ABSTRACT
Rice is one of the main foods consumed by half of the world’s population. The rice crop requires plenty of water, but upland rice is cultivated in a non-flooded environment, although its productivity is lower than that of lowland rice. Rice grains mostly consist of starch, which is synthesized from the non-structural carbohydrates imported from the vegetative organs. The long-term storage of carbohydrates plays a remarkable role in maintaining the supply of photoassimilates during grain filling if photosynthesis does not meet energy demand. Therefore, the dynamics of non-structural carbohydrates is central to the productivity of rice crops. The present study aimed to determine the non-structural carbohydrate content and soluble carbohydrate profiles in different vegetative organs of upland rice of the genotype BRS Esmeralda. The content was determined at the end of vegetative development. The identification and quantification of carbohydrates were performed by high-performance anion-exchange chromatography with pulsed amperometric detection. Fully expanded leaf blades, expanding leaf blades, and expanding stems exhibited the soluble carbohydrate content of 59.7, 53.3, and 52.3 mg g-1 DM, respectively. The stem was found to be the main organ for the long-term storage of non-structural carbohydrates, wherein the starch content was 36.1 mg g-1 DM. It also contained soluble carbohydrates such as glucose, fructose, and sucrose. The non-structural carbohydrates were found in low amounts in the roots, showing that this organ does not store long-term carbohydrates.
Index terms:
Fructose; glucose; Oryza sativa; starch; sucrose
RESUMO
O arroz é um dos principais alimentos para metade da população mundial. A cultura do arroz possui alta demanda por água, mas o arroz de terras altas cresce e produz em ambientes não alagados, embora sua produtividade seja menor que a do arroz de várzea. Os grãos de arroz são constituídos majoritariamente por amido, sintetizado a partir de carboidratos não estruturais importados dos órgãos vegetativos. As reservas de carboidratos a longo prazo possuem papel fundamental na manutenção do suprimento de fotoassimilados para o enchimento dos grãos em momentos que a fotossíntese não atende essa demanda. Portanto a dinâmica dos carboidratos não estruturais é central para a produtividade. O objetivo deste estudo foi o de determinar os conteúdos de carboidratos não estruturais e os perfis de carboidratos solúveis em diferentes órgãos vegetativos do arroz de terras altas, genótipo BRS Esmeralda ao final do desenvolvimento vegetativo. Foram realizadas a análise qualitativa e a determinação das quantidades individuais dos carboidratos solúveis. Lâminas foliares completamente expandidas, lâminas foliares em expansão e caules apresentaram os maiores teores de carboidratos solúveis: 59,7, 53,3 e 52,3 mg g-1 MS, respectivamente. O caule é o principal órgão de armazenamento de carboidratos não estruturais a longo prazo, no qual o conteúdo de amido foi de 36.1 mg g-1 MS. Glicose, frutose e sacarose constituem os carboidratos solúveis. Carboidratos não estruturais foram encontrados em baixas quantidades nas raízes, evidenciando que este órgão não armazena reservas duradouras de carboidratos.
Termos para indexação:
Frutose; glicose; Oryza sativa; amido; sacarose
INTRODUCTION
Rice is one of the main foods consumed by half of the world’s population; therefore, its availability is crucial for food security (Fageria, 2007FAGERIA, N. K. Yield physiology of rice. Journal of Plant Nutrition, 30(6):843-879, 2007.; Oliveira; Pegoraro; Viana, 2020OLIVEIRA, A. C.; PEGORARO, C.; VIANA, V. E. The future of rice demand: Quality beyond productivity. Pelotas: Springer, 2020. 533p.). Asia is the main rice-producing and -consuming continent; rice is also an essential food in sub-Saharan Africa, Latin America, and the Caribbean (Muthayya et al., 2014MUTHAYYA, S. et al. An overview of global rice production, supply, trade, and consumption. Annals of the New York Academy of Sciences, 1324(1):7-14, 2014.).
The cultivated rice was originated from a semi-aquatic ancestor and hence is able to grow in the flooded environments (Lafitte; Bennett, 2002LAFITTE, H. R.; BENNETT, J. Requirements for aerobic rice: physiological and molecular considerations. In: BOUMAN, B. A. M. et al. Water-wise rice production. Los Baños: International Rice Research Institute, v.1, p. 259-274, 2002. ). It exhibits properties such as the presence of aerenchyma in the vegetative organs; an ability of rapid stem elongation; a shift in gene expression and metabolism that leads to the induction of enzymes mainly involved in carbohydrate metabolism, alcoholic fermentation, and the prevention and protection of injuries caused by post-submergence oxidative stress (Das; Uchimiya, 2002DAS, A.; UCHIMIYA, H. Oxygen stress and adaptation of a semi-aquatic plant: Rice (Oryza sativa). Journal of Plant Research, 115(5):315-320, 2002.; Steffens; Geske; Sauter, 2010STEFFENS, B.; GESKE, T.; SAUTER, M. Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytologist , 190(2):369-378, 2011.). Despite its origin, rice can grow in various environmental conditions. Such diversity is seen in the following rice-cultivation systems, classified on the basis of environmental water supply: irrigated, rainfed lowland, and upland systems, which contribute to approximately 75%, 19%, and 4% of the global rice production, respectively (International Rice Research Institute - IRRI, 2013INTERNATIONAL RICE RESEARCH INSTITUTE - IRRI. GRiSP (Global Rice Science Partnership). Rice almanac. 4th edition. Los Baños Philippines: International Rice Research Institute. 2013. 283p.). Irrigated and lowland rice predominates in Asia, whereas upland rice cultivation has gained local importance in Latin America and the Caribbean, and West Africa, where upland rice is grown in approximately 50% of the rice-cultivated areas (IRRI, 2013INTERNATIONAL RICE RESEARCH INSTITUTE - IRRI. GRiSP (Global Rice Science Partnership). Rice almanac. 4th edition. Los Baños Philippines: International Rice Research Institute. 2013. 283p.).
