the most relevant contributions of nonconventional magnetic resonance techniques to the imaging diagnosis of primary central nervous system lymphoma, the differential diagnosis of this disease, and the prognosis of patients. This paper aims to describe a wide range of presentations of primary central nervous system lymphoma, their appearance in imaging, and the differential diagnoses of this disease.
Keywords:
CNS lymphomas; Lymphomatosis cerebri; Neurolymphomatosis; Ocular lymphomas; Magnetic resonance imaging; Brain neoplasm; Nonconventional MR
Introduction
Primary central nervous system lymphomas (PCNSL) have a predictable imaging appearance in their classical presentation. Conventional magnetic resonance imaging (MRI) features can be suggestive of PCNSL but are not specific. Because of the form of presentation, the list of differential diagnoses is long, and mainly includes inflammatory/infectious diseases particularly of the central nervous system (CNS), toxoplasmosis in AIDS patients, and primary and secondary malignancies.11 Haque S, Law M, Abrey LE, Young RJ. Imaging of lymphoma of the central nervous system, spine, and orbit. Radiol Clin North Am. 2008;46(2):339-61, ix.–33 Iwamoto FM, DeAngelis LM. An update on primary central nervous system lymphoma. Hematol Oncol Clin North Am. 2006;20(6):1267-85.
Modern techniques of magnetic resonance have increased the clinical applicability of this imaging method because they provide important information about tumor metabolism, in vivo metabolite formation, water molecule diffusion, microvascular density, and blood–brain barrier (BBB) permeability. Although histology and an investigation of the cerebrospinal fluid for meningeal disease are still the gold standard in the diagnosis of CNS lymphoma, these advanced techniques can improve the diagnostic accuracy of PCNSL, particularly when the brain parenchyma is affected.11 Haque S, Law M, Abrey LE, Young RJ. Imaging of lymphoma of the central nervous system, spine, and orbit. Radiol Clin North Am. 2008;46(2):339-61, ix.,4-99 Haldorsen IS, Krakenes J, Krossnes BK, Mella O, Espeland A. CT and MR imaging features of primary central nervous system lymphoma in Norway, 1989-2003. AJNR Am J Neuroradiol. 2009;30(4):744-51.
This report is a review of the most relevant contributions of advanced MRI techniques to the imaging diagnosis of PCNSL, the differential diagnosis of this disease, and the prognosis of patients.
Contribution of nonconventional magnetic resonance techniques to the diagnosis of primary central nervous system lymphoma and the prognosis of patients
The various imaging methods that are currently available no longer exclusively provide descriptive anatomical information. Now advanced imaging techniques allow an assessment of functional parameters, thereby maximizing the potential of these techniques for an accurate diagnosis and treatment assessment.
Despite treatment advances, survival among PCNSL patients in the United States has remained poor. However, survival has improved over time in the subset of PCNSL patients who are human immunodeficiency virus (HIV)-negative.1010 Norden AD, Drappatz J, Wen PY, Claus EB. Survival among patients with primar central nervous system lymphoma, 1973-2004. J Neurooncol. 2011;101(3):487-93.
Nonconventional MRI techniques are useful in the clinical practice because they enhance conventional MRI by reinforcing the possibility of a diagnosis of PCNSL, by contributing to the prognosis of patients, and by allowing the early detection of disease recurrence.11 Haque S, Law M, Abrey LE, Young RJ. Imaging of lymphoma of the central nervous system, spine, and orbit. Radiol Clin North Am. 2008;46(2):339-61, ix.,88 Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol. 2011;32(6): 984-92.,1111 Raizer JJ, Koutcher JA, Abrey LE, Panageas KS, DeAngelis LM, Lis E, et al. Proton magnetic resonance spectroscopy in immunocompetent patients with primary central nervous system lymphoma. J Neurooncol. 2005;71(2):173-80.
Diffusion-weighted imaging
Diffusion-weighted imaging (DWI) reflects the macromolecular motion of intra- and extracellular water and is helpful in distinguishing between PCNSL and other tumors and tumor-mimicking lesions. Due to the hypercellularity of PCNSL, water diffusion is often restricted, which makes these tumors appear hyperintense on DWI and hypointense on apparent diffusion coefficient (ADC) maps. A decreased ADC value indicates increased cellularity.44 Zacharia TT, Law M, Naidich TP, Leeds NE. Central nervous system lymphoma characterization by diffusion-weighted imaging and MR spectroscopy. J Neuroimaging. 2008;18(4):411-7.,88 Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol. 2011;32(6): 984-92.,1212 Barajas RF Jr, Rubenstein JL, Chang JS, Hwang J, Cha S. Diffusion-weighted MR imaging derived apparent diffusion coefficient is predictive of clinical outcome in primary central nervous system lymphoma. AJNR Am J Neuroradiol. 2010;31(1):60-6. The technique that is generally used to determine the ADC value involves calculating the ADC ratio of the solid component of the tumor and comparing this ratio to that of the normal-appearing white matter. Restricted diffusion with an ADC threshold value ≤1.1 × 10−3 mm2/s has been recommended to differentiate PCNSL from other intracranial focal lesions.77 Al-Okaili RN, Krejza J, Woo JH, Wolf RL, O’Rourke DM, Judy KD. Intraaxial brain masses: MR imaging-based diagnostic strategy-initial experience. Radiology. 2007;243(2):539-50. An ADC threshold value >1.6 × 10−3 mm2/s increases the probability of a diagnosis of toxoplasmosis over PCNSL in immunocompromised patients. However, there has been significant overlapping in these values in necrotic ring-enhancing lesions.44 Zacharia TT, Law M, Naidich TP, Leeds NE. Central nervous system lymphoma characterization by diffusion-weighted imaging and MR spectroscopy. J Neuroimaging. 2008;18(4):411-7.,1313 Camacho DL, Smith JK, Castill M. Differentiation of toxoplasmosis and lymphoma in AIDS patients by using apparent diffusion coefficients. AJNR Am J Neuroradiol. 2003;24(4):633-7.
A recent report revealed that low ADC values in pre-therapeutic tumors were predictive of shorter progression-free survival and overall survival. Furthermore, increasing ADC values during treatment suggest a positive response to therapy, whereas decreasing ADC values suggest tumor progression.1212 Barajas RF Jr, Rubenstein JL, Chang JS, Hwang J, Cha S. Diffusion-weighted MR imaging derived apparent diffusion coefficient is predictive of clinical outcome in primary central nervous system lymphoma. AJNR Am J Neuroradiol. 2010;31(1):60-6.
Diffusion tensor imaging (DTI) takes advantage of highly ordered white matter fibers, which have a directionally ordered structure due to preferential water diffusion along the longitudinal axis of axons and myelin sheaths. This technique has recently emerged as a sensitive tool for the detection of alterations in the structure of white matter. Additionally, different degrees of cellularity and cellular organization may affect the fractional anisotropy (FA) value. The FA values for PCNSL are significantly lower than those for glioblastoma; therefore, these values can help in the differentiation of these tumors.1414 Toh CH, Castillo M, Wong AM, Wei KC, Wong HF, Ng SH, et al. Primary cerebral lymphoma and glioblastoma multiforme: differences in diffusion characteristics evaluated with diffusion tensor imaging. AJNR Am J Neuroradiol. 2008;29(3):471-5.
