Abstract
- Pt-Rh/Al2O3 catalysts were prepared by successive incipient impregnations or coimpregnation. Characterization was achieved by H2 chemisorption and transmission electron microscopy. It was verified that method of preparation, ratio of metal weights and sequence of deposition are factors that result in very distinct catalysts.
Preparation methods; dispersion; catalysts
Pt-Rh/g Al2O3 Influence of Catalyst Preparation Methods on Metallic Particle Dispersion and Size Distribution
N.M. da Fonseca1,G. Djega-Mariadassou2, J.M. Manoli2, J. Felcman3,
D.S. Cunha4,** To whom correspondence should be addressed. To whom correspondence should be addressed. and G.M. da Cruz5
1White Matins Gases Industriais S.A., VPSA Plant Dev. and Degn Mgr, Rua Mayrink Veiga, 9
Centro - Rio de Janeiro 20.090-050 - Brazil
2Laboratoire de Réactivité de Surface Université P & M Curie (Paris VI), CNRS URA 1106,
4 place Jussieu, Tour 54-55, Casier 178, 75252 Paris Cedex 05, France
3Pontifícia Universidade Católica do Rio de Janeiro, Rua Marquês de São Vicente, 225
Rio de Janeiro 22453-900 Brazil
4Laboratório Associado de Combustão e Propulsão (LCP), Instituto Nacional de Pesquisas Espaciais (INPE), Caixa Postal 01, Cachoeira Paulista, SP, 12630-000 Brazil
Fax: (+ 55-12) 561-1992, Phone: (+55-12) 560-9219
e-mail: david@tupi.lcp.br
5Faculdade de Engenharia Química de Lorena - FAENQUIL, Rodovia Itajubá - Lorena, Km 74,5
Lorena 12600-000 Brazil
(Received: November 5, 1997; Accepted: March 27, 1998)
Abstract - Pt-Rh/Al2O3 catalysts were prepared by successive incipient impregnations or coimpregnation. Characterization was achieved by H2 chemisorption and transmission electron microscopy. It was verified that method of preparation, ratio of metal weights and sequence of deposition are factors that result in very distinct catalysts.
Keywords: Preparation methods, dispersion, catalysts.
INTRODUCTION
Supported metallic catalysts are used on a large scale for oil refining, to promote the conversion of automotive gas exhaust, carbon monoxide hydrogenation, and many other processes
(1). It is known from the literature that supported metallic catalysts, combining group VIII metals by several methods and in different proportions, have greatly improved the control of pollution originating from combustion engines
(2). Of these catalysts, supported Pt-Rh bimetallic catalysts have an outstanding role
(3).
The purpose of this work is to investigate the preparation conditions which yield good metal dispersion and a relatively narrow range of metallic particle sizes, even when the total metal content impregnated on the support is high.
EXPERIMENTAL PART
Catalyst Preparation
Pt/Al
2O
3 and Rh/Al
2O
3 catalysts, containing 1, 2, 3, 4, 6 or 9 metal weight percent, were prepared by the incipient impregnation method using gamma-alumina as the support (total specific surface area = 260 m
2/g and pore volume = 0.90 ml/g), which had been previously activated by calcination in air at 873 K during 4 hours. H
2PtCl
6 and RhCl
3 solutions, with 0.5 N free acidity, were used. After impregnation, the solids were dried in air at 383 K during 30 minutes, and later submitted to reduction in an H
2 flux by increasing the temperature slowly up to 423 K during two hours and then more quickly up to 673 K, and maintainig this temperature for four more hours.
Bimetallic catalysts were prepared by two different methods: a) successive impregnations: monometallic catalysts containing 1, 2, 3, 4 or 6 wt% of Pt or Rh, which had already been reduced, were impregnated a second time with a solution of the second metal, Rh or Pt, respectively. Then they were subjected to another thermal treatment similar to the first. Weight ratios of the first metal (M1) to the second (M2), M1/M2, were equal to 1/2 and 2/1. Total metal weight percentages in these catalysts were 3, 6 and 9 wt%; b) coimpregnation: bimetallic catalysts, with compositions identical to those prepared by successive impregnations (total metal contents of 3, 6 and 9 wt%, with weight ratios M1/M2 = 1/2 and 2/1), were prepared by a single impregnation with a mixture of Pt and Rh solutions, as described earlier. After impregnation, the catalysts were subjected to the same thermal treatment as that performed for the monometallic catalysts.
Catalyst Characterization
The catalysts were characterized by H
2 chemisorption at 298 K and by high ultra resolution transmission electron microscopy.
Chemical adsorptions were performed in a classic volumetric device after the following treatment: H2 (60 ml/min.) at 673 K during one hour; He (60 ml/min) at 673 K during one hour and high vacuum (0.13 x 10-2 Pa) during 30 minutes at room temperature (298 K).
Characterization by transmission electron microscopy was performed in a JEM 120 device at the Paris VI University. Pictures were taken at an enlargement of 330,000. Subsequently, photographs were enlarged four times.
