Abstract
Cytogenetic studies were carried out on samples of Parapteronotus hasemani, Sternarchogiton preto and Sternarchorhamphus muelleri (Apteronotidae, Gymnotiformes) from the Amazon basin. The first two species exhibited both a 2n = 52 karyotype, but differed in their karyotypic formulae, distribution of constitutive heterochromatin, and chromosomal location of the NOR. The third species, Sternarchorhamphus muelleri, was found to have a 2n = 32 karyotype. In all three species the DAPI and chromomycin A3 staining results were consistent with the C-banding results and nucleolar organizer region (NOR) localization. The 18S rDNA probe confirmed that there was only one pair of ribosomal DNA cistron bearers per species. The telomeric probe did not reveal interstitial telomeric sequences (ITS). The karyotypic differences among these species can be used for taxonomic identification. These data will be useful in future studies of these fishes and help understanding the phylogenetic relationships and chromosomal evolution of the Apteronotidae.
fluorochromes; FISH; chromosomal rearrangements; biodiversity
Chromosomal diversity in three species of electric fish (Apteronotidae, Gymnotiformes) from the Amazon Basin
Fernando Henrique Ramos SilvaI; Julio Cesar PieczarkaI; Adauto Lima CardosoI; Patrícia Corrêa da SilvaI; Jonas Alves de OliveiraII; Cleusa Yoshiko NagamachiI
IInstituto de Ciências Biológicas, Universidade Federal do Pará, Belém, PA, Brazil
IIInstituto de Desenvolvimento Sustentável Mamirauá, Tefé, AM, Brazil
Send correspondence to Send correspondence to: Cleusa Y. Nagamachi Instituto de Ciências Biológicas, Universidade Federal do Pará, Campus Guamá Avenida Perimetral, sn., Guamá 66075-900 Belém, PA, Brazil E-mail: cleusanagamachi@gmail.com
ABSTRACT
Cytogenetic studies were carried out on samples of Parapteronotus hasemani, Sternarchogiton preto and Sternarchorhamphus muelleri (Apteronotidae, Gymnotiformes) from the Amazon basin. The first two species exhibited both a 2n = 52 karyotype, but differed in their karyotypic formulae, distribution of constitutive heterochromatin, and chromosomal location of the NOR. The third species, Sternarchorhamphus muelleri, was found to have a 2n = 32 karyotype. In all three species the DAPI and chromomycin A3 staining results were consistent with the C-banding results and nucleolar organizer region (NOR) localization. The 18S rDNA probe confirmed that there was only one pair of ribosomal DNA cistron bearers per species. The telomeric probe did not reveal interstitial telomeric sequences (ITS). The karyotypic differences among these species can be used for taxonomic identification. These data will be useful in future studies of these fishes and help understanding the phylogenetic relationships and chromosomal evolution of the Apteronotidae.
Keywords: fluorochromes, FISH, chromosomal rearrangements, biodiversity.
Introduction
Apteronotidae is the Gymnotiformes family with the largest number of formally described species: 86 species divided into 15 genera (De Santana, 2007; De Santana and Vari, 2009, 2010a,b; Albert and Crampton, 2009; De Santana and Crampton, 2010). The species of this family are found in rivers from Panama to northern Argentina, including rivers that flow into the Pacific Ocean (eastern Colombia), the Orinoco, Maracaibo, Magdalena, Guyana shield, the Amazon, and the Paraná-Paraguay and San Francisco basins (Mago-Leccia, 1994). Intra-and interspecific variations in size and shape of the head have been observed, probably related to trophic specialization and/or aggression between males (Cox-Fernandes, 1998; Albert, 2001; Cox-Fernandes et al., 2002).
