ABSTRACT:
A set of attributes endows the soils with distinctive characteristics and astute understanding is required in order to formulate suitable strategies for soil management. The aim of this study was to physically, chemically and mineralogically characterize samples of the main soil classes in Minas Gerais, Brazil, determine the point of zero salt effect (PZSE) and the point of zero charge (PZC), and ascertain the correlation between these factors and soil attributes. The soils evaluated presented different textural classes ranging from loamy sand (Entisol) to very clayey (some Oxisols and Ultisols). The soils differed substantially in terms of fertility, presenting a range from dystrophic (low fertility, base saturation < 50 %) to eutrophic character (fertility, base saturation ≥ 50 %), even within the same soil class, such as the Oxisols, which suggests the concurrence of the parent material. Highly weathered soils are predominant in Minas Gerais and these soils are composed predominantly of kaolinite, gibbsite, goethite and hematite. Traces of hydroxy-Al interlayered vermiculite and illite were also found in the Oxisols, Ultisols and Inceptisols. A correlation between the PZSE and the PZC in the A horizon was observed. A high degree of correlation was observed between the PZC and the exchangeable aluminum and the ratio of iron obtained by ammonium oxalate and dithionite-citrate (Feo/Fed) in both the A and B horizons of soil classes. The results obtained reinforce the importance of knowledge of soil attributes to the adoption of practices such as the management of phosphate fertilization in clayey soils and liming in soils rich in aluminum.
Keywords:
soil attributes; weathered soils; secondary minerals; mineralogical composition; point of zero charge
Introduction
Due to the soil diversity in Brazil, soil characterization is essential to better agricultural or environmental usage and subsequent management. Mineralogical studies are very important to understanding the physical and chemical properties of the soil ( Camargo et al., 2008Camargo, L.A.; Marques Júnior, J.; Pereira, G.T.; Horvat, R.A. 2008. Spatial variability of mineralogical attributes of an oxisol under different relief forms. I. Clay fraction mineralogy. Revista Brasileira de Ciência do Solo 32: 2269-2277 (in Portuguese, with abstract in English). ; Almeida et al., 2021Almeida, C.C.; Fontes, M.P.F.; Dias, A.C.; Pereira, T.C.T.; Ker, J.C. 2021. Adsorption and desorption of arsenic and its immobilization in soils. Scientia Agricola 78: e20180386. ). Phosphorous adsorption is mainly attributed to gibbsite, goethite and hematite, the predominant minerals in highly weathered soils ( Fontes and Weed, 1996Fontes, M.P.F.; Weed, S.B. 1996. Phosphate adsorption by clays from Brazilian Oxisols: relationships with specific surface area and mineralogy. Geoderma 72: 37-51. ; Schaefer et al., 2008Schaefer, C.; Fabris, J.; Ker, J. 2008. Minerals in the clay fraction of Brazilian Latosols (Oxisols): a review. Clay Minerals 43: 137-154. ; Fontes, 2012Fontes, M.P. 2012. Behavior of heavy metals in soils: individual and multiple competitive adsorption. p. 77-118. In: Selim, H.M., ed. Competitive sorption and transport of heavy metals in soils and geological media. CRC Press, Boca Raton, FL, USA. ). Electrochemical properties of soils are fundamental to an understanding of the physico-chemical phenomena ( Camêlo et al., 2018Camêlo, D.L.; Ker, J.C.; Fontes, M.P.F.; Costa, A.C.S.; Corrêa, M.M.; Leopold, M. 2018. Mineralogy, magnetic susceptibility and geochemistry of Fe-rich Oxisols developed from several parent materials. Scientia Agricola 75: 410-419. ) that affect soil fertility, availability of nutrients for plants, phytoavailability and metal mobility ( Fontes and Alleoni, 2006Fontes, M.P.F.; Alleoni, L.R.F. 2006. Electrochemical attributes and availability of nutrients, toxic elements, and heavy metals in tropical soils. Scientia Agricola 63: 589-608. ).