Further studies that combine histological correlation techniques with imaging findings are needed to determine the impact of the measurement of ADC on patient prognosis and treatment planning. However, we recommend performing a careful ADC analysis of different solid parts of the tumor before treatment and after treatment as an additional noninvasive tool to estimate local in vivo responses.
Perfusion-weighted imaging
Perfusion MRI can be used to estimate the nutritive delivery of arterial blood to the capillary bed in biological tissue using signal intensity–time curves to determine the capillary density in dynamic susceptibility contrast (DSC) T2-weighted echoplanar sequences. The post-processing of acquired data allows for a semiquantitative analysis of physiological parameters, particularly of the relative cerebral blood volume (rCBV), relative cerebral blood flow (rCBF), and time-to-peak (TTP). Another perfusion MRI technique that can be used to evaluate vascular permeability in a dynamic contrast-enhanced (DCE) T1-weighted sequence is to simultaneously administer an intravenous injection of a paramagnetic agent (gadolinium).
PCNSL are associated with mildly increased or occasionally decreased perfusion that is usually higher than that in most focal brain inflammatory, demyelinating and infectious diseases but lower than that in high-grade gliomas (rCBV > 1.75).77 Al-Okaili RN, Krejza J, Woo JH, Wolf RL, O’Rourke DM, Judy KD. Intraaxial brain masses: MR imaging-based diagnostic strategy-initial experience. Radiology. 2007;243(2):539-50. PCNSL lesions are usually strongly enhanced due to the disruption of the BBB and the fast leakage across the BBB; however, these lesions show a low rCBV, which is in agreement with histological data that demonstrate a lack of neoangiogenesis in lymphomas, mainly when they are highly cellular or there is considerable perivascular or intravascular tumor infiltration.55 Lee IH, Kim ST, Kim HJ, Kim KH, Jeon P, Byun HS. Analysis of perfusion weighted image of CNS lymphoma. Eur J Radiol. 2010;76(1):48-51.
The percentage of signal intensity that is recovered at the end of the first pass of the contrast agent relative to baseline is associated with contrast agent leakage, the size of the intravascular space, and the rate of blood flow. It is shown as a characteristic signal intensity--time curve on DSC-MRI. The mean percentage of signal intensity recovery is high (113.15 ± 41.59) in lymphomas, intermediate in glioblastomas (78.22 ± 14.27), and low in metastases (53.46 ± 12.87). This value is an additional sensitive and specific MRI parameter that can be used to differentiate between malignant brain tumors.66 Mangla R, Kolar B, Zhu T, Zhong J, Almast J, Ekholm S. Percentage signal recovery derived from MR dynamic susceptibility contrast imaging is useful to differentiate common enhancing malignant lesions of the brain. AJNR Am J Neuroradiol. 2011;32(6):1004-10.
Perfusion computed tomography (CT) is available but involves ionizing radiation and a more limited area of the brain. In contrast to MRI, only a few studies have been published on perfusion CT in brain tumors. However, the diagnostic value of perfusion CT in brain tumors may increase in the future due to its advantages related to spatial resolution and the linear correlation between the contrast concentration and the attenuation of the tissue.1515 Cianfoni A, Colosimo C, Basile M, Wintermark M, Bonomo L. Brain perfusion CT: principles, technique and clinical applications. Radiol Med. 2007;112(8):1225-43.
Magnetic resonance spectroscopy
Proton magnetic resonance spectroscopy (MRS) allows for the semiquantitative evaluation of in vivo tissue to measure metabolites, such as N-acetylaspartate (NAA), choline (Cho), creatinine (Cr), lactate (Lac), and lipids (Lip). In PCNSL, MRS has demonstrated elevated Lip and Lac peaks, high Cho/Cr ratios, decreased NAA levels and high Cho/NAA ratios (Figure 1).44 Zacharia TT, Law M, Naidich TP, Leeds NE. Central nervous system lymphoma characterization by diffusion-weighted imaging and MR spectroscopy. J Neuroimaging. 2008;18(4):411-7.,1111 Raizer JJ, Koutcher JA, Abrey LE, Panageas KS, DeAngelis LM, Lis E, et al. Proton magnetic resonance spectroscopy in immunocompetent patients with primary central nervous system lymphoma. J Neurooncol. 2005;71(2):173-80.,1616 Braks E, Urbach H, Pels H, Traber FBlock W,Schild HH. Primary central nervous system immunocytoma: MRI and spectroscopy. Neuroradiology. 2000;42(10):738-41.,1717 Taillibert S, Guillevin R, Menuel C, Sanson M, Hoang-Xuan K, Chiras J, et al. Brain lymphoma: usefulness of the magnetic resonance spectroscopy. J Neurooncol. 2008;86(2):225-9. The presence of Lac or Lip at baseline has been associated with poor progression-free and overall survival.1111 Raizer JJ, Koutcher JA, Abrey LE, Panageas KS, DeAngelis LM, Lis E, et al. Proton magnetic resonance spectroscopy in immunocompetent patients with primary central nervous system lymphoma. J Neurooncol. 2005;71(2):173-80.
Diffuse large B-cell lymphoma in the corpus callosum. An axial T1 image after intravenous gadolinium administration (A) shows a homogeneous enhanced mass in the splenium of the corpus callosum, which is predominantly in the right hemisphere (arrow). An axial apparent diffusion coefficient map (B) confirmed a very low signal intensity in the solid lesion. Note the hyperintensity of the perilesional vasogenic edema. Proton magnetic resonance spectroscopy (C) shows decreased N-acetylaspartate levels and high choline/N-acetylaspartate and choline/creatinine ratios. Note the increased lipid and lactate peaks (0.9–1.3 ppm). A magnetic resonance perfusion sequence (dynamic susceptibility contrast magnetic resonance image T2*) (D) confirmed the absence of neoangiogenesis (low relative cerebral blood volume). Note the high percentage of signal intensity recovery (the ascending part of the curve above the baseline as indicated by the vertical arrowheads).
PCNSL grow rapidly and behave similar to high-grade brain tumors with evidence of high cell membrane turnover on MRS (a high Cho peak), neuronal damage (decreased NAA levels), and anaerobiosis (high lactate levels).44 Zacharia TT, Law M, Naidich TP, Leeds NE. Central nervous system lymphoma characterization by diffusion-weighted imaging and MR spectroscopy. J Neuroimaging. 2008;18(4):411-7.,1111 Raizer JJ, Koutcher JA, Abrey LE, Panageas KS, DeAngelis LM, Lis E, et al. Proton magnetic resonance spectroscopy in immunocompetent patients with primary central nervous system lymphoma. J Neurooncol. 2005;71(2):173-80.,1616 Braks E, Urbach H, Pels H, Traber FBlock W,Schild HH. Primary central nervous system immunocytoma: MRI and spectroscopy. Neuroradiology. 2000;42(10):738-41.,1717 Taillibert S, Guillevin R, Menuel C, Sanson M, Hoang-Xuan K, Chiras J, et al. Brain lymphoma: usefulness of the magnetic resonance spectroscopy. J Neurooncol. 2008;86(2):225-9. These findings are similar to those for high-grade gliomas and metastases; however, the presence of this MRS pattern may help in the differential diagnosis of brain toxoplasmosis in immunocompromised patients, which typically exhibits elevated Lip peaks and no significant increases in Cho or its ratios.11 Haque S, Law M, Abrey LE, Young RJ. Imaging of lymphoma of the central nervous system, spine, and orbit. Radiol Clin North Am. 2008;46(2):339-61, ix.,44 Zacharia TT, Law M, Naidich TP, Leeds NE. Central nervous system lymphoma characterization by diffusion-weighted imaging and MR spectroscopy. J Neuroimaging. 2008;18(4):411-7.,1111 Raizer JJ, Koutcher JA, Abrey LE, Panageas KS, DeAngelis LM, Lis E, et al. Proton magnetic resonance spectroscopy in immunocompetent patients with primary central nervous system lymphoma. J Neurooncol. 2005;71(2):173-80.