A few species of the order Gymnotiformes have been cytogenetically analyzed and have shown highly diverse karyotypes, with differences in both chromosome structure and number (Artoni et al., 2000). Among the studied members of this order, the chromosome number varies from 2n=24in Apteronotus albifrons (Howell, 1972; Almeida-Toledo et al., 1981; Mendes et al., 2012) to 2n = 54 in Gymnotus carapo, G. mamiraua, G. inaequilabiatus and G. paraguensis (Lacerda and Maistro, 2007; Milhomem et al., 2007, 2008, 2012a, 2012b; Scacchetti et al., 2011).
Although Apteronotidae is the Gymnotiformes family with the largest number of described species, chromosomal information is available only for Apteronotus albifrons, which has 2n = 24 chromosomes (Howell, 1972; Almeida-Toledo et al., 1981; Mendes et al., 2012). Reportedly, Apteronotus albifrons from the Parana River also has B chromosomes, which were found as microchromosomes (Mendes et al., 2012).
Here, we made the cytogenetic characterization of three additional Apteronotidae species, Sternarchorhamphus muelleri, Parapteronotus hasemani and Sternarchogiton preto, in an effort to increase the amount of chromosomal information available for representatives of this family, to allow comparative analyses and provide new insights into the possible mechanisms underlying the diversification of these species.
Material and Methods
We analyzed two males of species Sternarchorhamphus muelleri (MPEG 22759) from the Anequara river (Abaetetuba -PA; 1º40'42.6" S and 49º00'16.6" W), 15 specimens (10 males and five females) of Parapteronotus hasemani (IDSMIctio 059, IDSMIctio 0637, IDSMIctio 0746, IDSMIctio 0747, IDSMIctio 0754, IDSMIctio 0758, IDSMIctio 01001, IDSMIctio 01869, IDSMIctio 02047, IDSMIctio 02056, IDSMIctio 02057, IDSMIctio 02058, IDSMIctio 02095, IDSMIctio 02129, MPEG 22757) from the rivers of the Reserva de Desenvolvimento Sustentável Mamirauá (RDSM) -AM (3º2'50,2" S e 64º51'26,6" W), and five specimens of Sternarchogiton preto (one male, one female and three of unidentified sex; MPEG 22758) collected in the Caripetuba river (Abaetetuba -PA; 1º37'23,49"S e 48º55'33"W) (Figure 1). The specimens were deposited in the Instituto de Desenvolvimento Sustentável Mamirauá (IDSM) and the Museu Paraense Emílio Goeldi (MPEG). Figure 2 shows an example of each species studied.
Mitotic chromosomes were obtained according to the method described by Bertollo et al. (1978) and analyzed by conventional staining (Giemsa), C-banding (Sumner, 1972), Ag-NOR (Howell and Black, 1980), CMA3 (Schweizer, 1980), and DAPI (Pieczarka et al., 2006) staining. Fluorescent in situ hybridization (FISH) was performed with telomeric probes (All Telomere, Oncor) and 18S rDNA probes obtained from species Prochilodus argentus (Hatanaka and Galetti Jr, 2004) and labeled with biotin or digoxigenin by nick translation. Hybridization was detected with avidin-(Cy3 or FITC) or anti-digoxigenin-(Cy3 or FITC). The morphological classification of the chromosomes was made as described by Levan et al. (1964).
Results
The species Sternarchorhamphus muelleri was found to have a karyotype of 2n = 32 (28m/sm+4st/a) and a fundamental number (FN) of 60. C-banding showed the presence of constitutive heterochromatin (CH) in the centromeric regions of most chromosomes, except for pairs 7, 11, 12, 13, 14 and 16, where the banding was almost imperceptible (suggesting that there was relatively little CH). Pairs 1, 3 and 10 had pericentromeric heterochromatic blocks, and pair 15 had a heterochromatic block in the proximal region of the long arm. A nucleolar organizer region (NOR) was found in the interstitial region of the long arm of pair 15, and showed heteromorphism between the homologs (Figure 3A, B).