The soils of Minas Gerais represent a large part of those most commonly found in Brazil, such as Oxisols, Ultisols, and Inceptisols. The climatic conditions existing in the state favor the formation and stability of kaolinite, in which the Oxisols and Ultisols stand out.
The factors that favor the formation of kaolinite are those related to warm and humid climate conditions, with moderate drainage, without excessive leaching and low pH ( Fontes et al., 2001Fontes, M.P.F.; Camargo, O.A.; Sposito, G. 2001. Electrochemistry of colloidal particles and its relationship with the mineralogy of highly weathered soils. Scientia Agricola 58: 627-646. ). These authors mention that under moderate drainage and leaching conditions, the tendency is towards a strong loss of cations and moderate loss of silica, which allows for the formation of kaolinite. Furthermore, in drainage conditions that favor very strong leaching an almost complete desilication can occur, favoring the formation of aluminum (Al) and iron (Fe) oxy-hydroxides. Thus, kaolinite, Fe and Al oxy-hydroxides are the most common constituents found in tropical soils. They exert great influence on certain physical and chemical characteristics of soils, such as aggregate stability and ion exchange reactions, in addition to being determinant in their pigmentation.
Thus, the study of soil attributes provides significant information on the weathering process, losses of nutrients and organic matter, availability of nutrients for plants, and potential environmental changes among others. Consequently, this study aimed to physically, chemically, electrochemically and mineralogically characterize samples of the main soil classes in the state of Minas Gerais, Brazil.
Materials and Methods
Soil samples
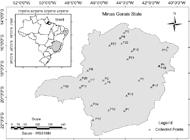
Twenty-three representative profiles of the main soil classes in the state of Minas Gerais, Brazil ( Figure 1 ), were selected at depths of 0 to 20 cm and 50 to 70 cm ( Embrapa, 2013Empresa Brasileira de Pesquisa Agropecuária [EMBRAPA]. 2013. Brazilian System of Soil Classification = Sistema Brasileiro de Classificação de Solos. Embrapa, Brasília, DF, Brazil (in Portuguese). ). After collection, the soil samples were air-dried, gently crumbled and sieved through a 2-mm mesh to obtain the air-dried fine earth (ADFE).
The soils were classified according to the Brazilian System of Soil Classification ( Embrapa, 2013Empresa Brasileira de Pesquisa Agropecuária [EMBRAPA]. 2013. Brazilian System of Soil Classification = Sistema Brasileiro de Classificação de Solos. Embrapa, Brasília, DF, Brazil (in Portuguese). ) and up to the category level of the subgroup ( Table 1 ) based on Soil Taxonomy ( Soil Survey Staff, 2014Soil Survey Staff. 2014. Keys to Soil Taxonomy. USDA-Natural Resources Conservation Service, Washington, DC, USA. ).
Soil classification according to the Brazilian System of Soil Classification (SiBCS) and U.S. Soil Taxonomy ( Soil Survey Staff, 2014Soil Survey Staff. 2014. Keys to Soil Taxonomy. USDA-Natural Resources Conservation Service, Washington, DC, USA. ).
Physical analysis
Texture, water-dispersible clay (WDC), and the degree of flocculation (DF) were determined ( Embrapa, 2017Empresa Brasileira de Pesquisa Agropecuária [EMBRAPA]. 2017. Manual of Soil Analysis Methods = Manual de Métodos de Análise de Solo. Embrapa Solos, Rio de Janeiro, RJ, Brazil (in Portuguese). ). For WDC analysis, 10 g of ADFE were weighed in a container with 200 mL of deionized water. These recipients were capped and agitated for 16 h at 50 rpm. The sedimentation time was calculated based on Stokes' Law.