Differential diagnosis
The early diagnosis of PCNSL is crucial to define therapeutic procedures. When PCNSL are suspected based on imaging, a tissue biopsy is mandatory and gross tumor resection must be avoided.1818 Ferreri AJ. How I treat primary CNS lymphoma. Blood. 2011;118(13):510-22. The nonconventional MRI features of PCNSL (Table 1) and mimicking brain lesions should be known in order to support the suspicion.
Findings obtained using nonconventional magnetic resonance techniques that are useful for differentiating PCNSL from mimic lesions.
The imaging features of primary central nervous system lymphoma and other focal brain lesions and the differential diagnosis of these lesions
PCNSL is associated with a higher nuclear-to-cytoplasm ratio and a relatively low intratumoral water content, which indicate prominent CT hyperdensity and hypointensity signals in T2-weighted images. These imaging features are important for distinguishing between PCNSL and other focal brain lesions that usually demonstrate a higher water content (hyperintense on T2-weighted images) such as gliomas, metastases, and tumefactive demyelinating lesions.1414 Toh CH, Castillo M, Wong AM, Wei KC, Wong HF, Ng SH, et al. Primary cerebral lymphoma and glioblastoma multiforme: differences in diffusion characteristics evaluated with diffusion tensor imaging. AJNR Am J Neuroradiol. 2008;29(3):471-5.,1919 Zalneraitis EL, Vonsattel JP. Case 26-1998. A 15-year-old girl with hemiparesis, slurred speech, and an intracranial lesion. N Engl J Med. 1998;339(8):542-9.
Neurosarcoidosis rarely presents as a brain tumor-like lesion but exhibits hyposignal intensity on T2-weighted images and disseminates perivascularly with variable contrast enhancement without necrosis. Therefore, the differential diagnosis of PCNSL is crucial.
Metastatic diseases related to melanoma and renal cell, breast, and lung carcinomas are more likely to have blood products than PCNSL. Moreover, in contrast to PCNSL, brain metastases are usually located at the gray-white matter junctions (hematogenously disseminated) and they often incite marked peritumoral edema.11 Haque S, Law M, Abrey LE, Young RJ. Imaging of lymphoma of the central nervous system, spine, and orbit. Radiol Clin North Am. 2008;46(2):339-61, ix.
Several high-grade gliomas may exhibit hyposignal intensity on T2 and often contain blood products with infiltrated margins and heterogeneous contrast enhancement, which makes it difficult to differentiate gliomas from lymphomas. The classical 'mirror image' or 'butterfly pattern' that is due to the symmetrical involvement of the genu or the splenium of the corpus callosum has been observed in PCNSL.1414 Toh CH, Castillo M, Wong AM, Wei KC, Wong HF, Ng SH, et al. Primary cerebral lymphoma and glioblastoma multiforme: differences in diffusion characteristics evaluated with diffusion tensor imaging. AJNR Am J Neuroradiol. 2008;29(3):471-5. Subependymal disease may occur in both PCNSL and high-grade glioma.11 Haque S, Law M, Abrey LE, Young RJ. Imaging of lymphoma of the central nervous system, spine, and orbit. Radiol Clin North Am. 2008;46(2):339-61, ix. PCNSL lesions often have more restricted diffusion and lower ADC values than high-grade gliomas and metastases.88 Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol. 2011;32(6): 984-92.
Peripheral enhancement patterns of PCNSL, including 'ring-like' and 'open ring-like' aspects, must be distinguished from those of classical brain demyelination.1919 Zalneraitis EL, Vonsattel JP. Case 26-1998. A 15-year-old girl with hemiparesis, slurred speech, and an intracranial lesion. N Engl J Med. 1998;339(8):542-9. PCNSL have solid portions with thicker and non-uniform enhancement, which is not restricted to periventricular zones but affects deep gray matter as a single lesion or multiple lesions.2020 Zhang D, Hu LB, Henning TD, Ravarani EM, Zou LG, Feng XY, et al. MRI findings of primary CNS lymphoma in 26 immunocompetent patients. Korean J Radiol. 2010;11(3):269-77. Moreover, high Cho levels and Cho ratios (Cho/NAA > 1.9) on MRS, areas of restricted water molecule diffusion on DWI, the absence of high rCBV levels (<1.75) and a high percentage of signal intensity recovery above the baseline on DSC-MRI would favor a PCNSL diagnosis over other diagnostic possibilities.44 Zacharia TT, Law M, Naidich TP, Leeds NE. Central nervous system lymphoma characterization by diffusion-weighted imaging and MR spectroscopy. J Neuroimaging. 2008;18(4):411-7.,66 Mangla R, Kolar B, Zhu T, Zhong J, Almast J, Ekholm S. Percentage signal recovery derived from MR dynamic susceptibility contrast imaging is useful to differentiate common enhancing malignant lesions of the brain. AJNR Am J Neuroradiol. 2011;32(6):1004-10.,77 Al-Okaili RN, Krejza J, Woo JH, Wolf RL, O’Rourke DM, Judy KD. Intraaxial brain masses: MR imaging-based diagnostic strategy-initial experience. Radiology. 2007;243(2):539-50.,1111 Raizer JJ, Koutcher JA, Abrey LE, Panageas KS, DeAngelis LM, Lis E, et al. Proton magnetic resonance spectroscopy in immunocompetent patients with primary central nervous system lymphoma. J Neurooncol. 2005;71(2):173-80.,1717 Taillibert S, Guillevin R, Menuel C, Sanson M, Hoang-Xuan K, Chiras J, et al. Brain lymphoma: usefulness of the magnetic resonance spectroscopy. J Neurooncol. 2008;86(2):225-9.