The karyotype of Parapteronotus hasemani was 2n = 52 (36m/sm+16st/a) and FN = 88. CH was observed in the centromeric regions of all chromosomes. Pairs 1, 22 and 23 also had heterochromatic blocks in the proximal regions of their long arms. Pairs 2, 3, 5 and 6 had large heterochromatic blocks that spanned nearly the entire length of their short arms. Pairs 4 and 12 had heterochromatic blocks in their pericentromeric regions. A NOR was found on the distal short arm of pair 3; it coincided with a secondary constriction and showed heteromorphism between the homologs (Figure 3C, D).
Sternarchogiton preto had a 2n = 52 (38m/sm+14st/a) karyotype and FN = 90. CH was observed in the centromeric region of most chromosomes, except for pairs 5, 9, 12, 16 and 20, where the banding was almost imperceptible. In addition, pair 1 had a heterochromatic block throughout the long arm, pair 2 had a heterochromatic block in the proximal region of the long arm, and pair 3 had a heterochromatic block on the long arm. Pairs 4, 8, 21, 23 and 24 had heterochromatic blocks on their short arms. A NOR was observed in the distal region of the long arm of pair 3, within the heterochromatic block, and showed heteromorphism between the homologs (Figure 3E, F).
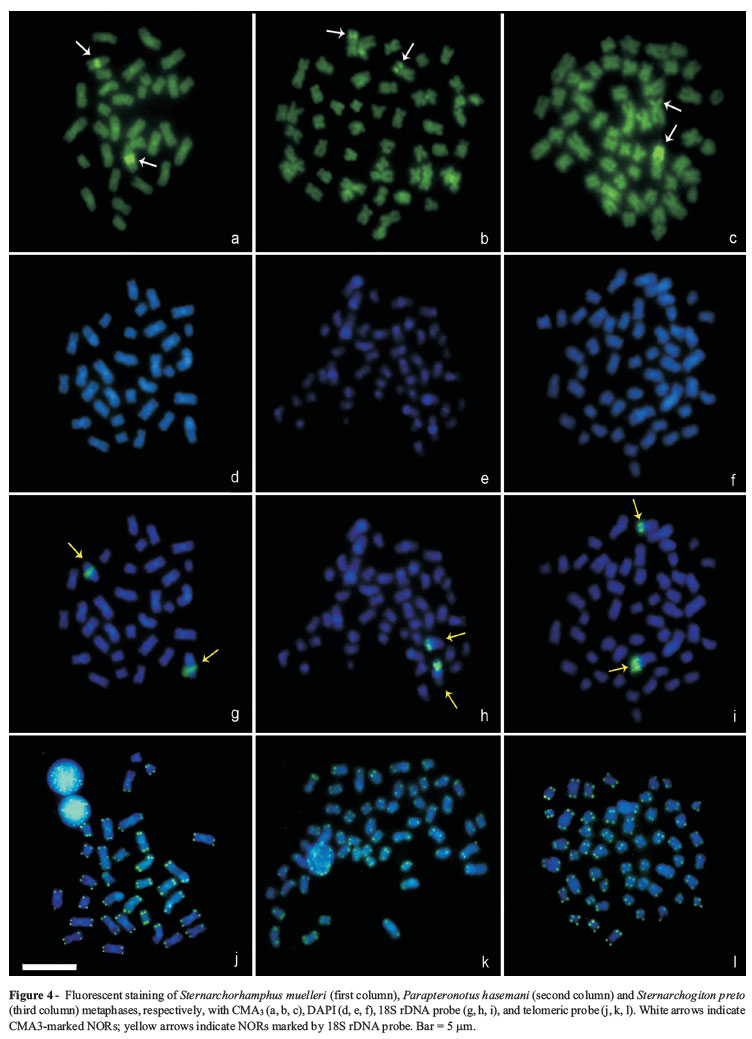
The three studied species showed similar results in the fluorochrome, CMA3 and DAPI staining, as well as FISH analysis with 18S rDNA and telomere repeat probes. CMA3 labeled the NORs in all three species, showing that the ribosomal genes were interspersed with GC-rich sequences. DAPI staining revealed that the CH was AT-rich, and FISH with 18S rDNA probes showed that these sites were localized in a single pair per species, coincident with the NOR. Finally, FISH using the telomeric probes did not indicate the presence of any interstitial telomeric sequences (Figure 4A-L).