Soil fertility assessment
The following analyses were performed: pH in water and 1 mol L−1 KCl; total organic carbon (TOC) ( Yeomans and Bremner, 1988Yeomans, J.C.; Bremner, J.M. 1988. A rapid and precise method for routine determination of organic carbon in soil. Communications in Soil Science and Plant Analysis 19: 1467-1476. ); available phosphorous (P), sodium (Na+), and potassium ( K+) after extraction with 0.5 mol L−1 HCl + 0.0125 mol L−1 H2SO4 (Mehlich-1); calcium (Ca2+) and magnesium (Mg2+) by atomic absorption spectroscopy and Al3+ by titration after extraction with 1 mol L−1 KCl; potential acidity (H+Al) by titration after extraction with 0.5 mol L−1 Ca(CH3COO2) at pH 7.0, and remaining P ( Alvarez and Fonseca, 1990Alvarez, V.V.H.; Fonseca, D.M. 1990. Definition of phosphorus levels to determine the maximum phosphate adsorption capacity and the response curves for greenhouse experiments. Revista Brasileira de Ciência do Solo 14: 49-55 (in Portuguese, with abstract in English). ). The micronutrients iron (Fe) and manganese (Mn) were extracted by means of a chelating solution (DTPA), and the chemical elements were determined by atomic absorption spectrophotometry. Boron (B) was extracted with hot water and determined by colorimetry. All chemical analyses were based on the Brazilian Agricultural Research Corporation's methods ( Embrapa, 2017Empresa Brasileira de Pesquisa Agropecuária [EMBRAPA]. 2017. Manual of Soil Analysis Methods = Manual de Métodos de Análise de Solo. Embrapa Solos, Rio de Janeiro, RJ, Brazil (in Portuguese). ).
Mineralogical analyses
Firstly, the clay and silt fractions were dispersed and separated by sedimentation and the sand fraction segregated by sieving from ADFE ( Embrapa, 2017Empresa Brasileira de Pesquisa Agropecuária [EMBRAPA]. 2017. Manual of Soil Analysis Methods = Manual de Métodos de Análise de Solo. Embrapa Solos, Rio de Janeiro, RJ, Brazil (in Portuguese). ).
X-ray diffraction (XRD) analyses were conducted on sand, silt and clay fractions on an X'pert PRO diffractometer (CoKα radiation) in the ranges of 4 to 50 °2θ (natural slides) and 5 to 30 °2θ (clay slides with treatments), with intervals of 0.02 °2θ to 1 step s−1, voltage of 40 kV, and current of 30 mA. The deferrified slides were prepared both with and without treatment. The samples were saturated with 1.0 mol L−1 KCl and MgCl2 solutions. KCl-impregnated slides were read before and after heating in a muffle at 550 °C for 3 h. In MgCl2-impregnated slides, the reading was taken before and after adding one drop of glycerol.
The Fe forms were determined by the methods of dithionite-citrate (Fed)( Coffin, 1963Coffin, D. 1963. A method for the determination of free iron in soils and clays. Canadian Journal of Soil Science 43: 7-17. ), and 0.2 mol L−1 ammonium acid oxalate at pH 3.0 (Feo)( McKeague and Day, 1966McKeague, J.; Day, J. 1966. Dithionite-and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Canadian Journal of Soil Science 46: 13-22. ). The Fe was quantified by an atomic absorption spectrophotometer.
Thermogravimetric determinations
The thermogravimetric analyses (TGA) were conducted by a Shimadzu TGA 50 Thermal Analyzer. For this process, the samples were placed in an alumina cell. The thermobalance operated with a constant flow of nitrogen atmosphere and the analysis temperature range was between the ambient temperature and 800 °C, at a heating rate of 10 °C min−1.
Point of zero Charge
The point of zero charge (PZC) was estimated from the pH values, according to the following equation: ( Keng and Uehara, 1974Keng, J.C.W.; Uehara, G. 1974. Chemistry, mineralogy and taxonomy of Oxisols and Uttisols. Proceedings of Soil and Crop Sciences Society 33: 119-126. ).