Despite lower productivity compared with irrigated and lowland rice, upland rice exhibits drought tolerance (Xia et al., 2019XIA, H. et al. Bi-directional selection in upland rice leads to its adaptive differentiation from lowland rice in drought resistance and productivity. Molecular Plant, 12(2):170-184, 2019.), enabling its cultivation in areas with limited water supply. Nevertheless, even for upland rice, environmental factors such as water deficit, high temperature, and nitrogen deficiency can trigger a plant stress response, and thus affect its yield (Fageria; Moreira; Coelho, 2011FAGERIA, N. K.; MOREIRA, A.; COELHO, A. M. Yield and yield components of upland rice as influenced by nitrogen sources. Journal of Plant Nutrition , 34(3):361-370, 2011.; You et al., 2016YOU, C. et al. Effect of removing superior spikelets on grain filling of inferior spikelets in rice. Frontiers in Plant Science , 7:1161, 2016.; Lanna et al., 2021LANNA, A. C. et al. Upland rice: Phenotypic diversity for drought tolerance. Scientia Agricola, 78(5):e20190338, 2021.).
During environmental stress, photosynthesis can be affected via different mechanisms such as reducing stomatal conductance and carboxylation; oxidative events that lead to damage to the photosynthetic apparatus; and repression of its regeneration (Singh; Takhur, 2018SINGH, J.; THAKUR, J. K. Photosynthesis and abiotic stress in plants. In: VATS, S. Biotic and abiotic stress tolerance in plants. Singapore: Springer, p. 27-46, 2018. ). To overcome these effects of environmental stress on photoassimilate synthesis, reserve compounds are crucial to ensure the sufficient carbon supply for metabolic activities and during the grain-filling phase (MacNeill et al., 2017MACNEILL, G. J. et al. Starch as a source, starch as a sink: The bifunctional role of starch in carbon allocation. Journal of Experimental Botany , 68(16):4433-4453, 2017.).
Carbohydrates are the main carbon reservoir in plants; however, most are structural carbohydrates, including cellulose, hemicelluloses, and pectins (Schädel et al., 2010SCHÄDEL, C. et al. Quantification and monosaccharide composition of hemicelluloses from different plant functional types. Plant Physiology and Biochemistry, 48(1):1-8, 2010.). Non-structural carbohydrates are dynamic and active in plant metabolism and perform various functions, such as supplying energy and substrates for metabolism and development, source-to-sink carbon transport, osmoregulation, molecular signaling, and protection (Halford et al., 2011HALFORD, N. G. et al. Sugars in crop plants. Annals of Applied Biology, 158(1):1-25, 2011.; Quentin et al., 2015QUENTIN, A. G. et al. Non-structural carbohydrates in woody plants compared among laboratories. Tree Physiology , 35(11):1146-1165, 2015.). The ubiquitous non-structural carbohydrates in plants include soluble carbohydrates such as glucose, fructose, and sucrose and water-insoluble polysaccharide such as starch.
Non-structural carbohydrates are synthesized in the mesophyll cells of leaves. At the end of photosynthesis, one part of the generated triose phosphates is used for the regeneration of ribulose-1,5-bisphosphate for maintaining the Calvin-Benson cycle. The remaining triose phosphates are used for transient starch biosynthesis in chloroplasts or sucrose synthesis in the cytosol. The sucrose that is not used for metabolism is transported through phloem to the sinks along the plant body, where it is metabolized to supply energy or carbon compounds for long-term storage (Ludewig; Flüge, 2013LUDEWIG, F.; FLÜGGE, U. Role of metabolite transporters in source-sink carbon allocation. Frontiers in Plant Science , 4:231, 2013.). Stems and leaf sheaths are the prominent sinks and long-term storage sites in the vegetative development of cereals; these long-term reserves are important for stabilizing the grain yield (Li et al., 2012LI, H. et al. Agronomic and physiological performance of high-yielding wheat and rice in the lower reaches of Yangtze River of China. Field Crops Research, 133(1):119-129, 2012.; Slewinski, 2012SLEWINSKI, T. L. Non-structural carbohydrate partitioning in grass stems: A target to increase yield stability, stress tolerance, and biofuel production. Journal of Experimental Botany , 63(13):4647-4670, 2012.; Deng et al., 2016DENG, F. et al. Polyaspartate urea and nitrogen management affect non-structural carbohydrates and yield of rice. Crop Science, 56(6):3272-3285, 2016.; Stella; Bregaglio; Confalonieri, 2016STELLA, T.; BREGAGLIO, S.; CONFALONIERI, R. A model to simulate the dynamics of carbohydrate remobilization during rice grain filling. Ecological Modelling, 320(1):366-371, 2016.).
Rice grains consist of 80%-90% of starch, which is synthesized during grain filling. In the grain-filling phase, sucrose reaches the developing grains through the phloem, and is rapidly hydrolyzed to provide substrates for starch synthesis (Braun; Wang; Ruan, 2014BRAUN, D. M.; WANG, L.; RUAN, Y. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signaling to enhance crop yield and food security. Journal of Experimental Botany, 65(7):1713-1735, 2014.). Sucrose is transported from the photosynthetically active leaves and from the long-term reserves present in the stems or leaf sheaths, depending on the rice genotype and environment (Yoshida, 1972YOSHIDA, S. Physiological aspects of grain yield. Annual Review of Plant Physiology, 23(1):437-464, 1972.). The carbohydrates transported from long-term storage organs contribute up to 28% of the total grain starch, and this reserve is more stable to daily environmental fluctuations than those generated by photosynthesis (Cock; Yoshida, 1972COCK, J. H.; YOSHIDA, S. Accumulation of 14C-labelled carbohydrate before flowering and its subsequent redistribution and respiration in the rice plant. Japanese Journal of Crop Science, 41(2):226-234, 1972.; Pan et al., 2011PAN, J. et al. Relationships of non-structural carbohydrates accumulation and translocation with yield formation in rice recombinant inbred lines under two nitrogen levels. Physiologia Plantarum, 141(4):321-331, 2011.).