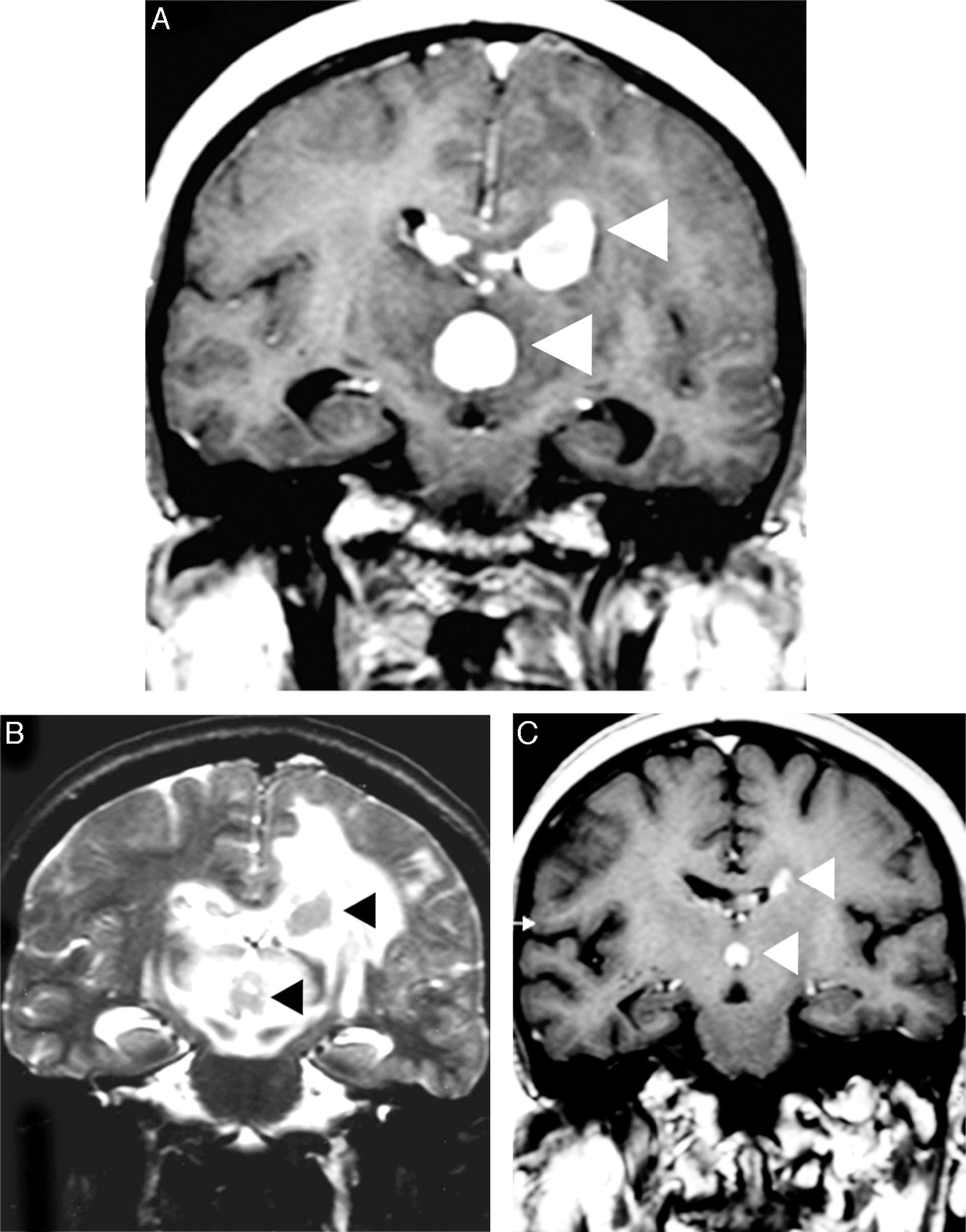
When PCNSL are suspected, corticotherapy should be avoided or discontinued because tumor shrinkage results in radiographic regression in approximately 40% of patients ('the vanishing tumor'), which is suggestive of PCNSL (Figure 2). The detection of a 'vanishing tumor' should not be considered in the diagnosis of PCNSL because sarcoidosis, brain demyelination, acute encephalomyelitis, and other malignancies (more rarely) can exhibit dramatic responses to steroids.33 Iwamoto FM, DeAngelis LM. An update on primary central nervous system lymphoma. Hematol Oncol Clin North Am. 2006;20(6):1267-85.,1818 Ferreri AJ. How I treat primary CNS lymphoma. Blood. 2011;118(13):510-22.
Corticotherapy effects in a primary central nervous system lymphoma. An axial T1 image after intravenous gadolinium administration (A) shows multifocal primary central nervous system lymphoma as deep homogeneous enhanced masses in the corpus callosum, which are adjacent to the third ventricle (arrowheads). A comparative T2-weighted image (B) depicts nodular hypointensity in the lesions with perilesional vasogenic edema (arrowheads). A comparative T1 image after intravenous gadolinium administration (C) was obtained after corticotherapy and demonstrated evident tumor shrinkage ('vanishing tumors').
A DTI analysis may be used to distinguish between PCNSL and glioblastoma. A quantitative analysis of FA and ADC values found that these values were significantly decreased when the solid portions of the tumors were compared with normal-appearing white matter in the contralateral hemisphere. The FA and ADC values in primary cerebral lymphoma were significantly lower than those in glioblastoma.1414 Toh CH, Castillo M, Wong AM, Wei KC, Wong HF, Ng SH, et al. Primary cerebral lymphoma and glioblastoma multiforme: differences in diffusion characteristics evaluated with diffusion tensor imaging. AJNR Am J Neuroradiol. 2008;29(3):471-5.
The MRS findings for PCNSL, high-grade gliomas and tumefactive demyelinating lesions may be similar; therefore, we recommend a careful interpretation of this technique. It is useful to consider all of the conventional and nonconventional features to support the clinical presumption of each disease, to make a histological diagnosis, and to define therapeutic procedures.11 Haque S, Law M, Abrey LE, Young RJ. Imaging of lymphoma of the central nervous system, spine, and orbit. Radiol Clin North Am. 2008;46(2):339-61, ix.,44 Zacharia TT, Law M, Naidich TP, Leeds NE. Central nervous system lymphoma characterization by diffusion-weighted imaging and MR spectroscopy. J Neuroimaging. 2008;18(4):411-7.,66 Mangla R, Kolar B, Zhu T, Zhong J, Almast J, Ekholm S. Percentage signal recovery derived from MR dynamic susceptibility contrast imaging is useful to differentiate common enhancing malignant lesions of the brain. AJNR Am J Neuroradiol. 2011;32(6):1004-10.,77 Al-Okaili RN, Krejza J, Woo JH, Wolf RL, O’Rourke DM, Judy KD. Intraaxial brain masses: MR imaging-based diagnostic strategy-initial experience. Radiology. 2007;243(2):539-50.
Distinguishing between PCNSL and infectious brain lesions is difficult but clinically relevant, particularly in immunocompromised patients. In this setting, nonconventional MRI may be used to differentiate focal brain lesions in PCNSL from neurotoxoplasmosis, cryptococcosis, tuberculosis and intracranial abscesses.2121 Thurnher MM, Rieger A, Kleibl-Popov C, Settinek U, Henk C, Haberler C, et al. Primary central nervous system lymphoma in AIDS: a wider spectrum of CT and MRI findings. Neuroradiology. 2001;43(1):29-35. DWI with ADC maps is useful to distinguish between toxoplasmosis and PCNSL. Early toxoplasmosis may be associated with areas of restricted diffusion on DWI; however, toxoplasmosis lesions have significantly greater diffusion than in PCNSL. ADC ratios that ranged from 1.0 to 1.6 have been reported for both PCNSL and toxoplasmosis, whereas ADC ratios greater than 1.6 were associated solely with toxoplasmosis.1313 Camacho DL, Smith JK, Castill M. Differentiation of toxoplasmosis and lymphoma in AIDS patients by using apparent diffusion coefficients. AJNR Am J Neuroradiol. 2003;24(4):633-7. In addition, toxoplasmosis may be indicated by the presence of Lip and Lac and a decrease in the NAA/Cr ratio on MRS. Conversely, markedly elevated Cho levels would favor a PCNSL diagnosis.44 Zacharia TT, Law M, Naidich TP, Leeds NE. Central nervous system lymphoma characterization by diffusion-weighted imaging and MR spectroscopy. J Neuroimaging. 2008;18(4):411-7.,1111 Raizer JJ, Koutcher JA, Abrey LE, Panageas KS, DeAngelis LM, Lis E, et al. Proton magnetic resonance spectroscopy in immunocompetent patients with primary central nervous system lymphoma. J Neurooncol. 2005;71(2):173-80. The accuracy of MRS is inversely proportional to the extension of necrosis (Figure 3).