Point of Zero Salt Effect
The point of zero salt effect (PZSE) was determined by potentiometric titration ( Fontes et al., 2001Fontes, M.P.F.; Camargo, O.A.; Sposito, G. 2001. Electrochemistry of colloidal particles and its relationship with the mineralogy of highly weathered soils. Scientia Agricola 58: 627-646. ) by the traditional method with modifications ( Raij and Peech, 1972Raij, B.V.; Peech, M. 1972. Eletrochemical properties of some Brazilian soils. Soil Science Society of America Proceedings 36: 587-593. ). A mass of 4 g of each soil sample was placed in 50 mL containers with 12.50 mL sodium nitrate solution at concentrations of 0.002, 0.02, and 0.2 mol L−1. The samples were acidified using increasing volumes of acid solution (0.5, 1.0, 2.0, and 3.0 mL 0.1 mol L−1 HCl) and titrated with a basic solution (0.5, 1.0, 2.0, and 3.0 mL 0.1 mol L−1 NaOH). Subsequently, distilled water was added to obtain a final volume of 25 mL in each container and dilution of the sodium nitrate concentration to 0.001, 0.01, and 0.1 mol L−1. The material was sealed, stirred, and allowed to stand for 24 h. The PZSE was calculated considering the pH value at which the curves crossed (pH = PZSE).
Statistical analysis
Pearson's correlation coefficient was determined to verify the degree of association between variables ( p < 0.01 and p < 0.05). The PZSE and estimated PZC data were analyzed by the Student t-test ( p < 0.05) in order to compare the means.
Results and Discussion
Soil physical characteristics
The studied soils differed substantially as regards texture even within the same soil class ( Table 2 ). For clay content, Oxisols (P1 to P12) had the highest average value, then Ultisols (P13 to P18), Inceptisols (P19 to P22) and Entisols (P23). Higher clay content was found in P2 and in the subsurface diagnostic horizons of the P6 ( Table 2 ). These results are in agreement with those found by other Brazilian authors ( Matos et al., 2001Matos, A.; Fontes, M.; Costa, L.; Martinez, M. 2001. Mobility of heavy metals as related to soil chemical and mineralogical characteristics of brazilian soils. Environmental Pollution 111: 429-435. ; Mello et al., 2006Mello, J.; Roy, W.; Talbott, J.; Stucki, J. 2006. Mineralogy and arsenic mobility in arsenic-rich Brazilian soils and sediments. Journal of Soils and Sediments 6: 9-19. ; Pacheco et al., 2018Pacheco, A.A.; Ker, J.C.; Schaefer, C.E.G.R.; Fontes, M.P.F.; Andrade, F.V.; Martins, E.d.S.; Oliveira, F.S.D. 2018. Mineralogy, micromorphology, and genesis of soils with varying drainage along a hillslope on granitic rocks of the Atlantic Forest Biome, Brazil. Revista Brasileira de Ciência do Solo 42: e0170291. ).
The clayey soils showed a predominance of clay minerals with a majority of pH-dependent charges, which enhances P adsorption, especially at low pH values. Phosphate adsorption capacity has been shown to be dependent on the variation in the Fe oxide mineralogy of the clay fraction ( Fontes and Weed, 1996Fontes, M.P.F.; Weed, S.B. 1996. Phosphate adsorption by clays from Brazilian Oxisols: relationships with specific surface area and mineralogy. Geoderma 72: 37-51. ).
The silt/clay ratio and WDC were inversely related to the degree of soil weathering, i.e. the higher the silt/clay ratio and WDC, the less the pedogenesis ( Resende et al., 2007Resende, M.; Curi, N.; Rezende, S.B.; Corrêa, G.F. 2007. Pedology: Basis for Distinguishing Environments = Pedologia: Base para Distinção de Ambientes. 5ed. UFLA, Lavras, MG, Brazil (in Portuguese). ). A direct relationship was observed with DF since the higher this variable is, the greater the pedogenesis. In general, the Oxisols had a lower silt/clay ratio, lower WDC, and higher DF, followed by Ultisols and Inceptisols ( Table 2 ). The silt/clay ratio of the Ultisols (0.57) was close to the average value found for the Oxisols (0.40), showing that soils of both classes are at an advanced weathering stage.
