Abstract
Polymerization shrinkage is a critical factor affecting the longevity and acceptability of dental composite resins. The aim of this work was to evaluate the effect of light intensity and irradiation time on the polymerization process of a photo cured dental composite resin by measuring the Vickers hardness number (VHN) and the volumetric polymerization shrinkage. Samples were prepared using a dental manual light-curing unit. The samples were submitted to irradiation times of 5, 10, 20 and 40 s, using 200 and 400 mW.cm-2 light intensities. Vickers hardness number was obtained at four different moments after photoactivation (immediate, 1 h, 24 h and 168 h). After this, volumetric polymerization shrinkage values were obtained through a specific density method. The values were analyzed by ANOVA and Duncan's (p = 0.05). Results showed increase in hardness values from the immediate reading to 1 h and 24 h readings. After 24 h no changes were observed regardless the light intensities or activation times. The hardness values were always smaller for the 200 mW.cm-2 light intensity, except for the 40 s irradiation time. No significant differences were detected in volumetric polymerization shrinkage considering the light intensity (p = 0.539) and the activation time (p = 0.637) factors. In conclusion the polymerization of the material does not terminate immediately after photoactivation and the increase of irradiation time can compensate a lower light intensity. Different combinations between light intensity and irradiation time, i.e., different amounts of energy given to the system, have not affected the polymerization shrinkage.
photoactivation; irradiation time; light intensity; polymerization shrinkage; hardness
ARTICLES PRESENTED AT THE XV CBECIMAT, NATAL - RN, NOVEMBER DE 2002
Effect of light intensity and irradiation time on the polymerization process of a dental composite resin
José Augusto César DiscacciatiII, * * e-mail: jacdiscacciati@uol.com.br ; Alisson Discacciati NevesI; Rodrigo Lambert OréficeIII; Flávio Juliano Garcia Santos PimentaII; Herbert Haueisen SanderII
IDepartment of Dentistry - UNIMONTES, Brazil
IIDental School, Federal University of Minas Gerais - MG, Brazil
IIIDepart. of Metallurgical and Materials Engineering Federal University of Minas Gerais - Brazil
ABSTRACT
Polymerization shrinkage is a critical factor affecting the longevity and acceptability of dental composite resins. The aim of this work was to evaluate the effect of light intensity and irradiation time on the polymerization process of a photo cured dental composite resin by measuring the Vickers hardness number (VHN) and the volumetric polymerization shrinkage. Samples were prepared using a dental manual light-curing unit. The samples were submitted to irradiation times of 5, 10, 20 and 40 s, using 200 and 400 mW.cm-2 light intensities. Vickers hardness number was obtained at four different moments after photoactivation (immediate, 1 h, 24 h and 168 h). After this, volumetric polymerization shrinkage values were obtained through a specific density method. The values were analyzed by ANOVA and Duncan's (p = 0.05). Results showed increase in hardness values from the immediate reading to 1 h and 24 h readings. After 24 h no changes were observed regardless the light intensities or activation times. The hardness values were always smaller for the 200 mW.cm-2 light intensity, except for the 40 s irradiation time. No significant differences were detected in volumetric polymerization shrinkage considering the light intensity (p = 0.539) and the activation time (p = 0.637) factors. In conclusion the polymerization of the material does not terminate immediately after photoactivation and the increase of irradiation time can compensate a lower light intensity. Different combinations between light intensity and irradiation time, i.e., different amounts of energy given to the system, have not affected the polymerization shrinkage.
Keywords: photoactivation, irradiation time, light intensity, polymerization shrinkage, hardness
1. Introduction
An important advance in the dental composite resin field has been observed in the last years. The incorporation of higher contents of filler and the inclusion of multifunctional monomers have resulted in materials with higher mechanical properties1. The polymerization of methacrylate monomers is a complex process that forms a copolymeric network2. Monomer molecules are first incorporated into polymer chains as units containing pendant bonds. Further propagation can proceed by addition of the next monomer molecule and by intramolecular or intermolecular attack of the radical site on the pendant double bond. Intramolecular attack leads to a cyclization, whereas intermolecular leads to network formation. The apparent reactivity of pendant double bonds on the same chain is initially enhanced as compared to monomeric double bonds due to their larger concentration in the vicinity of the radical site. This leads to extensive cyclization and formation of compact structures - microgels. These compact, internally crosslinked molecules formed at the beginning of polymerization causes a delay in the gel point conversion. Many of the unreacted pendant double bonds become entrapped in the microgel regions and their apparent reactivity decreases due to steric hindrance. In densely crosslinked poly(dimethacrylates), trapped free radicals are stable even in the presence of large amounts of unreacted double bonds. This low degree of monomer conversion can affect the mechanical properties and chemical stability of the polymer network. Further reaction (macrogelation) may occur by the chemical joining of microgel particles3.