A focal brain lesion in an AIDS patient (toxoplasmosis versus lymphoma). An axial T1 image after intravenous gadolinium administration (A) shows a large periventricular lesion in the left thalamus with extensive necrosis (asterisk) and thick peripheral enhancement. An axial apparent diffusion coefficient map (B) confirmed a very low signal intensity in the solid portion of the lesion (arrowheads) compared with the central area of necrosis (asterisk). Additionally, note the hyperintensity of the perilesional vasogenic edema. Proton magnetic resonance spectroscopy (C) confirmed the presence of elevated lipid and lactate (0.9–1.3 ppm) and choline levels with reduced N-acetylaspartate levels. A magnetic resonance perfusion sequence (dynamic susceptibility contrast magnetic resonance image T2*) (D) confirmed the absence of neoangiogenesis (low relative cerebral blood volume). These features supported the diagnosis of lymphoma.
The conventional and nonconventional MRI and pathological features of CNS post-transplantation lymphoproliferative disorder (PTLD) are similar to those of PCNSL and have been observed in immunocompromised patients.2222 Buell JF, Gross TG, Hanaway MJ, Trofe J, Roy-Chaudhury P,First MR, et al. Posttransplant lymphoproliferative disorder: significance of central nervous system involvement. Transplant Proc. 2005;37(2):954-5.–2424 Senzolo M, Ferronato C, Burra P.Neurologic complications after solid organ transplantation. Transpl Int. 2009;22(3):269-78. An imaging pattern that is associated with CNS PTLD includes peripherally enhancing intraparenchymal lesions, which are usually multifocal supratentorial masses that may extend to the ependymal surface leptomeninges with nodular enhancement. Imaging plays a crucial role in diagnosing disease, guiding biopsy and monitoring disease responses following therapy.2525 Lake W,Chang JE, Kennedy T, Morgan A, Salamat S, Baskaya M. A case series of primary central nervous system post-transplant lymphoproliferative disorder: imaging and clinical characteristics. Neurosurgery. 2013;72(6):960-70 [discussion 970].
After solid organ transplantation, patients are at risk of developing several neurological complications, including neurotoxicity due to immunosuppressive drugs, seizures, encephalopathy, stroke, opportunistic infections, and PTLD.2424 Senzolo M, Ferronato C, Burra P.Neurologic complications after solid organ transplantation. Transpl Int. 2009;22(3):269-78. Because of immunosuppression, transplant patients are at risk for developing several CNS infections that may lead to meningitis, encephalitis, and focal abscesses. Several CNS infections share many overlapping imaging features with PTLD, particularly in the first months after transplantation. Nonconventional MRI helps the prompt diagnosis of disease, the guidance of biopsy and the initiation of appropriate treatment.
Peculiar presentations of primary central nervous system lymphomas
Leptomeningeal infiltration in the course of lymphoma, particularly in systemic disease, is not rare. However, this imaging appearance is nonspecific and may alternatively occur after CNS invasion by infection or in several other primary and systemic malignancies (meningeal carcinomatosis). Associated periventricular masses, mainly with subependymal infiltration, may corroborate a diagnosis of CNS lymphoma in a differential diagnosis.11 Haque S, Law M, Abrey LE, Young RJ. Imaging of lymphoma of the central nervous system, spine, and orbit. Radiol Clin North Am. 2008;46(2):339-61, ix. Dural-based lymphoma, particularly the mucosa-associated lymphoid tissue (MALT) subtype, mimics meningiomas; therefore, a careful interpretation of imaging findings in the clinical scenario is recommended. Few reports have described the typical presentation of intracranial MALT lymphoma-mimicking globular meningiomas (Figure 4).11 Haque S, Law M, Abrey LE, Young RJ. Imaging of lymphoma of the central nervous system, spine, and orbit. Radiol Clin North Am. 2008;46(2):339-61, ix.,2626 Iwamoto FM, Abrey LE. Primary dural lymphomas: a review. Neurosurg Focus. 2006;21(5):E5.–2828 Abboud H, Carpentier A, Martin-Duverneuil N, Kujas M, Hoang-Xuan K. MALT lymphoma presenting as a meningioma. J Neurooncol. 2005;75(2):221.
Mucosa-associated lymphoid tissue (MALT) lymphoma mimicking meningioma. A sagittal T2-weighted image (A) shows a solid extra-axial lesion that infiltrated the dura mater in the parietal region (arrowheads). A comparative image on a T1-weighted sequence after intravenous gadolinium administration (B) confirmed homogeneous contrast enhancement that was associated with an extensive dura tail, which mimicked the appearance of a meningioma (arrowhead).
The diagnostic possibility of a cranial vault lymphoma is greater when the lesion involves the scalp, the skull bone, and the pachymeninges, mainly in the absence of bone erosion (Figure 5). However, this pattern of lesion is not specific to PCNSL because it has been observed in systemic lymphoma and in many mimic lesions, including metastatic infiltration from prostate carcinoma and breast cancer, osteomyelitis, meningioma, plasma cell tumors, and histiocytosis.2929 da Rocha AJ, da Rocha TM, da Silva CJ, Paes RP, Bruniera P, Chiattone CS. Cranial vault lymphoma: a systematic review of five patients. J Neurooncol. 2010;100(1):9-15.
A cranial vault lymphoma. An axial T2-weighted image (A) shows a small multicompartmental lesion in the occipitoparietal region (arrow). Axial diffusion-weighted imaging (B) shows restricted water molecule diffusion in the solid portions of the lesion. A comparative T1 image after intravenous gadolinium administration (C) shows an enhanced lesion that involves the scalp, the skull bone, and the pachymeninges and extends to the leptomeninges (arrowhead). A non-enhanced computed tomography scan (D) confirmed the absence of bone erosion.