In the soils, the silt + clay ratio was higher in Inceptisols, followed by Oxisols and Ultisols ( Table 2 ). In the Oxisols, the lower contents of silt + clay found in P3 and P10 are due to the parent material being derived from granitic rock ( Ministério das Minas e Energia, 1982Ministério das Minas e Energia. 1982. Project RadamBrazil: Geology, Geomorphology, Pedology, Vegetation, Potential Land Use = Projeto RadamBrasil: Geologia, Geomorfologia, Pedologia, Vegetação e Uso Potencial da Terra. Ministério das Minas e Energia, Departamento Nacional de Produção Mineral, Rio de Janeiro, RJ, Brazil (in Portuguese). ), as observed in the collection sites located in Itacarambi (P3) and Prata (P10), while the highest contents (in excess of 90 %) were found in an Oxisol (P2) derived from mafic/ultramafic rocks of the Macaúbas Group ( Ministério das Minas e Energia, 1982Ministério das Minas e Energia. 1982. Project RadamBrazil: Geology, Geomorphology, Pedology, Vegetation, Potential Land Use = Projeto RadamBrasil: Geologia, Geomorfologia, Pedologia, Vegetação e Uso Potencial da Terra. Ministério das Minas e Energia, Departamento Nacional de Produção Mineral, Rio de Janeiro, RJ, Brazil (in Portuguese). ), located in Capão, MG. In the Ultisols, the silt + clay contents indicate a higher participation of finer particles in the diagnostic horizon.
For the Inceptisols, results of silt + clay presented maximum contents of 96 %, with a large participation of the first fraction, even in the diagnostic horizon. High WDC contents, associated with a low DF and high silt/clay ratio ( Table 2 ), suggest a lower pedogenesis and water percolation capacity in the Inceptisols, due to the discontinuity of the porous system related to coarser texture and the mineralogy of the clay fraction, which is predominantly kaolinitic and micaceous. According to Mello et al. (2006)Mello, J.; Roy, W.; Talbott, J.; Stucki, J. 2006. Mineralogy and arsenic mobility in arsenic-rich Brazilian soils and sediments. Journal of Soils and Sediments 6: 9-19. , the Inceptisols are subject to erosion, leading to the eventual translocation of particles to lowlands and streams where ferric oxy-hydroxides may be reduced and release toxic metals, such as arsenic, into the environment.
In the P20, unlike the other Inceptisols, silt and clay fractions together reached a percentage of 40 % ( Table 2 ) since the greater part of the parent material is from sandstone rocks belonging to the Araxá Group. Soils developed from this geology are usually gravelly, sometimes stony, with a medium texture and low-activity clay ( Ministério das Minas e Energia, 1982Ministério das Minas e Energia. 1982. Project RadamBrazil: Geology, Geomorphology, Pedology, Vegetation, Potential Land Use = Projeto RadamBrasil: Geologia, Geomorfologia, Pedologia, Vegetação e Uso Potencial da Terra. Ministério das Minas e Energia, Departamento Nacional de Produção Mineral, Rio de Janeiro, RJ, Brazil (in Portuguese). ). In this profile, it is possible that the highest clay contents found in the Bi horizon were favored by the coarser texture of the parent material, with an eventual illuviation of fine materials, which, however, does not allow for classifying it as a Bt horizon ( Embrapa, 2013Empresa Brasileira de Pesquisa Agropecuária [EMBRAPA]. 2013. Brazilian System of Soil Classification = Sistema Brasileiro de Classificação de Solos. Embrapa, Brasília, DF, Brazil (in Portuguese). ).
P22 corresponds to a soil derived from calcareous rocks from the Lagoa do Jacaré Formation (Bambuí Group) ( Ministério das Minas e Energia, 1982Ministério das Minas e Energia. 1982. Project RadamBrazil: Geology, Geomorphology, Pedology, Vegetation, Potential Land Use = Projeto RadamBrasil: Geologia, Geomorfologia, Pedologia, Vegetação e Uso Potencial da Terra. Ministério das Minas e Energia, Departamento Nacional de Produção Mineral, Rio de Janeiro, RJ, Brazil (in Portuguese). ) and has a finer grain size compared to P20. The silt/clay ratio is of the order of 1.6 ( Table 2 ), reflecting the parent material and a low weathering of these soils possibly due to higher material resistance and lower water infiltration capacity. In the region of Montes Claros, in the north of Minas Gerais, where the profile P21 was collected, there is a great influence of the pelitic material of the Bambuí Group, with soils tending to present characteristics related to the sub-horizontality of the parent material.