Several studies have shown the role of non-structural carbohydrates in maintaining the rice yield and in responses to abiotic factors (Das; Sarkar; Ismail, 2005DAS, K. K.; SARKAR, R. K.; ISMAIL, A. M. Elongation ability and non-structural carbohydrate levels in relation to submergence tolerance in rice. Plant Science, 168(1):131-136, 2005.; Pan et al., 2011PAN, J. et al. Relationships of non-structural carbohydrates accumulation and translocation with yield formation in rice recombinant inbred lines under two nitrogen levels. Physiologia Plantarum, 141(4):321-331, 2011.). These studies have quantified the non-structural carbohydrates, but sugar profiling has not been performed and thus restricts the broader understanding of non-structural carbohydrate dynamics. The qualitative analyses of carbohydrates in the vegetative organs and grains of rice have been performed (Shanmugavelan et al., 2013SHANMUGAVELAN, P. et al. Evaluation of sugar content and composition in commonly consumed Korean vegetables, fruits, cereals, seed plants, and leaves by HPLC-ELSD. Carbohydrate Research, 380(1):112-117, 2013.; Wang et al., 2016WANG, D. R. et al. Robust phenotyping strategies for evaluation of stem non-structural carbohydrates (NSC) in rice. Journal of Experimental Botany , 67(21):6125-6138, 2016.; Hu et al., 2017HU, X. et al. Determination of soluble sugar profile in rice. Journal of Chromatography B, 1058:19-23, 2017.); however, those in the roots of rice plants need to be studied.
Therefore, the present study aimed to evaluate the non-structural carbohydrate content in the vegetative organs of upland rice at the end of the vegetative growth. The study findings will improve the understanding of non-structural carbohydrate dynamics during the crucial stages for rice yield, the effect of environmental factors, and the yield potential of upland rice plants.
MATERIAL AND METHODS
Plant material collection
The upland rice genotype BRS Esmeralda, developed by Embrapa, was used. BRS Esmeralda is a vigorously-growing plant with a height of 95-108 cm, an average sowing-to-flowering cycle of 77 days, and a total cycle of 105-110 days. Another noteworthy characteristic of BRS Esmeralda is delayed leaf senescence because of the presence of the stay-green trait. All these features are responsible for the high productivity of rice (Castro et al., 2014CASTRO, A. P. et al. BRS Esmeralda: Cultivar de arroz de terras altas com elevada produtividade e maior tolerância à seca. (Embrapa Arroz e Feijão. Comunicado técnico, 215). Santo Antônio de Goiás: Embrapa Arroz e Feijão, 2014. 4p.).
Based on the preliminary chemical analysis, the soil had a pH of 5.8 in H2O and 4.5 in 0.01 M CaCl2, and minerals such as 27.7 mmolc dm−3 Ca, 11.2 mmolc dm−3 Mg, 0 mmolc dm−3 Al, 17 mmolc dm−3 H + Al, 1.6 mmolc dm−3 P, 100 mmolc dm−3 K, 1.1 mg dm−3 Cu, 1.8 mg dm−3 Zn, 11.3 mg dm−3 Fe, 27.2 mg dm−3 Mn, and 42.7 g dm−3 soil organic matter.
We cultivated the BRS Esmeralda plants in plastic trays (30 × 15 × 10 cm) with 3 kg of NPK-fertilized soil (5 g of NPK formulation 5% N, 30% P, 15% K + Zn and 3 g of ammonium sulfate). During the experimental period, the following temperature ranges were used: 28 °C to 32 °C for maximal and 18 °C to 23 °C for minimal. The relative air humidity ranged from 60% to 70%. The trays were irrigated to soil capacity every day. After 20 days of planting, 1.5 g of ammonium sulfate was applied as topdressing. The trays were kept in a greenhouse located at Embrapa Arroz e Feijão in the municipality of Santo Antônio de Goiás, Goiás, Brazil (16° 28’00” S, 49° 17’00” W), at an altitude of 823 m.
The plants were collected at the end of the vegetative phase (V6 in BRS Esmeralda) that occurred 50 days after sowing. This phase was selected because of the intense remobilization of stored carbohydrates to subside reproductive development (Yoshida, 1981YOSHIDA, S. Fundamentals of rice crop science. Los Baños, Laguna, Philippines: The International Rice Research Institute, 1981. 269p.). The plants were collected between 9:00 and 10:00 am, and immediately separated into fully expanded leaf blades (FEL); expanding leaf blades (EXL); culms with leaf sheaths, denoted as stems (STE), and roots (ROT), which constituted the treatment groups.
Experimental design
The experimental design was a completely randomized design. The treatment groups consisted of plant organs such as FEL, EXL, STE, and ROT, with four replicates of each treatment group (n = 16). Each plot consisted of a combination of organs of three plants.
Sample processing
Immediately after the separation, the plant parts were cut into small pieces and were enzymatically inactivated in a microwave oven (Consul, model Facilite 32 L) at the maximal power and with intervals of 10 s up to 1 min. The samples were then oven-dried at 50 °C for 24 h. After complete dehydration, the samples were macerated using a mortar and pestle, using liquid nitrogen, to obtain a fine powder. The sample processing and the extraction of non-structural carbohydrates were performed as described by Quentin et al. (2015QUENTIN, A. G. et al. Non-structural carbohydrates in woody plants compared among laboratories. Tree Physiology , 35(11):1146-1165, 2015.) and Landhäusser et al. (2018LANDHÄUSSER, S. M. et al. Standardized protocols and procedures can precisely and accurately quantify non-structural carbohydrates. Tree Physiology, 38(12):1764-1778, 2018.).