RESULTS AND DISCUSSION
Table 1 summarizes the main results obtained for the characterization of the monometallic catalysts, Pt/Al
2O
3 and Rh/Al
2O
3, by H
2 chemisorption. The assumed adsorption stoichiometry was one hydrogen atom to one metal atom on the surface (H/M
s).
From the experimental results presented in Table 1, we can observe that:
- the Pt/Al2O3 catalysts have dispersion values around 30%;
- the Rh/Al2O3 catalysts have dispersion values around 4 %; and
- the rhodium dispersion values on alumina are much smaller than those for platinum on the same support. Such a conclusion is in accord with that of Fiedorow et al.(4), who compare the sintering rates of Pt, Ir and Rh on alumina in hydrogen and oxygen atmospheres. These authors observed that in oxygen, Rh is more stable than Pt. On the other hand, in hydrogen Pt is more stable than Rh.
Tables 2 and 3 present the composition and the results of characterization obtained by H2 chemisorption for the Pt-Rh/Al2O3 catalysts prepared by successive impregnations and coimpregnation.
2O3 and Rh/Al2O3, obtained by H2 chemisorption(a) metallic dispersion; (b)

0
p
= average diameter of metallic particles (92/D
(%)
).
(a) Pt was added before Rh; (b) Rh was adedd before Pt.
By observing the dispersion values in Tables 2 and 3, one can verify that:
- the introduction of platinum after rhodium gives larger values of metallic phase dispersion for most of the preparations;
- whatever the sequence of deposition for the metals, a Pt/Rh ratio equal to 2 yields, in general, larger values for metallic phase dispersion than a Pt/Rh ratio equal to 1/2; and
- the bimetallic catalysts prepared by successive impregnations and those prepared by coimpregnation present dispersion values in a range varying from 4 to 30 wt%, similar to those presented by monometallic catalysts.
Table 4 shows the effect of the increase in reduction temperature from 673 K to 773 K on the dispersion of the bimetallic catalysts with 6 wt% total metal content, prepared by either coimpregnation or successive impregnations. Such materials were selected because they are representative of the whole series.
By comparing the results shown in Tables 2, 3 and 4, it is possible to verify that the majority of the bimetallic catalysts presents a significant decrease in dispersion values, which means that a sintering process must certainly have occurred in the metallic phase, when the reduction took place at 773 K in comparison to that which occurred at 673 K.
Aiming at complementing the information obtained from the gas volumetry characterizations, the transmission electron microscopy technique was used for eight bimetallic catalysts.
Table 5 presents the results of the metallic particle size distribution for the catalysts characterized by transmission electron microscopy (TEM).
The first three catalysts, prepared by successive impregnations, were first impregnated with platinum, followed by rhodium, on alumina. The 4Pt-2Rh catalyst has a total metallic content of 6 wt%, and its particles tend to be small, which is characteristic of 4Pt catalysts and thus favors a good dispersion for the second metal deposited, rhodium. In other words, when added first, platinum has the tendency to increase rhodium dispersion. However, this effect seems to be restricted only to catalysts containing up to a total metal content of 6 wt%, since in the case of the 6Pt-3Rh and 3Pt-6Rh catalysts, both having 9 wt% total metal contents, Table 5 shows a drastic decrease in the number of particles with dp £ 1 nm, and an increase in the number with larger diameters, including those with dp > 10 nm, and even those with dp > 50 nm.
When the catalysts have rhodium as the first metal impregnated, followed by platinum, the phenomenon of platinum influencing the dispersion of rhodium does not seem to be limited to the total metal content of 6 wt%, since the 6Rh-3Pt and 3Rh-6Pt catalysts that have 9 wt% contents, present very different dispersion. The majority of the particles of the former have large dimensions, which is not only the result of a bad dispersion of the first metal, rhodium, but also of not presenting small particles of the second metal, platinum. In other words, in the case of the 6Rh-3Pt catalyst, a good platinum dispersion does not occur when this is the second metal to be impregnated. When one inverts these metal contents to 3Rh-6Pt, the phenomenon observed is just the opposite, with platinum redispersing the rhodium during the second thermal treatment in H2.
Thus, one can conclude that, in the case of the preparations with successive impregnations, the following parameters favor a larger dispersion of the metallic phase:
-
Pt-Rh Catalysts
- Pt content larger than Rh content; and
- total metallic content up to 6 wt%.
-
Rh-Pt Catalysts
- Pt content larger than Rh content.
The results shown in Table 5, for catalysts prepared by coimpregnation are hard to interpret; because the two metal solutions were introduced in a single impregnation, it would be expected that the metal present in a larger proportion would be the one to have a greater influence on the metallic phase dispersion state. This fact really occurs with the 4Pt+2Rh(co) catalyst. The opposite situation is observed with the 6Pt+3Rh(co) and 3Pt+6Rh(co) catalysts. It is possible that the total metal content of 9 wt% interfered in the platinum role by favoring the dispersion of the metallic phase, as occurred with the Pt-Rh catalysts prepared by successive impregnations.