Discussion
Family Apteronotidae is not only more diverse in its number of genera, species and morphological forms, it also displays the greatest variation in diploid number among the Gymnotiformes, ranging from 2n = 24 (16m/sm+8st/a) in Apteronotus albifrons (Howell, 1972; Almeida-Toledo et al., 1981; Mendes et al., 2012) to 2n = 52 in Parapteronotus hasemani (36m/sm+16st/a) and Sternarchogiton preto (38m/sm+14st/a) (this work). Sternarchorhamphus muelleri (2n = 32; 28m/sm+4st/a) has an intermediate diploid number (this work).
Parapteronotus hasemani and Sternarchogiton preto have the same diploid number (2n = 52), but differ in their karyotypic formulae. This difference can be explained by chromosomal inversions, which can change the morphology of chromosomes while maintaining the diploid number (Alves et al., 2003; De Oliveira et al., 2006). Karyotypes of 2n = 52 are also found in other Apteronotidae species (Sternarchorhynchus cramptoni, S. oxyrhynchus, Platyurosternachus macrostomus, Apteronotus bonapartii; unpublished data), while 2n = 50 is seen in two species of genus Adontosternarchus (A. clarkae and A. balaenops; unpublished data). This indicates that a higher diploid number is common in the family. Although they share the same diploid number, P. hasemani and S. preto display considerable differences in their C-banding patterns and the location of their NORs. The latter may be due to translocations of the NOR-bearing chromosomes.
The karyotype of Sternarchorhamphus muelleri (2n = 32) is quite different from those of the other two species in chromosome number and morphology, CH distribution and NOR location. The diploid number reduction appears to have occurred through chromosomal fusion events. Such events are believed to explain the reduction of 2n in genus Ancistrus (Loricariidae) (Alves et al., 2003; De Oliveira et al., 2009), and have been suggested for other groups of fishes (Cipriano et al., 2008; Margarido and Moreira-Filho, 2008). Similarly, fusion events could also explain the diploid number decrease in Apteronotus albifrons. The location of the NORs in this species is quite different from that seen in other species, as it typically appears in the interstitial region of the long arm of an acrocentric chromosome. This variation in the NOR-bearing chromosome may have arisen, in this case, through a pericentric inversion that moved the NOR to the middle of the chromosome arm.
Nagamachi et al. (2010) used chromosome painting to show that the degree of chromosomal rearrangement between karyotypes of two cryptic species of Gymnotus cf carapo (2n = 40 and 2n = 42) was much greater than that estimated using classical cytogenetics (Milhomem et al., 2008). Thus, the pericentric inversions and centric fusions that were believed to differentiate the karyotypes of these species may be an underestimate of the actual degree of genomic rearrangement at work.
CH was found in the centromeric regions of virtually all chromosomes of the analyzed species. DAPI staining revealed that this CH is AT-rich, which is consistent with reports concerning other Gymnotiformes (Milhomem et al., 2007, 2008, 2012a,b; Silva et al., 2008, 2009; Cardoso et al., 2011). The three species analyzed in the present study showed differing CH patterns, with several additional blocks in non-centromeric regions. Processes related to the dynamics of repetitive DNA, such as amplifications and translocations, might have been involved in the development of these blocks. Using microarray analysis, Lippman et al. (2004) showed that in Arabidopsis the heterochromatin is determined by transposable elements and related to tandem repeats. Transposons have been found in the heterochromatin of several fish groups, including Cichla kelberi (Teixeira et al., 2009), Hisonotus leucofrenatus (Ferreira et al., 2011), and Antarctic fishes of the suborder Notothenioidei (Ozouf-Costaz et al., 2004). Furthermore, transposons have been associated with the karyotypic variation observed in Erythrinus erythrinus (Cioffi et al., 2010), and the formation of the Y chromosome in Chionodraco roseofuscus (Capriglione et al., 2000).