Quantification of non-structural carbohydrates
The quantification of non-structural carbohydrates was performed as follows: exhaustive extraction of the total soluble carbohydrates, followed by the determination of starch content from the soluble carbohydrates-free plant material. For the quantification of soluble carbohydrate content, 0.030 g of fine powder obtained from the aerial organs and 0.300 g of fine powder obtained from the roots were weighed directly in 15-mL polypropylene tubes. For extraction, 3 mL of aqueous ethanol (80% v/v) was added to the plant material, and the tubes were vortexed and incubated in a water bath at 80 °C for 15 min. After centrifugation at 685g for 10 min, the supernatants were collected and pooled. For ensuring the exhaustive extraction of soluble carbohydrates, the same procedure was repeated four more times. All the pooled supernatants were combined, and the total volume was adjusted to 15 mL, comprising the extract of total soluble carbohydrates.
In a 5-mL aliquot of the extract, hydrophobic compounds were removed by adding 2 mL of distilled water and 3 mL of chloroform (5:2:3). The mixture was stirred vigorously and then centrifuged at 2739g for 5 min for phase separation. The aqueous phase was used to quantify the total soluble carbohydrates by the phenol-sulfuric acid method (Dubois et al., 1956DUBOIS, M. et al. Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3):350-356, 1956. ), and glucose was used as a standard. The absorbance was measured at 490 nm in triplicate for each sample.
After the exhaustive extraction of soluble carbohydrates, the resulting powder was oven-dried at 50 °C for 24 h. One aliquot of 0.030 g of each sample was separated from the plant material for roots, as the plant material was free from soluble sugars. This separation is not required for the analysis of other plant parts. Starch was extracted as described by Amaral et al. (2007AMARAL, L. I. V. do et al. Novo método enzimático rápido e sensível de extração e dosagem de amido em materiais vegetais. Hoehnea, 34(4):425-431, 2007.) with the following modifications: thermostable α-amylase diluted in acetate buffer (0.1M, pH 5.0) was added to the dried powder (0.4 mL, 60 U mL-1). After homogenization, the samples were incubated in a water bath at 75 °C for 30 min. This procedure was repeated one more time. After cooling down to room temperature, 0.4 mL (30 U mL-1) of amyloglucosidase diluted in acetate buffer (0.1M, pH 4.5) was added and incubated at 50 °C for 30 min. This step was also repeated one more time. The reaction was terminated by the addition of 0.1 mL of perchloric acid (0.8M), and then the samples were centrifuged at 11068g for 2 min. Glucose liberated in the supernatant after starch hydrolysis was quantified using glucose oxidase and peroxidase reagents (Labtest Diagnóstica S.A., Lagoa Santa, MG, Brazil; Ref. 133), incubated in 30 °C for 15 min. The absorbance was measured in triplicate for each sample using a microplate reader at 490 nm.
Identification and quantification of individual sugars
The aqueous phase of the soluble carbohydrate extract was concentrated using a rotary evaporator (39 °C), resuspended in 1 mL of ultrapure water (18.2 MΩ), and filtered through hydrophilic cellulose acetate membranes (0.45 µm) attached to syringes (Almeida et al., 2017ALMEIDA, L. V. et al. Seasonal changes of fructans in dimorphic roots of Ichthyothere terminalis (Spreng.) Blake (Asteraceae) growing in Cerrado. Science of The Total Environment, 598:404-412, 2017.). The identification and quantification of sugars were performed by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) using an ICS5000 chromatography system (Dionex, Thermo Fischer). The samples were injected and separated using a CarboPac PA-100 column (4 × 250 mm) attached to the respective guard column. Elution (1 mL min-1) was performed by sodium acetate gradient in a sodium hydroxide (100 mM), programmed as follows: 0-2 min, 5 mM; 2.1-8 min, 5-50 mM; 8.1-11 min, 50-150 mM; 11.1-15 min, 250 mM; and 15.1-20 min, 5 mM. The detector was configured according to the manufacturer’s instructions, set using the Chromeleon software (v. 6.8). The sugars were identified by comparing their retention time with the retention time of authentic standards in similar analytical conditions. The sugars were quantified by the external standard method using the calibration curves obtained from the pre-determined concentrations of authentic standards.
Statistical analyses
The data obtained for starch and total soluble carbohydrates, including glucose, fructose, and sucrose, were analyzed to normality by the Shapiro-Wilk test. The data were also tested to verify sphericity by the Mauchly method. As the data did not meet the parameters, the Greenhouse-Geisser correction method was used. The data were analyzed by repeated-measures analysis of variance, and the means were compared by the Bonferroni test. A p-value of <0.05 was considered statistically significant. All the statistical analyses were performed using the R software version 1.2.1335 (R core team, 2019R CORE TEAM. R: A language and environment for statistical computing. Version 1.2.1335. R Foundation for Statistical Computing, Vienna, Austria. 2019. Available in: <Available in: https://www.R-project.org/ >. Access in: April, 17, 2021.
https://www.R-project.org/...
) with the packages “dplyr” (Wicklam et al., 2021WICKHAM, H. et al. Dplyr: A Grammar of Data Manipulation. 2021. R package version 1.0.6. Available in: <Available in: https://cran.r-project.org/web/packages/dplyr/index.html >. Access in: April, 17, 2021.
https://cran.r-project.org/web/packages/...
), “ez” (Lawrence, 2016LAWRENCE, M. A. ez: Easy analysis and visualization of factorial experiments. 2016. R package version 4.4-0. Available in: <Available in: https://cran.r-project.org/web/packages/dplyr/index.html >. Access in: April, 17, 2021.
https://cran.r-project.org/web/packages/...