Light activated dental composite resins are now the most widely used restorative material. The main advantage of this activation mode over the chemical one is the control the operator has over the working time. However, a definite amount of energy, which is defined as power (light intensity) multiplied by photoactivation time, is necessary to obtain the optimal degree of monomer conversion and properties4. Moreover, the way this energy is delivered to the material has been recently proposed as a potential method to manipulate the progress of the polymerization reaction and the properties of the system5,6, i.e., monomer conversion, polymerization shrinkage and mechanical properties could possibly be tailored by altering photoactivation parameters such as light intensity and irradiation time.
Monomer conversion often results in a change in volume during the polymerization. For SAKAGUCHI et al.7, this post-gel shrinkage is the major problem in the clinical use of composites. Gaps may occur at the interface between the restoration and the remaining tooth structure when the enamel or dentine bond strength is inadequate to withstand the polymerization contractile stresses. This phenomenon may cause post-operative pain, marginal microleakage and secondary caries, which are the predominant reason for replacement of composite resin restorations8-10. If the bond strength is adequate, the contraction stress is transmitted to the remaining tooth structure and may result in enamel microcracks and cuspal deflection7.
The use of high light intensity units has been recommended almost universally5, since they would be able to enhance monomer conversion. Conversely, some authors do not indicate the use of high light intensity units because they believe that this type of units induce higher polymerization shrinkage and larger residual stress11-14.
Many methods have been studied aiming to reduce the effects of the polymerization shrinkage, such as: development of resins that do not shrink when they polymerize15 or that expand through a double ring open process16, and the use of the incremental filling technique17-19. Recently, other studies have evaluated the use of low light intensity units aiming to promote a smaller shrinkage stress polymerization2,4,6,11-14,20-24.
The best activation method is probably the one that promotes as little polymerization shrinkage as possible, and consequently the lowest shrinkage stress value, and that does not affect the integrity of the tooth and the mechanical properties of the material. Considering that a better understanding of the kinetics of the polymerization and consequently of the shrinkage stress is still necessary, the aim of this study was to monitor the polymerization process of a photo cured dental composite resin by measuring the Vickers hardness after the end of the photoactivation period, and the volumetric polymerization shrinkage, as a function of different light intensities and irradiation times.
2. Materials and Methods
A photo cured dental composite resin based on the combination of Bis-GMA, UDMA and Bis-EMA, containing 60 vol% of inorganic filler, was used in this study (commercial name: Filtek Z250 - shade B2, batch no 1370, from 3M, St. Paul, MN, USA). A manual light-curing unit (Ultralux Electronic, Dabi Atlante, Ribeirão Preto, SP, Brazil), equipped with one halogen lamp (75 watts), was used for the photoactivation.
Samples with dimensions of 10 mm in diameter and 1 mm in thickness were prepared in a stainless steel o-ring placed between two glass slides. To ensure that the composite resin would be well distributed within the mold, 0.5 Kgf was applied for 30 s to the material. Glass slides were used to prevent inhibition of surface polymerization due to the presence of oxygen. The samples were then photo-cured from the upper surface being submitted to irradiation times of 5, 10, 20 and 40 s, using 200 and 400 mW.cm-2 light intensities.
Considering constant the size of each specimen, the amount of energy (En, given in Joules) given to the system in each different combination was calculated by the product of the light intensity (LI) and the irradiation time (IT).