Souza et al. (2009) analyzed species of genus Peckoltia (Siluriformes: Loricariidae) and proposed possible homeologies among some pairs with similar C-banding patterns, morphologies and NOR localizations. In the karyotypes of P. hasemani, S. muelleri and S. preto,we identified chromosomes (pair 1 in the first two species and pair 2 in the latter) that resembled one another in chromosome morphology, size and HC distribution (Figure 5).
Heteromorphisms in NOR size (by 2-fold or more) have been described in some Gymnotiformes species, and the NORs of Eigenmannia sp and E. virescens were found to be larger than those of the other examined species. Tandem duplications, unequal crossovers involving repeated regions, and/or accidental duplications might explain these variations (Foresti et al., 1981).
The NOR regions of the tested species stained positive with CMA3, revealing that the ribosomal genes are interspersed with GC-rich sequences (Pendás et al., 1993). Our results resemble those found in other Gymnotiformes and additional fish species (Artoni and Bertollo, 1999; Milhomem et al., 2007, 2008, 2012a,b; Silva et al., 2008, 2009; Cardoso et al., 2011).
FISH with telomeric probes failed to show any evidence of interstitial telomeric sequences (ITSs). This may reflect the absence of chromosomal rearrangements involving the telomeres (Silva et al., 2009). Alternatively, telomeric sequences away from the chromosomal ends may have undergone sequence changes that hinder probe hybridization (Albuín et al., 1996).
Accumulating evidence suggests that variability in diploid number and karyotype formula can be explained by chromosome rearrangements, such as fusions (which can decrease the diploid number) and inversions. According to the phylogenies of Alves-Gomes et al. (1995) and Albert (2001), the family Gymnotidae, represented by Gymnotus and Electrophorus, occupies the basal position among the other Gymnotiformes. The species in this family have diploid numbers ranging from 34 to 54, with a higher occurrence of 2n above 50 (for review, see Milhomem et al., 2012a,b). Thus, we believe that the higher diploid numbers (2n = 50 or 52) are basal in Apteronotidae and that the karyotypes of Apteronotus albifrons and Sternarchorhamphus muelleri underwent rearrangements that decreased their 2n values. Their CH patterns are also quite variable, showing additional blocks that are characteristic to each species. The NORs varied in size and location on the chromosome, but all three species had a single NOR.
It is therefore likely that some homeologies exist among these species. Together, the results presented herein add new information that may prove valuable in future studies of this group, facilitating taxonomic identification, and increasing our understanding of the chromosomal evolution and phylogenetic relationships of the Apteronotidae.
Acknowledgments
Most of this research was supported by FAPESPA (Pará State Research Foundation) through a grant from the National Excellence in Research Program (PRONEX, TO 011/2008) for a project coordinated by JCP. Further funding and support came from UFPA, CNPq and CAPES. This study is part of the Master dissertation of FHRS, who was the recipient of a CAPES Master Scholarship. ALC and PCS had CAPES Master and CAPES Doctoral Scholarships, respectively. We thank the IDSM (Instituto de Desenvolvimento Sustentável Mamirauá) for logistic support during sample collection, and Dr. Carlos David Canabarro Machado De Santana for taxonomic identification of the samples. Sample collection was authorized under IBAMA (Instituto Brasileiro do Meio Ambiente) permit 020/2005 (IBAMA Registration: 207419).
Received: April 13, 2014
Accepted: June 24, 2014.
Associate Editor: Yatiyo Yonenaga-Yassuda
- Albert JS (2001) Species diversity and phylogenetic systematic of American knifefishes (Gymnotiformes, Teleostei). Misc Publ Mus Zool Univ Mich 190:1-127.