A non-enhancing presentation of PCNSL was found in 1% of patients in a large series of immunocompetent patients and was documented in immunocompromised patients.2121 Thurnher MM, Rieger A, Kleibl-Popov C, Settinek U, Henk C, Haberler C, et al. Primary central nervous system lymphoma in AIDS: a wider spectrum of CT and MRI findings. Neuroradiology. 2001;43(1):29-35.,3030 Kuker W,Nagele T, Korfel A, Heckl S, Thiel E, Bamberg M, et al. Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients. J Neurooncol. 2005;72(2):169-77.,3131 Lachenmayer ML, Blasius E, Niehusmann P, Kovacs A, Stuplich M, Eichler O, et al. Non-enhancing primary CNS lymphoma. J Neurooncol. 2011;101(2):343-4. 'Lymphomatosis cerebri' is frequently misdiagnosed as diffuse nonspecific leukoencephalopathy, Binswanger's disease (subcortical ischemic vascular dementia), infectious encephalomyelitis including progressive multifocal leukoencephalopathy, toxic or metabolic abnormalities, unknown autoimmune diseases, or neoplasms such as gliomatosis cerebri.99 Haldorsen IS, Krakenes J, Krossnes BK, Mella O, Espeland A. CT and MR imaging features of primary central nervous system lymphoma in Norway, 1989-2003. AJNR Am J Neuroradiol. 2009;30(4):744-51.,3232 Bakshi R, Mazziotta JC, Mischel PS, Jahan R, Seligson DB, Vinters HV. Lymphomatosis cerebri presenting as a rapidly progressive dementia: clinical, neuroimaging and pathologic findings. Dement Geriatr Cogn Disord. 1999;10(2):152-7.–3535 de Toledo M, Lopez-Valdes E, Ferreiro M, Cervera JL, Ramos A, Cabello A, et al. Lymphomatosis cerebri as the cause of leukoencephalopathy. Rev Neurol. 2008;46(11):667-70. DWI and MRS may support a presumptive diagnosis of brain infiltrative neoplasia; however, an accurate diagnosis is only possible after a brain biopsy.3636 Fischer L, Koch A, Schlegel U, Koch HC, Wenzel R, Schroder N, et al. Non-enhancing relapse of a primary CNS lymphoma with multiple diffusion-restricted lesions. J Neurooncol. 2011;102(1):163-6. MRS may show increased Cho levels and Cho ratios (Cho/NAA > 1.9), which suggest infiltrative neoplastic disease (Figure 6).3535 de Toledo M, Lopez-Valdes E, Ferreiro M, Cervera JL, Ramos A, Cabello A, et al. Lymphomatosis cerebri as the cause of leukoencephalopathy. Rev Neurol. 2008;46(11):667-70. DWI is variable and can depict heterogeneous areas with restricted water molecule movement. Perfusion MRI techniques are not helpful because the rCBV is not increased and there is no abnormal permeability of the BBB.
Lymphomatosis cerebri in an immunocompetent patient. An axial fluid-attenuated inversion recovery image (A) shows unspecific diffuse hyperintense areas on both brain hemispheres that cross the splenium of the corpus callosum with no mass effect. No contrast enhancement was observed (not shown). A comparative fluid-attenuated inversion recovery image (B) was obtained after two months without specific therapy confirming the extensive progression of the disease, and exhibiting confluent hyperintensity of both brain hemispheres, predominantly of the left. Minimal mass effect and no gadolinium-enhanced areas were observed. A proton magnetic resonance spectroscopy study (C) was useful to support a presumptive diagnosis and demonstrated increased choline levels and an increased choline/N-acetylaspartate ratio (>1.9), which were associated with reduced N-acetylaspartate levels and elevated lactate peaks.
Intravascular lymphoma is often misdiagnosed as multifocal demyelination, septic encephalopathy, infectious lesions, or multifocal and recurrent strokes due to embolic diseases or CNS vasculitis (systemic or primary angiitis).3434 Moussouttas M. Intravascular lymphomatosis presenting as posterior leukoencephalopathy. Arch Neurol. 2002;59(4):640-1.,3737 Beristain X, Azzarelli B. The neurological masquerade of intravascular lymphomatosis. Arch Neurol. 2002;59(3):439-43. Cerebral angiography may reveal multivessel segmental stenoses and dilatations ('bead-like pattern') that are consistent with vasculitis.3838 Song DK, Boulis NM, McKeever PE, Quint DJ. Angiotropic large cell lymphoma with imaging characteristics of CNS vasculitis. AJNR Am J Neuroradiol. 2002;23(2):239-42. Intravascular lymphoma may follow a relapsing-remitting or relapsing-progressive clinical course due to fluctuating ischemic damage caused by the small vascular occlusions.11 Haque S, Law M, Abrey LE, Young RJ. Imaging of lymphoma of the central nervous system, spine, and orbit. Radiol Clin North Am. 2008;46(2):339-61, ix.,3737 Beristain X, Azzarelli B. The neurological masquerade of intravascular lymphomatosis. Arch Neurol. 2002;59(3):439-43. A correct presumptive diagnosis may be supported by persistent and progressive DWI abnormalities that are associated with variable gadolinium enhancement.3636 Fischer L, Koch A, Schlegel U, Koch HC, Wenzel R, Schroder N, et al. Non-enhancing relapse of a primary CNS lymphoma with multiple diffusion-restricted lesions. J Neurooncol. 2011;102(1):163-6. An extensive imaging investigation is not enough to confirm intravascular lymphoma, and an early brain biopsy is of paramount importance to achieve a definitive diagnosis and to define therapeutic procedures.3737 Beristain X, Azzarelli B. The neurological masquerade of intravascular lymphomatosis. Arch Neurol. 2002;59(3):439-43.,3939 Lozsadi DA, Wieshmann U, Enevoldson TP. Neurological presentation of intravascular lymphoma: report of two cases and discussion of diagnostic challenges. Eur J Neurol. 2005;12(9):710-4.,4040 Konikkara JJ, Perurena OH, Warach S, Bauserman SC. A 62-year-old man with fluctuating neurological deficits and skin lesions. JAMA Neurol. 2013;70(1):120-4.
When primary intraocular lymphomas are considered, the imaging features are not specific and are frequently inconclusive. Conversely, the imaging features of orbital lymphomas often mimic other diseases. These diseases include metastatic disease, inflammatory pseudotumors, sarcoidosis, histiocytosis including Erdheim-Chester disease, meningiomas, cavernous hemangioma, ocular melanomas, and primary lacrimal gland lesions.11 Haque S, Law M, Abrey LE, Young RJ. Imaging of lymphoma of the central nervous system, spine, and orbit. Radiol Clin North Am. 2008;46(2):339-61, ix.,4141 McKelvie PA. Ocular adnexal lymphomas: a review. Adv Anat Pathol. 2010;17(4):251-61. In contrast to orbital pseudotumors, primary orbital lesions are painless.4242 Yan J, Wu Z, Li Y.The differentiation of idiopathic inflammatory pseudotumor from lymphoid tumors of orbit: analysis of 319 cases. Orbit. 2004;23(4):245-54.
A DWI analysis is useful to distinguish between lymphomas and idiopathic orbital inflammatory pseudotumors, optic nerve sheath meningiomas and gliomas.4343 Fatima Z, Ichikawa T, Ishigame K, Motosugi U, Waqar AB, Hori M, et al. Orbital masses: the usefulness of diffusion-weighted imaging in lesion categorization. Clin Neuroradiol. 2014;24(2):129-34.,4444 Sepahdari AR, Politi LS, Aakalu VK, Kim HJ, Abdel Razek AA. Diffusion-weighted imaging of orbital masses: multi-institutional data support a 2-ADC Threshold model to categorize lesions as benign, malignant, or indeterminate. AJNR Am J Neuroradiol. 2014;35(1):170-5. The superior lateral quadrant and the extraconal space are typical locations of orbital lymphomas. An intriguing pattern is characterized by the infiltrative behavior of the tumor, which shapes the contour of the eyeball and typically crosses the bony limits. Osteolysis may not be present. Effective treatment responses were observed in the follow-up imaging of orbital lymphomas; however, several cases of local relapse were found.4545 Priego G, Majos C, Climent FMuntane A. Orbital lymphoma: imaging features and differential diagnosis Insights Imaging. 2012;3(4):337-44.