), “reshape” (Wickham, 2007WICKHAM, H. Reshaping data with the reshape package. Journal of Statistical Software, 21(12):1-20, 2007.), and “rstatix” (Kassambara, 2020KASSAMBARA, A. Rstatix: Pipe-friendly framework for basic statistical tests. 2020. R package version 0.6.0. Available in: <Available in: https://cran.r-project.org/web/packages/rstatix/index.html >. Access in: April, 17, 2021.
https://cran.r-project.org/web/packages/...
).
RESULTS AND DISCUSSION
The total soluble carbohydrate content differed in different plant organs (Figure 1) at the time of vegetative growth. The soluble carbohydrate content in FEL, EXL, and STE was similar (10.4-12.05 mg.g-1 DM), and was higher than that of ROT (4.8 mg g-1 DM) (Table 1, Figure 1a).
Box-plot of total soluble carbohydrate (a) and starch (b) contents in different plant organs of upland rice of the genotype BRS Esmeralda at the time of vegetative growth (n = 4). F statistics and significance levels from repeated-measures analysis of variance, * p <0.0001.
Rice leaves have a complex morphology and are differentiated into three functionally different parts such as leaf blade, sheath, and ligule (Chaffey, 2000CHAFFEY, N. Physiological anatomy and function of the membranous grass ligule. New Phytologist, 146(1):5-21, 2000.). Leaf blades are the regions with the highest rates of gas exchange and photosynthetic activity; therefore, the highest levels of soluble carbohydrates were observed in them (Figure 1a). Leaf sheaths are protective and storage structures and perform photosynthesis at lower rates (17% to 25% of photosynthesis) (Sadok et al., 2020SADOK, W. et al. Sheathing the blade: Significant contribution of sheaths to daytime and nighttime gas exchange in a grass crop. Plant, Cell & Environment , 43(8):1844-1861, 2020.).
The leaf sheaths and stems of rice plants are the vegetative sites for long-term storage before grain filling (Wang et al., 2020WANG, G. et al. Expression profile of the carbon reserve remobilization from the source to sink in rice in response to soil drying during grain filling. Food and Energy Security, 9(3):e204, 2020.). In the present study, the soluble carbohydrate content in leaf sheaths and stems was similar to that in photosynthetically active leaves (Figure 1a); therefore, this pool of soluble carbohydrates represents the photoassimilates that originated in leaf sheaths combined with the soluble carbohydrates transported from leaf blades. In contrast, roots are the heterotrophic organs (Geiger; Shieh, 1993GEIGER, D. R.; SHIEH, W. J. Sink strength: Learning to measure, measuring to learn. Plant, Cell & Environment, 16(9):1017-1018, 1993.) that depend on the photoassimilates transported from the aerial organs. In this study, half of the total soluble carbohydrates were found in ROT (Figure 1a).
Soluble carbohydrates and starch constitute the main carbon reserve in the vegetative organs of rice and other plants of the Poaceae family (Slewinski, 2012). In this study, similar to the soluble carbohydrates, the starch content differed in the vegetative organs of upland rice. The highest starch content of 36.1 mg.g-1 DM was found in STE (Figure 1b). This content is the highest among all the non-structural carbohydrates quantified in this study. In the case of other vegetative organs, EXL exhibited higher starch content than that of FEL, and FEL exhibited higher starch content than that of ROT (Table 1, Figure 1b).
Starch, a polysaccharide, is synthesized from soluble carbohydrates and is classified into the following two types: 1. reserve starch, which is stored in amyloplasts for long-term energy storage; and 2. transient starch, which is synthesized in chloroplasts and metabolized in the plant circadian cycle (Lloyd; Kossmann, 2015LLOYD, J. R.; KOSSMANN, J. Transitory and storage starch metabolism: Two sides of the same coin?. Current Opinion in Biotechnology, 32(1):143-148, 2015.). In the BRS Esmeralda rice genotype, the highest starch content was found in STE (Figure 1b); therefore, it represents the long-term reserve and confirms the remarkable role of this region as a storage organ (Wang et al., 2020WANG, G. et al. Expression profile of the carbon reserve remobilization from the source to sink in rice in response to soil drying during grain filling. Food and Energy Security, 9(3):e204, 2020.).
During photosynthesis, starch is not only a sugar-sink in the chloroplasts of leaves but also a sugar source, as it is remobilized to supply energy when photosynthesis does not meet the energy demand (MacNeill et al., 2017MACNEILL, G. J. et al. Starch as a source, starch as a sink: The bifunctional role of starch in carbon allocation. Journal of Experimental Botany , 68(16):4433-4453, 2017.). We performed sampling in the morning, and hence the starch content in FEL and EXL (Figure 1b) represents the newly synthesized as well as the residual starch of the night period. The use of transitory starch reserves is regulated in such a way that it is not entirely depleted in the night (MacNeill et al., 2017MACNEILL, G. J. et al. Starch as a source, starch as a sink: The bifunctional role of starch in carbon allocation. Journal of Experimental Botany , 68(16):4433-4453, 2017.). The starch present in photosynthetic organs, including leaf blades, represents a relevant fraction of carbon assimilated during the day, which is used to support the metabolism and carbon transport at night; therefore, it represents a short-term reserve (Zeeman; Smith; Smith, 2007ZEEMAN, S. C.; SMITH, S. M.; SMITH, A. M. The diurnal metabolism of leaf starch. Biochemical Journal, 401(1):13-28, 2007.). In contrast to aerial organs, the low starch content in ROT indicates that these organs are not used for starch storage in this rice genotype.