- Albert JS and Crampton WGR (2009) A new species of electric knifefish, genus Compsaraia (Gymnotiformes, Apteronotidae) from the Amazon River, with extreme sexual dimorphism in snout and jaw length. Syst Biodiv 7:81-92.
- Albuín M, Martínez P and Sánchez L (1996) Localization of the repetitive telomeric sequence (TTAGGG)n in four salmonid species. Genome 39:1035-1038.
- Almeida-Toledo LF, Foresti F and Toledo-Filho SA (1981) Constitutive heterochromatin and nucleolus organizer in the knifefish Apteronotus albifrons (Pisces, Apteronotidae). Experientia 37:953-954.
- Alves AL, Oliveira C and Foresti F (2003) Karyotype variability in eight species of the subfamilies Loricariinae and Ancistrinae (Teleostei, Siluriformes, Loricariidae). Caryologia 56:57-63.
- Alves-Gomes JA, Ortí G, Haygood M, Meyer A and Heiligenberg W (1995) Phylogenetic analysis of the South American electric fishes (Order Gymnotiformes) and the evolution of their electrogenic system: A synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Mol Biol Evol 12:298-318.
- Artoni RF and Bertollo LAC (1999) Nature and distribuition of constitutive heterochromatin in fishes, genus Hypostomus (Loricariidae). Genetica 106:209-214.
- Artoni RF, Vicari MR and Bertollo LAC (2000) Citogenética de peixes Neotropicais: Métodos, resultados e perspectivas. Biol Health Sci 6:43-60.
- Bertollo LAC, Takashi CS and Moreira-Filho O (1978) Cytotaxonomic considerations on Hoplias lacerdae (Pisces, Erythrinidae). Braz J Genet 1:103-120.
- Capriglione T, Odierna G, Canapa A, Caputo V, Cerioni PN and Olmo E (2000) Characterization of Tc1 Transposon-like sequences in Notothenioids. Ital J Zool 67:123-126.
- Cardoso AL, Pieczarka JC, Feldberg E, Milhomem SSR, Moreira-Almeida T, Silva DS, Silva PC and Nagamachi CY (2011) Chromosomal characterization of two species of genus Steatogenys (Gymnotiformes, Rhamphichthyoidea, Steatogenini) from the Amazon basin: Sex chromosomes and correlation with Gymnotiformes phylogeny. Rev Fish Biol Fisheries 21:613-621.
- Cioffi MB, Martins C and Bertollo LAC (2010) Chromosome spreading of associated transposable elements and ribosomal DNA in the fish Erythrinus erythrinus Implications for genome change and karyoevolution in fish. BMC Evol Biol 10:e271.
- Cipriano RR, Fenocchio AS, Artoni RF, Molina W, Noleto RB, Kantek DLZ and Cestari MM (2008) Chromosomal studies of five species of the marine fishes from the Paranaguá Bay and the karyotypic diversity in the marine Teleostei of the Brazilian coast. Braz Arch Biol Technol 51:303-314.
- Cox-Fernandes C (1998) Sex-related morphological variation in two Apteronotid fishes (Gymnotiformes) from the Amazon River Basin. Copeia 1998:730-735.
- Cox-Fernandes C, Lundberg JG and Riginos C (2002) Larges to fall electric fish snouts: Hypermorphic facial growth in male Apteronotus hasemani and the identity of Apteronotus anas (Gymnotiformes, Apteronotidae). Copeia 2002:52-61.
- De Santana CDC (2007) Sistemática e Biogeografia da Família Apteronotidae (Otophysi, Gymnotiformes). Tese de Doutorado, INPA/UFAM, Manaus.
- De Santana CDC and Crampton WGR (2010) A review of the South American electric fish genus Porotergus (Gymnotiformes, Apteronotidae) with the description of a new species. Copeia 2010:165-175.
- De Santana CDC and Vari RP (2009) The South American electric fish genus Platyurosternarchus (Gymnotiformes, Apteronotidae). Copeia 2009:233-244.