The Entisols (P23) presented 7 % clay and 5 % silt ( Table 2 ), indicating a large participation of coarse and fine sand in the soil and presenting as main negative influences low water retention capacity and nutritional reserve for plants.
Soil chemical characteristics
In the studied soils, except for the B horizon in P11, the values of pH in water were higher compared to those observed for the pH in KCl, indicating a balance of negative charges on the surface of soil colloids ( Table 3 ). The balance of charges has been commonly used as an indicator of the soil net charge ( Mekaru and Uehara, 1972Mekaru, T.; Uehara, G. 1972. Anion adsorption in ferruginous tropical soils. Soil Science Society of America Journal 36: 296-300. ).
Most of the profiles from P1 to P5, taken from the Oxisols, are dystrophic and acid, except for P3, which presents a eutrophic profile ( Table 3 ). This Eutrustox is located near Itacarambi, suggesting the participation of a carbonate material from the Sete Lagoas Formation (Bambuí Group) ( Ministério das Minas e Energia, 1982Ministério das Minas e Energia. 1982. Project RadamBrazil: Geology, Geomorphology, Pedology, Vegetation, Potential Land Use = Projeto RadamBrasil: Geologia, Geomorfologia, Pedologia, Vegetação e Uso Potencial da Terra. Ministério das Minas e Energia, Departamento Nacional de Produção Mineral, Rio de Janeiro, RJ, Brazil (in Portuguese). ). Its high base saturation (in excess of 80 %) is mainly due to the high contents of K+ and relatively high contents of Mg2+ and Ca2+, considered very good according to criteria established for the state of Minas Gerais ( Ribeiro et al., 1999aRibeiro, A.C.; Guimarães, P.T.G.; Alvarez, V.H.V. 1999a. Recommendations for the use of correctives and fertilizers in Minas Gerais: 5thApproach = Recomendações para uso de corretivos e fertilizantes em Minas Gerais. 5ª Aproximação. Comissão de Fertilidade do Solo do Estado de Minas Gerais, Viçosa, MG, Brazil (in Portuguese). ). Brazilian Eutrophic Oxisols are in the minority and show unusually large base saturation in areas under rustic regimes where the parent material is particularly rich in bases ( Schaeffer et al., 2008Schaefer, C.; Fabris, J.; Ker, J. 2008. Minerals in the clay fraction of Brazilian Latosols (Oxisols): a review. Clay Minerals 43: 137-154. ).
The Oxisols P1, P2, P4, and P5, in addition to being dystrophic, present high to very high Al saturation, except P5. However, exchangeable acidity lower than 4 cmolc dm−3 is not enough to classify a soil given the aluminum criterion ( Embrapa, 2013Empresa Brasileira de Pesquisa Agropecuária [EMBRAPA]. 2013. Brazilian System of Soil Classification = Sistema Brasileiro de Classificação de Solos. Embrapa, Brasília, DF, Brazil (in Portuguese). ). These results are in agreement with Simas et al. (2005)Simas, F.N.; Schaefer, C.E.; Fernandes Filho, E.I.; Chagas, A.C.; Brandao, P.C. 2005. Chemistry, mineralogy and micropedology of highland soils on crystalline rocks of Serra da Mantiqueira, southeastern Brazil. Geoderma 125: 187-201. .
In relation to pH, the soils presented acidity from very high (pH H2O ≤ 5) to low alkalinity (pH H2O between 7.1 and 7.8), according to Ribeiro et al. (1999a). The highest pH value corresponds to the surface horizon of P14, which is probably due to the agricultural management. Acidic soils can support high-level agriculture when correctly managed, as was evident in a number of regions in southern Minas ( Fontes and Alleoni, 2006Fontes, M.P.F.; Alleoni, L.R.F. 2006. Electrochemical attributes and availability of nutrients, toxic elements, and heavy metals in tropical soils. Scientia Agricola 63: 589-608. ).