Environmental conditions such as submergence can cause alterations in the relative content of soluble carbohydrates and starch in the vegetative organs of lowland rice. During such environmental conditions, the source-sink balance is altered because of the reduction in photosynthesis and the shifts in the development triggered by the induction of rapid stem elongation (Nurrahma et al., 2021NURRAHMA, A. H. I. et al. Analysis of non-structural carbohydrate in relation with shoot elongation of rice under complete submergence. Sustainability, 13(2):670, 2021.). Rice yield is sensitive to high temperatures during the night period. The ability of storage and remobilization of non-structural carbohydrates from the storage sites, allied to genotype, environment, and their interaction, are the key stability factors during environmental challenges (Moura et al., 2017MOURA, D. S. et al. Non-structural carbohydrates accumulation in contrasting rice genotypes subjected to high night temperatures. Journal of Agricultural Science, 9(12):302-315, 2017.).
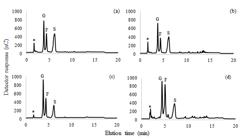
The HPAEC-PAD analysis of soluble carbohydrate content showed that glucose, fructose, and sucrose are the main sugars in all the vegetative organs of the upland rice (Figure 2 HPAEC-PAD). These sugars are commonly reported in the vegetative organs of other plants also. In temperate grasses, fructans are also found in the water-soluble pool (Livingston; Hincha; Heyer, 2009LIVINGSTON, D. P.; HINCHA, D. K.; HEYER, A. G. Fructan and its relationship to abiotic stress tolerance in plants. Cellular and Molecular Life Sciences, 66(13):2007-2023, 2009.). In addition, maltose, raffinose, sugar alcohols, and maltose-based oligosaccharides are found in the vegetative aerial organs of tropical native grasses (Moraes et al., 2013MORAES, M. G. et al. Diversity of non-structural carbohydrates in grasses (Poaceae) from Brazil. Grass and Forage Science, 68(1):165-177, 2013.).
Soluble carbohydrate profile in fully expanded leaf blades (a), expanding leaf blades (b), stems (c), and roots (d) of upland rice of the genotype BRS Esmeralda. The chromatograms were obtained by high-performance anion-exchange chromatography with pulsed amperometric detection. Root extracts were prepared with ten times the amount of plant material compared with the extracts of the other organs. Peak labels stand for glucose (G), fructose (F), and sucrose (S). * represents unidentified peaks.
Glucose and fructose contents differed among the vegetative organs but had the same distribution profile (Figure 3 a, b). Glucose and fructose contents in ROT were lower than those in FEL and STE (Table 2). Sucrose was the major component of the soluble carbohydrate fraction in all the vegetative organs (Figure 3), and its content in ROT was lower than that in all the aerial organs (Table 2).
Box-plot of glucose (a), fructose (b), and sucrose (c) contents in different plant organs of upland rice of the genotype BRS Esmeralda at the time of vegetative phase (n = 4). F statistics and significance levels from repeated-measures analysis of variance, * p <0.0001.
After the use of photoassimilates for metabolism, the photosynthetically-active leaves produce sucrose, which is stored in the vacuoles or is transported through phloem to sink tissues (Ludewig; Flüge, 2013LUDEWIG, F.; FLÜGGE, U. Role of metabolite transporters in source-sink carbon allocation. Frontiers in Plant Science , 4:231, 2013.). In all the aerial organs, the highest content was observed for sucrose, followed by glucose and fructose (Figure 3a, b). The difference between the sucrose distribution patterns among the plant organs was statistically insignificant. STE exhibited the highest glucose and fructose contents but the lowest sucrose content compared with the other aerial organs (Figure 3 c).
As sucrose and monosaccharides such as glucose and fructose are interconvertible, the presence of all of these sugars at the same sites was obvious in all the vegetative organs (Figure 2). The relationships between the different carbohydrate contents reflect the functions and dynamics of the plant organs and are important markers for the evaluation of crop productivity (Purdy et al., 2015PURDY, S. J. et al. Non-structural carbohydrate profiles and ratios between soluble sugars and starch serve as indicators of productivity for a bioenergy grass. AoB Plants, 7:plv032, 2015.). Sucrose occupies a central position in plant metabolism, as it is an essential carbon source for several cellular functions (Ruan et al., 2014RUAN, Y. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annual Review of Plant Biology, 65(1):33-67, 2014.). One of these roles includes the regulation of starch metabolism, as sucrose stimulates enzymes involved in starch biosynthesis, such as ADP-glucose pyrophosphorylase that catalyzes the formation of ADP-glucose and granule-bound starch synthase I that is involved in amylose extension (Yoon et al., 2021YOON, J. et al. Sucrose signaling in higher plants. Plant Science , 302(3):110703, 2021.).
As soon as the sucrose reaches the sink cells, it is unloaded from the phloem. Sucrose is rapidly hydrolyzed by cell wall invertases to release glucose and fructose or by sucrose synthase to release uridine diphosphate glucose and fructose. These products are produced for fulfilling the metabolic demands or for signaling processes (Braun; Wang; Ruan, 2014BRAUN, D. M.; WANG, L.; RUAN, Y. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signaling to enhance crop yield and food security. Journal of Experimental Botany, 65(7):1713-1735, 2014.). The present study showed the role of stems as the photoassimilate sink. Stems use a part of sucrose for starch synthesis (Figures 1 and 3). The difference between the relative proportions of carbohydrates indicates that as soon as sucrose reaches the storage organs, it is rapidly hydrolyzed into the substrates that are necessary for the synthesis of long-term storage starch.
In grasses, the storage of non-structural carbohydrates in stems is a strategy for mediating the source-sink interaction (Slewinski, 2012SLEWINSKI, T. L. Non-structural carbohydrate partitioning in grass stems: A target to increase yield stability, stress tolerance, and biofuel production. Journal of Experimental Botany , 63(13):4647-4670, 2012.). When the amount of photoassimilates exceeds the plant’s requirements for growth and metabolism, the excess sugars are stored in stems, and in the case of BRS Esmeralda, it is mainly stored as starch (Figure 1b). During grain filling, most of the carbon requirement is fulfilled by photosynthesis (You et al., 2016YOU, C. et al. Effect of removing superior spikelets on grain filling of inferior spikelets in rice. Frontiers in Plant Science , 7:1161, 2016.); however, during fluctuations in environmental carbon levels or abrupt impairment of photosynthesis, the reserves stored in stems are useful for grain filling (Slewinksi, 2012SLEWINSKI, T. L. Non-structural carbohydrate partitioning in grass stems: A target to increase yield stability, stress tolerance, and biofuel production. Journal of Experimental Botany , 63(13):4647-4670, 2012.).