- De Santana CDC and Vari RP (2010a) New rheophilic species of electric knifefish from the rapids and waterfalls of the lower Rio Xingu, Brazil (Gymnotiformes, Apteronotidae). Copeia 2010:160-164.
- De Santana CDC and Vari RP (2010b) Electric fishes of the genus Sternarchorhynchus (Teleostei, Ostariophysi, Gymnotiformes); phylogenetic and revisionary studies. Zool J Linn Soc 159:223-371.
- De Oliveira RR, Souza IL and Venere PC (2006) Karyotype description of three species of Loricariidae (Siluriformes) and occurrence of the ZZ/ZW sexual system in Hemiancistrus spilomma Cardoso and Lucinda, 2003. Neotrop Ichthyol 4:93-97.
- De Oliveira RR, Feldberg E, Dos Anjos MB and Zuanon J (2009) Mechanisms of chromosomal evolution and its possible relation to natural history characteristics in Ancistrus catfishes (Siluriformes, Loricariidae). J Fish Biol 75:2209-2225.
- Ferreira DC, Oliveira C and Foresti F (2011) A new dispersed element in the genome of the catfish Hisonotus leucofrenatus (Teleostei, Siluriformes, Hypoptopomatinae). Mobile Genet Elements 1:103-106.
- Foresti F, Almeida-Toledo LF and Toledo SAF (1981) Polymorphic nature of nucleolus organizer regions in fishes. Cytogenet Cell Genet 31:137-144.
- Hatanaka T AND Galetti Jr PM (2004) Mapping of 18S and 5S ribosomal RNA genes in the fish Prochilodus argenteus Agassiz, 1829 (Characiformes, Prochilodontidae). Genetica 122:239-244.
- Howell WM (1972) Somatic chromosomes of the Black Ghost Knifefish, Apteronotus albifrons (Pisces, Apteronotidae). Copeia 1972:191-193.
- Howell WM and Black DA (1980) Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: A 1-step method. Experientia 36:1014-1015.
- Lacerda MCV and Maistro EL (2007) Cytogenetic analysis of three sympatric Gymnotus species (Teleostei, Gymnotidae) from the Fundo stream, MG, Brazil. Cytologia 72:89-93.
- Levan A, Fredga K and Sandberg A (1964) A nomenclature for centromeric position on chromosomes. Hereditas 52:201-220.
- Lippman Z, Gendrel AV, Black M, Vaughn MW, Dedhia N, McCombie WR, Lavine K, Mitta LV, May B, Kasschau KD, et al. (2004) Role of transposable elements in heterochromatin and epigenetic control. Nature 430:471-476.
- Mago-Leccia F (1994) Electric fishes of the continental waters of America. Bibl de la Acad Cienc Fisicas, Matemat Nat, Caracas, Venezuela 29:1-206.
- Margarido VP and Moreira-Filho O (2008) Karyotypic differentiation through chromosome fusion and number reduction in Imparfinis hollandi (Ostariophysi, Heptapteridae). Genet Mol Biol 31:235-238.
- Mendes VP, Portela-Castro ALB and Júlio-Júnior HF (2012) First record of supernumerary (B) chromosomes in electric fish (Gymnotiformes) and the karyotype structure of three species of the same order from the upper Paraná River basin. Comp Cytogenet 6:1-16.
- Milhomem SSR, Pieczarka JC, Crampton WGR, Souza ACP, Carvalho Jr JR and Nagamachi CY (2007) Differences in karyotype between two sympatric of Gymnotus (Gymnotiformes, Gymnotidae) from the Easthern Amazon of Brazil. Zootaxa 1397:55-62.
- Milhomem SSR, Pieczarka JC, Crampton WGR, Silva DS, Souza ACP, Carvalho Jr JR and Nagamachi CY (2008) Chromosomal evidence for a criptic species in the Gymnotus carapo species-complex (Gymnotiformes, Gymnotidae) BMC Genet 9:e75.