Neurolymphomatosis is extremely rare; therefore, this diagnostic possibility is often overlooked. This disease is often misdiagnosed as chronic inflammatory demyelinating polyneuropathy. The possibility of concomitant lymphoma arises when thickened cranial nerves, cauda equinae roots and/or peripheral nerves are demonstrated, particularly on MRI. Neurolymphomatosis should be considered in various types of neuropathy even when the diagnostic criteria for chronic inflammatory demyelinating polyneuropathy are met, particularly in patients who complain of pain.4646 Tomita M, Koike H, Kawagashira Y Iijima M, Adachi H, Taguchi J, et al. Clinicopathological features of neuropathy associated with lymphoma. Brain. 2013;136 Pt 8:2563-78.
Metastatic tumors, including systemic lymphoma, may affect the leptomeningeal compartment with cranial nerve or root thickening and variable contrast enhancement that mimics the imaging appearance of neurolymphomatosis. Additionally, hypertrophy of the cauda equina nerve roots is a nonspecific finding and may be observed in hereditary and acquired demyelinating neuropathies or in secondary neoplasias due to infiltration of the nerve roots by the neoplasm. Hypertrophy of the cauda equina nerve roots has been described as a likely paraneoplastic phenomenon in an elderly patient with lymphoplasmacytic lymphoma.4747 Kumar N, Dyck PJ. Hypertrophy of the nerve roots of the cauda equina as a paraneoplastic manifestation of lymphoma. Arch Neurol. 2005;62(11):1776-7. In certain circumstances, the imaging pattern of neurofibromatosis may mimic the pattern of neurolymphomatosis; however, understanding the systemic features of this neurocutaneous syndrome will be useful to make an accurate diagnosis.
Primary lymphoma is a rare cause of myelopathies. Spinal MRI has demonstrated multifocal lesions with typical homogeneous gadolinium enhancement. Conus medullaris or cauda equina involvement is characteristic of primary intramedullary spinal cord lymphoma; however, a pathological confirmation often requires a CNS biopsy.4848 Flanagan EP, O’Neill BP, Porter AB, Lanzino G, Haberman TM, Keegan BM. Primary intramedullary spinal cord lymphoma. Neurology. 2011;77(8):784-91.,4949 Nakamizo T, Inoue H, Udaka F Oda M, Kawai M, Uemura K, et al. Magnetic resonance imaging of primary spinal intramedullary lymphoma. J Neuroimaging. 2002;12(2): 183-6. Lymphoma of the spine may often occur in the bony, extradural-epidural, intradural extramedullary, or intradural intramedullary compartments and may mimic several different diseases. These diseases include metastatic tumors, infectious diseases particularly tuberculosis and epidural empyema, and inflammatory conditions, such as sarcoidosis and demyelinating lesions.
Conclusions
PCNSL are distinctive and rare presentations of certain subtypes of lymphoma, generally the non-Hodgkin diffuse large B-cell type, and contrast-enhanced MRI is the imaging technique of choice to evaluate most patients and their variable presentations.
Modern neuroimaging techniques may help us suspect PCNSL earlier, which would result in timely treatment and less CNS damage. Radiologists and hematologists must be familiar with the role of these new advanced techniques, which may help in the differentiation of PCNSL and other mimic lesions.
REFERENCES
-
1Haque S, Law M, Abrey LE, Young RJ. Imaging of lymphoma of the central nervous system, spine, and orbit. Radiol Clin North Am. 2008;46(2):339-61, ix.
-
2Baehring JM, Longtine J, Hochberg FH. A new approach to the diagnosis and treatment of intravascular lymphoma. J Neurooncol. 2003;61(3):237-48.
-
3Iwamoto FM, DeAngelis LM. An update on primary central nervous system lymphoma. Hematol Oncol Clin North Am. 2006;20(6):1267-85.
-
4Zacharia TT, Law M, Naidich TP, Leeds NE. Central nervous system lymphoma characterization by diffusion-weighted imaging and MR spectroscopy. J Neuroimaging. 2008;18(4):411-7.
-
5Lee IH, Kim ST, Kim HJ, Kim KH, Jeon P, Byun HS. Analysis of perfusion weighted image of CNS lymphoma. Eur J Radiol. 2010;76(1):48-51.
-
6Mangla R, Kolar B, Zhu T, Zhong J, Almast J, Ekholm S. Percentage signal recovery derived from MR dynamic susceptibility contrast imaging is useful to differentiate common enhancing malignant lesions of the brain. AJNR Am J Neuroradiol. 2011;32(6):1004-10.
-
7Al-Okaili RN, Krejza J, Woo JH, Wolf RL, O’Rourke DM, Judy KD. Intraaxial brain masses: MR imaging-based diagnostic strategy-initial experience. Radiology. 2007;243(2):539-50.
-
8Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol. 2011;32(6): 984-92.
-
9Haldorsen IS, Krakenes J, Krossnes BK, Mella O, Espeland A. CT and MR imaging features of primary central nervous system lymphoma in Norway, 1989-2003. AJNR Am J Neuroradiol. 2009;30(4):744-51.
-
10Norden AD, Drappatz J, Wen PY, Claus EB. Survival among patients with primar central nervous system lymphoma, 1973-2004. J Neurooncol. 2011;101(3):487-93.
-
11Raizer JJ, Koutcher JA, Abrey LE, Panageas KS, DeAngelis LM, Lis E, et al. Proton magnetic resonance spectroscopy in immunocompetent patients with primary central nervous system lymphoma. J Neurooncol. 2005;71(2):173-80.
-
12Barajas RF Jr, Rubenstein JL, Chang JS, Hwang J, Cha S. Diffusion-weighted MR imaging derived apparent diffusion coefficient is predictive of clinical outcome in primary central nervous system lymphoma. AJNR Am J Neuroradiol. 2010;31(1):60-6.
-
13Camacho DL, Smith JK, Castill M. Differentiation of toxoplasmosis and lymphoma in AIDS patients by using apparent diffusion coefficients. AJNR Am J Neuroradiol. 2003;24(4):633-7.
-
14Toh CH, Castillo M, Wong AM, Wei KC, Wong HF, Ng SH, et al. Primary cerebral lymphoma and glioblastoma multiforme: differences in diffusion characteristics evaluated with diffusion tensor imaging. AJNR Am J Neuroradiol. 2008;29(3):471-5.
-
15Cianfoni A, Colosimo C, Basile M, Wintermark M, Bonomo L. Brain perfusion CT: principles, technique and clinical applications. Radiol Med. 2007;112(8):1225-43.
-
16Braks E, Urbach H, Pels H, Traber FBlock W,Schild HH. Primary central nervous system immunocytoma: MRI and spectroscopy. Neuroradiology. 2000;42(10):738-41.