The TOC content ( Table 3 ) in the surface layer also ranged from low (between 0.41 and 1.16 dag kg−1) to very high (> 4.06 dag kg−1), according to Ribeiro et al. (1999a). On the surface of organic matter colloids the variable charge is negative, below pH values commonly found in humid tropical soils, capable of attracting cationic species, increasing the cation exchangeable capacity (CEC) of soils with a high content of organic matter ( Fontes and Alleoni, 2006Fontes, M.P.F.; Alleoni, L.R.F. 2006. Electrochemical attributes and availability of nutrients, toxic elements, and heavy metals in tropical soils. Scientia Agricola 63: 589-608. ).
The P contents were low or very low ( Ribeiro et al., 1999bRibeiro, A.C., Guimarães, P.T.G.; Alvarez, V.H.V. 1999b. Recommendations for the Use of Correctives and Fertilizers in Minas Gerais: 5thApproach = Recomendações para Uso de Corretivos e Fertilizantes em Minas Gerais: 5ª Aproximação. 5ed. Comissão de Fertilidade do Solo do Estado de Minas Gerais, Viçosa, MG, Brasil (in Portuguese). ) in all the Oxisol profiles ( Table 3 ). In these soils, P adsorption reflects soil mineralogy. Crystalline Fe oxides are important adsorbents of P, with goethite apparently adsorbing more than hematite ( Fontes and Weed, 1996Fontes, M.P.F.; Weed, S.B. 1996. Phosphate adsorption by clays from Brazilian Oxisols: relationships with specific surface area and mineralogy. Geoderma 72: 37-51. ). According to these authors, gibbsite also plays a key role in adsorption, mainly related to mineral abundance. In this regard, the Oxisols P6 and P11 presented more pronounced goethite, hematite, and gibbsite ( Table 4 ), and consequently, showed lower contents of P-rem in relation to the other studied soils. Similarly, P12 presented very low P-rem contents due to the goethite mineralogy, with a significant presence of gibbsite, in addition to its very clayey texture. Remanescent phosphorus is inversely related to the phosphate adsorption capacity by soils. In other words, low P-rem values mean that the soils have a high capacity for adsorbing P and vice versa.
Another aspect involving the Oxisols P6 to P11 was the high affinity between micronutrients and Fe and Mn oxides. In general, the Fe and Mn contents were high, while Cu and Zn contents were considered higher ( Ribeiro et al., 1999bRibeiro, A.C., Guimarães, P.T.G.; Alvarez, V.H.V. 1999b. Recommendations for the Use of Correctives and Fertilizers in Minas Gerais: 5thApproach = Recomendações para Uso de Corretivos e Fertilizantes em Minas Gerais: 5ª Aproximação. 5ed. Comissão de Fertilidade do Solo do Estado de Minas Gerais, Viçosa, MG, Brasil (in Portuguese). ) in profiles P7 and P14, respectively. These two elements tend to concentrate in soils derived from basic igneous rocks, while B tends to predominate in sedimentary rocks ( Krauskopf, 1972Krauskopf, K. 1972. Geochemistry of micronutrients. p. 7-40. In: Mortvedt, J.J.; Giordano, P.M.; Lindsay, W.M., eds. Micronutrients in agriculture. Soil Science Society of America, Madison, WI, USA. ) which explains the very low contents of this element.
Higher values for P-rem ( Table 3 ), such as those presented by P23, followed by the Ultisols (P13 to P18), except for the very clayey horizon of P15, and Inceptisols (P19 to P22) are associated with more kaolinitic and less oxidic soils ( Table 4 ) ( Schaefer et al., 2008Schaefer, C.; Fabris, J.; Ker, J. 2008. Minerals in the clay fraction of Brazilian Latosols (Oxisols): a review. Clay Minerals 43: 137-154. ).