Before each photoactivation, the unit was maintained connected by 40 s to stabilize the light intensity, always checked using a radiometer (Demetron 100, Demetron, Danbury, CT, USA). After being removed from the mold, the samples were stored in dark and dry conditions at 37 °C. Vickers hardness number (VHN) was measured by indenting the lower surfaces of the samples at a load of 0.5 kgf for 15 s with a Microhardness Tester (Future Tech Corp., Tokyo, Japan). Hardness values were obtained at four different moments after photoactivation (immediate, 1 h, 24 h and 168 h). For the immediate indentation, the VHN tester was turned on one minute after the beginning of the photoactivation. For each sample, four subsequent measurements were made. After the last Vickers hardness number analysis, volumetric polymerization shrinkage was measured through a specific density method25. The specific gravity was calculated using the following relationship:
where: SPgr = specific gravity; A = weight of the disc in air; B = weight of the disc in water.
The volumetric shrinkage was calculated using the following relationship:
Samples were divided into eight groups according to the combination of two light intensities and four irradiation times. For all groups, the average values and standard deviations (SD) of four replications were calculated. The values were compared by two and three-way factorial analysis of variance (ANOVA) and Duncan's comparison test with significance of (p = 0.05).
3. Results
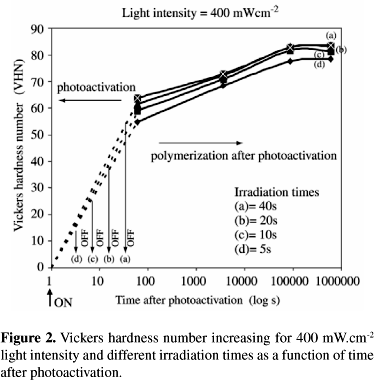
Figures 1 and 2 show typical shapes of kinetic curves for the polymerization of a methacrylate monomer. They show the increase of the Vickers hardness number as a function of the time after photoactivation, considering two light intensities and four different irradiation times (5, 10, 20 and 40 s). For all groups, Vickers hardness number values increased until the 24 h reading, which did not modify after this time.
Table 1 summarizes the VHN averages and standard deviation for each group, and the Duncan's groupings. Vickers hardness number varied from a high of 84 (400 mW.cm-2 - 40 s) to a low of 49 (200 mW.cm-2 - 5 s). At any moment after photoactivation, the Vickers hardness number values were smaller for the 200 mW.cm-2 light intensity, except for the 40 s irradiation time (groups G and H). Three-factor ANOVA indicated that the interaction among the three factors (F = 0.28; p = 0.979) and between light intensity and time after photoactivation (F = 1.21; p = 0.311) were not significant. On the other hand, the interactions between light intensity and irradiation time (F = 21.98; p = 0.000) and between irradiation time and time after photoactivation (F = 3.22; p = 0.001) were significant. The analysis also revealed that the Vickers hardness number was affected by light intensity (F = 191.18; p = 0.000), irradiation time (F = 231.98; p = 0.000) and time after photoactivation (F = 1783.92; p = 0.000).
Table 2 shows the averages of the volumetric polymerization shrinkage and standard deviations for each group. Two-factor ANOVA indicated that the interaction between light intensity and irradiation time (F = 0.21; p = 0.886) was not significant. The polymerization shrinkage was not affected by the light intensity (F = 0.39; p = 0.539) or irradiation time (F = 0.57; p = 0.637).
4. Discussion
The degree of monomer conversion in photoactivated composite resins depends on many factors. One of them is the quantity of free radicals formed during the irradiation. So, the energy delivered to the system and, consequently, the irradiation time and the light intensity are important factors in this process.
In this study, the degree of conversion of a photo cured dental composite resin was determined by the Vickers hardness number analysis, an indirect form to measure pendant double bonds22,26-28. The microhardness of composites can be correlated to the amount of residual double bonds measured, for example, by infrared spectroscopy27. In order to evaluate the kinetics of the polymerization process after photoactivation, hardness values were measured at different times after the photoactivation, considering different combinations of light intensities and irradiation times. Results showed that, for a specific thickness of the material used, it was observed that the monomer conversion does not reach a final value just after the light source is cut off. The composite resin reaches its maximum degree of polymerization actually at any moment between 1 h and 24 h. This result can be explained by the microgel/macrogelation theory3, that proposes that unreacted pendant double bonds and free radicals become temporally entrapped within polymer chains, being able to eventually react later.