From the results discussed above, one can conclude that the high metal content bimetallic catalysts, prepared by coimpregnation, present textural properties which are shown to be extremely sensitive to preparation parameters. At present, it is not possible to point out the most important parameter.
Table 6 presents a comparison of average diameters of metallic particles calculated by TEM, with the corresponding diameters obtained by H2 chemisorption. Three different values for the average characteristic dimension (0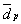 ), obtained by TEM, are shown. They were calculated particle length (L), area (A) and volume (V).
), obtained by TEM, are shown. They were calculated particle length (L), area (A) and volume (V).
One can verify that, for four of the eight catalysts analyzed, there is reasonable agreement between the values for
V and those obtained by hydrogen chemisorption ( QH).
QH).
In the case of the 6Pt-3Rh, 3Pt-6Rh, 6Pt+3Rh(co) and 3Pt+6Rh(co) catalysts, the best agreement is shown by
A, mainly because good agreement between H2 chemisorption characterization and V occurs only when the particle size distribution range is narrow, which is not the case with these four materials.
V occurs only when the particle size distribution range is narrow, which is not the case with these four materials.
From the results presented above, one can say that the reasonable agreement between the  values obtained by H2 chemisorption, and those for
values obtained by H2 chemisorption, and those for  V or
V or  A obtained by TEM is a result of a narrow distribution range for metallic particle size.
A obtained by TEM is a result of a narrow distribution range for metallic particle size.
The composition of the metallic particles, obtained by different preparation methods used in this research, will be discussed in a technical paper to be published in the future.
Results of the characterization bimetallic catalysts by TEM and comparison with that obtained by H
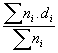
; (b)
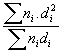
; (c)
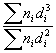
; (d)

QH = 92/D
(%)QH
CONCLUSIONS
Based on the discussions presented in this paper, one can conclude that:
- the results obtained by H2 chemisorption show that platinum disperses much more (~ 30 %) than rhodium (~ 4 %) on alumina for monometallic catalysts with total metal contents from 3 to 6 wt%;
- bimetallic catalysts go through a remarkable sintering process, when reducted in H2 at 773 K;
- values obtained for
QH and for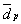 V or
V or 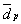 A are coherent enough to show that the bimetallic catalysts are very different from one another, as a function of the preparation methods used;
A are coherent enough to show that the bimetallic catalysts are very different from one another, as a function of the preparation methods used;
- when the successive impregnation preparation method is used, the introduction of Rh before Pt produces bimetallic solids which generally have higher dispersion than when the inverse sequence is used for the addition of the metals; and
- in addition to the sequence of adding metals, the parameters that have the most influence on the dispersion of the metallic phase for bimetallic catalysts are total metallic content and the weight proportions of Rh and Pt.
REFERENCES
Fiedorow, R.M.J.; Chahar, B.S. and Wanke, S.E., The Sintering of Supported Metal Catalysts. II Comparasion of Sintering Rates of Supported Pt, Ir and Rh Ctalysts, in Hydrogen and Oxygen, J. Catal., 51, 193-202 (1978).
Gates, B. C., Supported Metal Clusters: Synthesis, Structure and Catalysis, Chem. Rev., 95, 511-522 (1995).
Kreuzer, T.; Lox, E. S.; Lindner, D. and Leyer, J., Advanced Exhaust Gas Aftertreatment Systems for Gasoline and Diesel Fuelled Vehicles, Catal. Today, 29, 17-27 (1996).
Wang, T. and Schmidt, I., Intraparticle Redispersion of Rh and Pt-Rh Particles on SiO2 by Oxidation-Reduction Cycling, J. Catal., 70, 1987 (1981).
- Fiedorow, R.M.J.; Chahar, B.S. and Wanke, S.E., The Sintering of Supported Metal Catalysts. II Comparasion of Sintering Rates of Supported Pt, Ir and Rh Ctalysts, in Hydrogen and Oxygen, J. Catal., 51, 193-202 (1978).
- Gates, B. C., Supported Metal Clusters: Synthesis, Structure and Catalysis, Chem. Rev., 95, 511-522 (1995).
- Kreuzer, T.; Lox, E. S.; Lindner, D. and Leyer, J., Advanced Exhaust Gas Aftertreatment Systems for Gasoline and Diesel Fuelled Vehicles, Catal. Today, 29, 17-27 (1996).
- Wang, T. and Schmidt, I., Intraparticle Redispersion of Rh and Pt-Rh Particles on SiO2 by Oxidation-Reduction Cycling, J. Catal., 70, 1987 (1981).
Publication Dates
-
Publication in this collection
09 Oct 1998 -
Date of issue
June 1998
History
-
Accepted
27 Mar 1998 -
Received
05 Nov 1997


 1
1