CONCLUSIONS
The aerial organs of upland rice of the genotype BRS Esmeralda are the main sites for non-structural storage in the V6 phase. The non-structural carbohydrates such as glucose, fructose, sucrose, and starch were found in all the organs, but their relative contents were different. In the leaf blades, the pool of non-structural carbohydrates was transient and mainly consisted of soluble carbohydrates. The stems and leaf sheaths were found to be the main sites for the long-term storage of starch, whereas the roots did not store carbohydrates at all. This analysis of the distribution of the non-structural carbohydrates has strong implications in assessing the performance of rice crops as well as important markers for the evaluation of rice crop productivity.
ACKNOWLEDGMENTS
G. O. P. F and G. A. B are grateful to CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil), N. B. C is grateful to CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil), for the scholarships. All the authors are grateful to Dra. Marta Cristina Corsi di Fillipi from Embrapa Arroz e Feijão, Santo Antônio de Goiás, GO.
REFERENCES
- ALMEIDA, L. V. et al. Seasonal changes of fructans in dimorphic roots of Ichthyothere terminalis (Spreng.) Blake (Asteraceae) growing in Cerrado. Science of The Total Environment, 598:404-412, 2017.
- AMARAL, L. I. V. do et al. Novo método enzimático rápido e sensível de extração e dosagem de amido em materiais vegetais. Hoehnea, 34(4):425-431, 2007.
- BRAUN, D. M.; WANG, L.; RUAN, Y. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signaling to enhance crop yield and food security. Journal of Experimental Botany, 65(7):1713-1735, 2014.
- CASTRO, A. P. et al. BRS Esmeralda: Cultivar de arroz de terras altas com elevada produtividade e maior tolerância à seca. (Embrapa Arroz e Feijão. Comunicado técnico, 215). Santo Antônio de Goiás: Embrapa Arroz e Feijão, 2014. 4p.
- CHAFFEY, N. Physiological anatomy and function of the membranous grass ligule. New Phytologist, 146(1):5-21, 2000.
- COCK, J. H.; YOSHIDA, S. Accumulation of 14C-labelled carbohydrate before flowering and its subsequent redistribution and respiration in the rice plant. Japanese Journal of Crop Science, 41(2):226-234, 1972.
- DAS, K. K.; SARKAR, R. K.; ISMAIL, A. M. Elongation ability and non-structural carbohydrate levels in relation to submergence tolerance in rice. Plant Science, 168(1):131-136, 2005.
- DAS, A.; UCHIMIYA, H. Oxygen stress and adaptation of a semi-aquatic plant: Rice (Oryza sativa). Journal of Plant Research, 115(5):315-320, 2002.
- DENG, F. et al. Polyaspartate urea and nitrogen management affect non-structural carbohydrates and yield of rice. Crop Science, 56(6):3272-3285, 2016.
- DUBOIS, M. et al. Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3):350-356, 1956.
- FAGERIA, N. K. Yield physiology of rice. Journal of Plant Nutrition, 30(6):843-879, 2007.
- FAGERIA, N. K.; MOREIRA, A.; COELHO, A. M. Yield and yield components of upland rice as influenced by nitrogen sources. Journal of Plant Nutrition , 34(3):361-370, 2011.
- GEIGER, D. R.; SHIEH, W. J. Sink strength: Learning to measure, measuring to learn. Plant, Cell & Environment, 16(9):1017-1018, 1993.
- INTERNATIONAL RICE RESEARCH INSTITUTE - IRRI. GRiSP (Global Rice Science Partnership). Rice almanac. 4th edition. Los Baños Philippines: International Rice Research Institute. 2013. 283p.
- HALFORD, N. G. et al. Sugars in crop plants. Annals of Applied Biology, 158(1):1-25, 2011.
- HU, X. et al. Determination of soluble sugar profile in rice. Journal of Chromatography B, 1058:19-23, 2017.
- KASSAMBARA, A. Rstatix: Pipe-friendly framework for basic statistical tests. 2020. R package version 0.6.0. Available in: <Available in: https://cran.r-project.org/web/packages/rstatix/index.html >. Access in: April, 17, 2021.
» https://cran.r-project.org/web/packages/rstatix/index.html - LAFITTE, H. R.; BENNETT, J. Requirements for aerobic rice: physiological and molecular considerations. In: BOUMAN, B. A. M. et al. Water-wise rice production. Los Baños: International Rice Research Institute, v.1, p. 259-274, 2002.
- LANDHÄUSSER, S. M. et al. Standardized protocols and procedures can precisely and accurately quantify non-structural carbohydrates. Tree Physiology, 38(12):1764-1778, 2018.
- LANNA, A. C. et al. Upland rice: Phenotypic diversity for drought tolerance. Scientia Agricola, 78(5):e20190338, 2021.
- LAWRENCE, M. A. ez: Easy analysis and visualization of factorial experiments. 2016. R package version 4.4-0. Available in: <Available in: https://cran.r-project.org/web/packages/dplyr/index.html >. Access in: April, 17, 2021.
» https://cran.r-project.org/web/packages/dplyr/index.html - LI, H. et al. Agronomic and physiological performance of high-yielding wheat and rice in the lower reaches of Yangtze River of China. Field Crops Research, 133(1):119-129, 2012.