Results also showed that low light intensity values (200 mW.cm-2) and short irradiation times (5, 10 and 20 s) promoted both lower values of hardness (VHN) and degrees of conversion. By increasing the amount of energy delivered to the system, the VHN values could be increased, independently on the combination of IT and LI, up to 8 J, when no further increase in hardness was observed. Considering the groups that received the same energy, no significant difference in hardness values was observed. These findings are in accordance with those reported by Nomoto et al.20, who did not observe differences in the depth of cure and degree of conversion when the energy delivered to the system was kept constant, regardless of the light intensities and irradiation times. Although it was observed that the increase of the irradiation time can compensate low values of light intensity, as in group G, it was also observed that the light intensity has a more pronounced influence in the process than the irradiation time. For example, the energy delivered to groups D and E was the same, but only when high values of light intensity were used, it was possible to obtain values of hardness comparable to those obtained with higher energy. This result is evidence that, within a given energy, the light intensity can be the main factor in the photoactivation of multifunctional monomers. Our results agree with those that have associated higher degrees of conversion with high light intensity units, since (up to 20 s) the intensity of 400 mW.cm-2 produced higher values of hardness than the 200 mW.cm-2 light source. Higher values of hardness and double bond conversion could have been caused by a combination of both photo and thermal effects. High light intensities immobilize initially the system by increasing rapidly the viscosity, but also generate a temporary excess of free volume that enhances the mobility of the monomers. This enhanced mobility allows the system to reach higher degrees of conversion. This means that at high light intensities, most of the reaction will occur in the unrelaxed state (post-gel), whereas at low light intensities the sample is more able to relax during polymerization, thus reducing its free volume and internal mobility and thereby its final conversion. Moreover, the increase of the light intensity also increases the maximum temperature reached during polymerization, and consequently the mobility of the polymer chains. Therefore, it was evident that for low intensity sources if the irradiation time is increased, it is possible to get comparable degrees of conversion to the ones obtained by applying high light intensities. This combination (low light intensity and longer photoactivation times) is potentially very interesting, considering the fact that many studies have associated smaller polymerization shrinkage stress with low light intensity units2,4,6,11-14,20-24. According to these authors, the part of the polymerization that occurs before the gel point does not induce residual stress, because the volumetric change can be compensated by flow, since molecules can still slip into new positions and orientations. However, following gel formation, the polymerization process is accompanied by a rapid increase in elastic modulus, which means that subsequent shrinkage can induce stress within the polymer and distribute it into the boundary layers7. By postponing the gel point by slowing down the rate of polymerization, it would then be possible to allow the material to flow, diminishing the stress.
In this study, we measured the total volumetric polymerization shrinkage, i.e., the net change in volume occurred before and after the gel-point. Experimental results showed no statistical correlation between light intensity or irradiation time, i.e., the energy delivered to the system, and the dimensional change of the composite during polymerization. High values of energy delivered to the material during photoactivation yields higher levels of monomer to polymer conversion. Since the density of the polymer is higher than the density of the monomer system, volumetric shrinkage tends to be more pronounced for composites polymerized under high energy inputs. However, high energies during photoactivation also can lead to high viscosities at the very early stages of the polymerization that can restrict polymer chain accommodation and result in frozen free volume and lower overall densities. These two factors can overlap each other resulting in a situation where density of the composite is not highly affected by the energy delivered to the material during photoactivation (considering the conditions used in this work).
5. Conclusions
By using a visible light source, the polymerization process of a photo cured dental composite resin was monitored. For the material and methods used, the results showed that the polymerization reaction does not end up just after the photoactivation period. In general, irradiation using higher light intensities promotes greater monomer conversion and shortens the irradiation time needed to form an extensively reacted polymer network. Low light intensities associated with short irradiation times do not provide enough energy to the system and yield low degrees of conversion. However, materials produced by photoactivation processes that combine low light intensities and long irradiation times (Group G) were able to reach the maximum observed degree of conversion among all experimental groups. This combination of processing conditions can potentially reduce residual stresses due to polymerization shrinkage, by providing enough time to the system to flow and consequently release stress. No correlation was observed between the values of the total volumetric polymerization shrinkage and irradiation times or light intensities.
Acknowledgements
The authors would like to acknowledge the financial support from FAPEMIG (Ref: CDS 678/99) and CNPq/Brazil.
Received: January 30, 2003; Revised: October 13, 2003
Articles presented at the XV CBECIMAT, Natal - RN, November de 2002.
- 1. Hirata, R.; Mazzetto, A.H.; Yao, E. J. Bras. Clín. & Est. Em Odont., v. 4, p. 13-21, 2000.