- Milhomem SSR, Crampton WGR, Pieczarka JC, Shetka GH, Silva DS and Nagamachi CY (2012a) Gymnotus capanema, a new species of electric knife fish (Gymnotiformes, Gymnotidae) from eastern Amazonia, with comments on an unusual karyotype. J Fish Biol 80:802-815.
- Milhomem SSR, Crampton WGR, Pieczarka JC, Silva DS, Cardoso AL, Silva PC, Oliveira JA and Nagamachi CY (2012b) Chromosomal and electric signal diversity in three sympatric electric knifefish species (Gymnotus, Gymnotidae) from the Central Amazon Floodplain. Rev Fish Biol Fisheries 22:485-497.
- Nagamachi CY, Pieczarka JC, Milhomem SSR, O'Brien PCM, Souza ACP and Ferguson-Smith MA (2010) Multiple rearrangements in cryptic species of electric knifefish, Gymnotus carapo (Gymnotidae, Gymnotiformes) revealed by chromosome painting. BMC Genet 11:e28.
- Ozouf-Costaz C, Brandt J, Körting C, Pisano E, Bonillo C, Coutanceau JP and Volff JN (2004) Genome dynamics and chromosomal localization of the non-LTR retrotransposons Rex 1and Rex 3 in Antarctic fish. Antarct Sci 16:51-57.
- Pendás AM, Morán O and Garcia-Vásquez E (1993) Multichromosomal location of ribossomal RNA genes and heterochromatin association in brown trout. Chromosome Res 1:63-67.
- Pieczarka JC, Nagamachi CY, Souza ACP, Milhomem SSR, Castro RR and Nascimento AL (2006) An adaptation to DAPIbanding to fishes chromosomes. Caryologia 59:43-46.
- Scacchetti PC, Pansonato-Alves JC, Utsunomia R, Oliveira C and Foresti F (2011) Karyotypic diversity in four species of the genus Gymnotus Linnaeus, 1758 (Teleostei, Gymnotiformes, Gymnotidae): Physical mapping of ribosomal genes and telomeric sequences. Comp Cytogenet 5:223-235.
- Schweizer D (1980) Simultaneous fluorescent staining of R bands and specific heterocromatic regions (DA/DAPI bands) in human chromosomes. Cytogenet Cell Genet 27:190-193.
- Silva DS, Milhomem SSR, Sousa ACP, Pieczarka JC and Nagamachi CY (2008) A conserved karyotype of Sternopygus macrurus (Sternopygidae, Gymnotyformes) in the Amazon region: Differences from other hydrographic basins suggest cryptic speciation. Micron 39:1251-1254.
- Silva DS, Milhomem SSR, Pieczarka JC and Nagamachi CY (2009) Cytogenetic studies in Eigenmannia virescens (Sternopygidae, Gymnotiformes) and new inferences on the origin of sex chromosomes in the Eigenmannia genus. BMC Genet 10:74-81.
- Souza ACP, Nagamachi CY, Milhomem SSR, Feldberg E and Pieczarka JC (2009) Cytogenetic analysis in catfish species of the genus Peckoltia Miranda Ribeiro, 1912 (Teleostei, Siluriformes, Loricariidae). Comp Cytogenet 3:103-109.
- Sumner AT (1972) A simple technique for demonstrating centromeric heterocromatin. Exp Cell Res 75:304 -306.
- Teixeira WG, Ferreira IA, Cabral-De-Mello DC, Mazzuchelli J, Valente GT, Pinhal D, Poletto AB, Venere PC and Martins C (2009) Organization of repeated DNA elements in the genome of the cichlid fish Cichla kelberi and Its contributions to the knowledge of fish genomes. Cytogenet Genome Res 125:224-234.
Send correspondence to:
Publication Dates
-
Publication in this collection
03 Nov 2014 -
Date of issue
Dec 2014
History
-
Accepted
24 June 2014 -
Received
13 Apr 2014