-
17Taillibert S, Guillevin R, Menuel C, Sanson M, Hoang-Xuan K, Chiras J, et al. Brain lymphoma: usefulness of the magnetic resonance spectroscopy. J Neurooncol. 2008;86(2):225-9.
-
18Ferreri AJ. How I treat primary CNS lymphoma. Blood. 2011;118(13):510-22.
-
19Zalneraitis EL, Vonsattel JP. Case 26-1998. A 15-year-old girl with hemiparesis, slurred speech, and an intracranial lesion. N Engl J Med. 1998;339(8):542-9.
-
20Zhang D, Hu LB, Henning TD, Ravarani EM, Zou LG, Feng XY, et al. MRI findings of primary CNS lymphoma in 26 immunocompetent patients. Korean J Radiol. 2010;11(3):269-77.
-
21Thurnher MM, Rieger A, Kleibl-Popov C, Settinek U, Henk C, Haberler C, et al. Primary central nervous system lymphoma in AIDS: a wider spectrum of CT and MRI findings. Neuroradiology. 2001;43(1):29-35.
-
22Buell JF, Gross TG, Hanaway MJ, Trofe J, Roy-Chaudhury P,First MR, et al. Posttransplant lymphoproliferative disorder: significance of central nervous system involvement. Transplant Proc. 2005;37(2):954-5.
-
23Cavaliere R, Petroni G, Lopes MB, Schiff D, International Primary Central Nervous System Lymphoma Collaborative G. Primary central nervous system post-transplantation lymphoproliferative disorder: an International Primary Central Nervou System Lymphoma Collaborativ Group Report. Cancer. 2010;116(4):863-70.
-
24Senzolo M, Ferronato C, Burra P.Neurologic complications after solid organ transplantation. Transpl Int. 2009;22(3):269-78.
-
25Lake W,Chang JE, Kennedy T, Morgan A, Salamat S, Baskaya M. A case series of primary central nervous system post-transplant lymphoproliferative disorder: imaging and clinical characteristics. Neurosurgery. 2013;72(6):960-70 [discussion 970].
-
26Iwamoto FM, Abrey LE. Primary dural lymphomas: a review. Neurosurg Focus. 2006;21(5):E5.
-
27Rottnek M, Strauchen J, Moore F, Morgello S. Primary dural mucosa-associated lymphoid tissue-type lymphoma: case report and review of the literature. J Neurooncol. 2004;68(1):19-23.
-
28Abboud H, Carpentier A, Martin-Duverneuil N, Kujas M, Hoang-Xuan K. MALT lymphoma presenting as a meningioma. J Neurooncol. 2005;75(2):221.
-
29da Rocha AJ, da Rocha TM, da Silva CJ, Paes RP, Bruniera P, Chiattone CS. Cranial vault lymphoma: a systematic review of five patients. J Neurooncol. 2010;100(1):9-15.
-
30Kuker W,Nagele T, Korfel A, Heckl S, Thiel E, Bamberg M, et al. Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients. J Neurooncol. 2005;72(2):169-77.
-
31Lachenmayer ML, Blasius E, Niehusmann P, Kovacs A, Stuplich M, Eichler O, et al. Non-enhancing primary CNS lymphoma. J Neurooncol. 2011;101(2):343-4.
-
32Bakshi R, Mazziotta JC, Mischel PS, Jahan R, Seligson DB, Vinters HV. Lymphomatosis cerebri presenting as a rapidly progressive dementia: clinical, neuroimaging and pathologic findings. Dement Geriatr Cogn Disord. 1999;10(2):152-7.
-
33Kanai R, Shibuya M, Hata T, Hori M, Hirabayashi K, Terada T, et al. A case of 'lymphomatosis cerebri’ diagnosed in an early phase and treated by whole brain radiation: case report and literature review. J Neurooncol. 2008;86(1):83-8.
-
34Moussouttas M. Intravascular lymphomatosis presenting as posterior leukoencephalopathy. Arch Neurol. 2002;59(4):640-1.
-
35de Toledo M, Lopez-Valdes E, Ferreiro M, Cervera JL, Ramos A, Cabello A, et al. Lymphomatosis cerebri as the cause of leukoencephalopathy. Rev Neurol. 2008;46(11):667-70.
-
36Fischer L, Koch A, Schlegel U, Koch HC, Wenzel R, Schroder N, et al. Non-enhancing relapse of a primary CNS lymphoma with multiple diffusion-restricted lesions. J Neurooncol. 2011;102(1):163-6.
-
37Beristain X, Azzarelli B. The neurological masquerade of intravascular lymphomatosis. Arch Neurol. 2002;59(3):439-43.
-
38Song DK, Boulis NM, McKeever PE, Quint DJ. Angiotropic large cell lymphoma with imaging characteristics of CNS vasculitis. AJNR Am J Neuroradiol. 2002;23(2):239-42.
-
39Lozsadi DA, Wieshmann U, Enevoldson TP. Neurological presentation of intravascular lymphoma: report of two cases and discussion of diagnostic challenges. Eur J Neurol. 2005;12(9):710-4.
-
40Konikkara JJ, Perurena OH, Warach S, Bauserman SC. A 62-year-old man with fluctuating neurological deficits and skin lesions. JAMA Neurol. 2013;70(1):120-4.
-
41McKelvie PA. Ocular adnexal lymphomas: a review. Adv Anat Pathol. 2010;17(4):251-61.
-
42Yan J, Wu Z, Li Y.The differentiation of idiopathic inflammatory pseudotumor from lymphoid tumors of orbit: analysis of 319 cases. Orbit. 2004;23(4):245-54.
-
43Fatima Z, Ichikawa T, Ishigame K, Motosugi U, Waqar AB, Hori M, et al. Orbital masses: the usefulness of diffusion-weighted imaging in lesion categorization. Clin Neuroradiol. 2014;24(2):129-34.
-
44Sepahdari AR, Politi LS, Aakalu VK, Kim HJ, Abdel Razek AA. Diffusion-weighted imaging of orbital masses: multi-institutional data support a 2-ADC Threshold model to categorize lesions as benign, malignant, or indeterminate. AJNR Am J Neuroradiol. 2014;35(1):170-5.
-
45Priego G, Majos C, Climent FMuntane A. Orbital lymphoma: imaging features and differential diagnosis Insights Imaging. 2012;3(4):337-44.
-
46Tomita M, Koike H, Kawagashira Y Iijima M, Adachi H, Taguchi J, et al. Clinicopathological features of neuropathy associated with lymphoma. Brain. 2013;136 Pt 8:2563-78.
-
47Kumar N, Dyck PJ. Hypertrophy of the nerve roots of the cauda equina as a paraneoplastic manifestation of lymphoma. Arch Neurol. 2005;62(11):1776-7.
-
48Flanagan EP, O’Neill BP, Porter AB, Lanzino G, Haberman TM, Keegan BM. Primary intramedullary spinal cord lymphoma. Neurology. 2011;77(8):784-91.
-
49Nakamizo T, Inoue H, Udaka F Oda M, Kawai M, Uemura K, et al. Magnetic resonance imaging of primary spinal intramedullary lymphoma. J Neuroimaging. 2002;12(2): 183-6.
Publication Dates
-
Publication in this collection
Jan-Feb 2016
History
-
Received
1 July 2014 -
Accepted
30 Nov 2015