- LIVINGSTON, D. P.; HINCHA, D. K.; HEYER, A. G. Fructan and its relationship to abiotic stress tolerance in plants. Cellular and Molecular Life Sciences, 66(13):2007-2023, 2009.
- LLOYD, J. R.; KOSSMANN, J. Transitory and storage starch metabolism: Two sides of the same coin?. Current Opinion in Biotechnology, 32(1):143-148, 2015.
- LUDEWIG, F.; FLÜGGE, U. Role of metabolite transporters in source-sink carbon allocation. Frontiers in Plant Science , 4:231, 2013.
- MACNEILL, G. J. et al. Starch as a source, starch as a sink: The bifunctional role of starch in carbon allocation. Journal of Experimental Botany , 68(16):4433-4453, 2017.
- MORAES, M. G. et al. Diversity of non-structural carbohydrates in grasses (Poaceae) from Brazil. Grass and Forage Science, 68(1):165-177, 2013.
- MOURA, D. S. et al. Non-structural carbohydrates accumulation in contrasting rice genotypes subjected to high night temperatures. Journal of Agricultural Science, 9(12):302-315, 2017.
- MUTHAYYA, S. et al. An overview of global rice production, supply, trade, and consumption. Annals of the New York Academy of Sciences, 1324(1):7-14, 2014.
- NURRAHMA, A. H. I. et al. Analysis of non-structural carbohydrate in relation with shoot elongation of rice under complete submergence. Sustainability, 13(2):670, 2021.
- OLIVEIRA, A. C.; PEGORARO, C.; VIANA, V. E. The future of rice demand: Quality beyond productivity. Pelotas: Springer, 2020. 533p.
- PAN, J. et al. Relationships of non-structural carbohydrates accumulation and translocation with yield formation in rice recombinant inbred lines under two nitrogen levels. Physiologia Plantarum, 141(4):321-331, 2011.
- PURDY, S. J. et al. Non-structural carbohydrate profiles and ratios between soluble sugars and starch serve as indicators of productivity for a bioenergy grass. AoB Plants, 7:plv032, 2015.
- QUENTIN, A. G. et al. Non-structural carbohydrates in woody plants compared among laboratories. Tree Physiology , 35(11):1146-1165, 2015.
- R CORE TEAM. R: A language and environment for statistical computing. Version 1.2.1335. R Foundation for Statistical Computing, Vienna, Austria. 2019. Available in: <Available in: https://www.R-project.org/ >. Access in: April, 17, 2021.
» https://www.R-project.org/ - RUAN, Y. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annual Review of Plant Biology, 65(1):33-67, 2014.
- SADOK, W. et al. Sheathing the blade: Significant contribution of sheaths to daytime and nighttime gas exchange in a grass crop. Plant, Cell & Environment , 43(8):1844-1861, 2020.
- SCHÄDEL, C. et al. Quantification and monosaccharide composition of hemicelluloses from different plant functional types. Plant Physiology and Biochemistry, 48(1):1-8, 2010.
- SHANMUGAVELAN, P. et al. Evaluation of sugar content and composition in commonly consumed Korean vegetables, fruits, cereals, seed plants, and leaves by HPLC-ELSD. Carbohydrate Research, 380(1):112-117, 2013.
- SINGH, J.; THAKUR, J. K. Photosynthesis and abiotic stress in plants. In: VATS, S. Biotic and abiotic stress tolerance in plants. Singapore: Springer, p. 27-46, 2018.
- SLEWINSKI, T. L. Non-structural carbohydrate partitioning in grass stems: A target to increase yield stability, stress tolerance, and biofuel production. Journal of Experimental Botany , 63(13):4647-4670, 2012.
- STEFFENS, B.; GESKE, T.; SAUTER, M. Aerenchyma formation in the rice stem and its promotion by H2O2 New Phytologist , 190(2):369-378, 2011.
- STELLA, T.; BREGAGLIO, S.; CONFALONIERI, R. A model to simulate the dynamics of carbohydrate remobilization during rice grain filling. Ecological Modelling, 320(1):366-371, 2016.
- WANG, D. R. et al. Robust phenotyping strategies for evaluation of stem non-structural carbohydrates (NSC) in rice. Journal of Experimental Botany , 67(21):6125-6138, 2016.
- WANG, G. et al. Expression profile of the carbon reserve remobilization from the source to sink in rice in response to soil drying during grain filling. Food and Energy Security, 9(3):e204, 2020.
- WICKHAM, H. Reshaping data with the reshape package. Journal of Statistical Software, 21(12):1-20, 2007.
- WICKHAM, H. et al. Dplyr: A Grammar of Data Manipulation. 2021. R package version 1.0.6. Available in: <Available in: https://cran.r-project.org/web/packages/dplyr/index.html >. Access in: April, 17, 2021.
» https://cran.r-project.org/web/packages/dplyr/index.html - XIA, H. et al. Bi-directional selection in upland rice leads to its adaptive differentiation from lowland rice in drought resistance and productivity. Molecular Plant, 12(2):170-184, 2019.
- YOON, J. et al. Sucrose signaling in higher plants. Plant Science , 302(3):110703, 2021.
- YOSHIDA, S. Physiological aspects of grain yield. Annual Review of Plant Physiology, 23(1):437-464, 1972.
- YOSHIDA, S. Fundamentals of rice crop science. Los Baños, Laguna, Philippines: The International Rice Research Institute, 1981. 269p.
- YOU, C. et al. Effect of removing superior spikelets on grain filling of inferior spikelets in rice. Frontiers in Plant Science , 7:1161, 2016.
- ZEEMAN, S. C.; SMITH, S. M.; SMITH, A. M. The diurnal metabolism of leaf starch. Biochemical Journal, 401(1):13-28, 2007.
Publication Dates
-
Publication in this collection
29 Oct 2021 -
Date of issue
2021
History
-
Received
20 Apr 2021 -
Accepted
08 July 2021