- 2. Asmussen, E.; Peutzfeldt, A. Dent. Mat., v. 19, p. 466-470, 2003.
- 3. Andrzejewska, E. Prog. Polym. Sci., v. 26, p. 605-665, 2001.
- 4. Dietschi, D.; Marret, N.; Krejci, I. Dent. Mat., v. 19, p. 493-500, 2003.
- 5. Rueggeberg, F.A.; Jordan, D.M. Int. J. Prosthet., v. 6, p. 364-370, 1993.
- 6. Lovell, L.G.; Newman, S.M.; Donaldson, M.M.; Bowman, C.N. Dent. Mat., v. 19, p. 458-465, 2003.
- 7. Sakaguchi, R.L.; Sasik, C.T.; Bunczak, M.A.; Douglas, W.H. J. Dent., v. 19, p. 312-316, 1991.
- 8. Bausch, J.R.; De Lange, K.; Davidson, C.L.; Peters, A.; De Gee, A.L. J. Prosthet. Dent., v. 48, p. 59-67, 1982.
- 9. I. Mjor. In: Amalgam and composite resin restorations: longevity and reasons for replacement. Chicago: Quintessence. 1989, Chap 2.
- 10. Kroese, H.J.P.; Plasschaert, A.J.M.; van't Hof, M.A.; Truin, G.J. J. Dent. Res., v. 69, p. 1270-1274, 1990.
- 11. Uno, S.; Asmussen, E. Scand. J. Dent. Res., v. 99, p. 440-444, 1991.
- 12. Unterbrink, G.L.; Muessner, R. J. Dent., v. 23, p. 183-189, 1995.
- 13. Feilzer, A.J.; Dooren, L.H.; De Gee, A.J.; Davidson, C.L. Eur. J. Oral. Sci., v. 103, p. 322-326, 1995.
- 14. Sílicas, N.; Eliades, G.; Watts, D.C. Dent. Mat., v. 16, p. 292-296, 2000.
- 15. Eick, J.D.; Robinson, S.J.; Byerley, T.J.; Chappelow, C.C. Quint. Int., v. 24, p. 632-640, 1993.
- 16. Stansbury, J.W. J. Dent. Res., v. 71, p. 1408-1412, 1992.
- 17. Lutz, F.; Krejci, I.; Barbakow, F. Dent. Mat., v. 7, p. 107-113, 1991.
- 18. Bertolotti, R. Pract. Period. Aesthet. Dent., v. 3, p. 53-58, 1991.
- 19. Segura, A.; Donly, K.J. J. Oral. Rehabilit., v. 20, p. 495-499, 1993.
- 20. Nomoto, R.; Uchida, K.; Hirasawa, T. Dent. Mat. J., v. 13, p. 198-205, 1994.
- 21. Yoshikawa, T.; Borrow, M.F.; Tagami, J. Dent. Mat., v. 17, p. 259-266, 2001.
- 22. Park, S.H.; Krejci, I.; Lutz, F. Oper. Dent., v. 27, p. 30-37, 2002.
- 23. Muangmingsuk, A.; Senawongse, P.; Yudhasaraprasithi, S. Am. J. Dent., v. 16, p. 117-119, 2003.
- 24. Uno, S.; Tanaka, T.; Natzuizaka, A.; Abo, T. Dent. Mat., v. 19, p. 147-152, 2003.
- 25. Puckett, A.D.; Smith, R. J Prosthet Dent., v. 68, p. 56-58, 1992.
- 26. Discacciati, J.A.C.; Neves, A.D.; Yared, K.F.G.; Oréfice, R.L.; Jansen, W.C. J. Dent. Res., v. 81, p. B-207, 2002.
- 27. Oréfice, R.L.; Discacciati, J.A.C.; Neves, A.D.; Mansur, H.S.; Jansen, W.C. Pol. Test., v. 22, p. 77-81, 2003.
- 28. Cohen, M.E.; Leonard, D.L.; Charlton, D.G.; Roberts, H.W.; Gragain Jr, J. Dent. Mat. In press, 2003.
Publication Dates
-
Publication in this collection
29 June 2004 -
Date of issue
June 2004
History
-
Received
30 Jan 2003 -
Reviewed
13 Oct 2003