For the Ultisols, variable values of base saturation and Al saturation occur because part of these soils are eutrophic (P14 and P17) and the others (P13, P15, P16, and PV18) are dystrophic or alic ( Table 3 ), according to Embrapa (2013)Empresa Brasileira de Pesquisa Agropecuária [EMBRAPA]. 2013. Brazilian System of Soil Classification = Sistema Brasileiro de Classificação de Solos. Embrapa, Brasília, DF, Brazil (in Portuguese). . However, one of the most pronounced characteristics is related to the micronutrient content in the superficial horizons of these soils, which are among the highest of all the studied soils. Nutrient cycling in the A horizon, as well as the affinity of certain elements for organic matter, may possibly be responsible for this accumulation.
In eutrophic soils, the contents of Ca2+ and Mg2+ are also high in all profiles, especially in the superficial horizon. Even in dystrophic Ultisols, it is common for the surface horizon to have high base saturation, giving an epi-eutrophic character to these soils (P13 and P16) ( Embrapa, 2013Empresa Brasileira de Pesquisa Agropecuária [EMBRAPA]. 2013. Brazilian System of Soil Classification = Sistema Brasileiro de Classificação de Solos. Embrapa, Brasília, DF, Brazil (in Portuguese). ). This influences soil pH values, which tend to be higher than in the Oxisols, especially in P14, which is classified as having low alkalinity according to Ribeiro et al. (1999a).
The Inceptisols presented low to high acidity (Ribeiro et al., 1999b) with an average pH value for the profiles of approximately 5.5 ( Table 3 ). These values are above the estimated soil PZC values ( Table 5 ), with a greater predominance of negative charges in the pH range when compared to other soil classes, which is probably due to the higher presence of minerals with negative charges resulting from isomorphic substitution (IS), such as micas. The high contents of P-rem in these soils may be related, among other factors, to this existing negative charge balance, a high amount of silt and the lower relative amount of Fe oxides, and the virtual absence of gibbsite.
Mineralogical and electrochemical characteristics of the studied soils and Fe contents obtained from the clay fraction by three successive extractions of dithionite-citrate (Fed) and a single extraction of ammonium oxalate (Feo).
The set of physical, chemical and mineralogical characteristics gives the soil a specific capacity to retain anionic species. The Inceptisols, due to their lower Fe and Al content, especially gibbsite, were the soil class with the lowest adsorption of anionic species ( Almeida et al., 2021Almeida, C.C.; Fontes, M.P.F.; Dias, A.C.; Pereira, T.C.T.; Ker, J.C. 2021. Adsorption and desorption of arsenic and its immobilization in soils. Scientia Agricola 78: e20180386. ).
The Inceptisols, represented by the profiles P19, P20 and P21, presented high Al3+ contents ( Table 3 ), mainly P20, whose values were higher than 4 cmolc dm−3 and Al saturation higher than 50 % were sufficient to be classified as having an Al character ( Embrapa, 2013Empresa Brasileira de Pesquisa Agropecuária [EMBRAPA]. 2013. Brazilian System of Soil Classification = Sistema Brasileiro de Classificação de Solos. Embrapa, Brasília, DF, Brazil (in Portuguese). ). High Al contents are not always due to intense soil weathering, but to the pelitic material i.e., the low contents of bases and the presence of Al in the mica and feldspar structure causes young soils, developed from pelitic rocks, to have high Al3+ contents whereas, in older soils, Al contents in solution tend to be lower due to the formation of gibbsite ( Resende et al., 2007Resende, M.; Curi, N.; Rezende, S.B.; Corrêa, G.F. 2007. Pedology: Basis for Distinguishing Environments = Pedologia: Base para Distinção de Ambientes. 5ed. UFLA, Lavras, MG, Brazil (in Portuguese). ). Thus, these soils presented a low sum of bases, with Ca2+ and Mg2+ contents considered very low/medium (Ribeiro et al., 1999b) in all soil profiles.
On the other hand, high K+ contents were found. This element is considered the most common in soils derived from pelitic rocks, which is a consequence of the micaceous mineralogy and probably of a higher substitution of Al by Si, allowing for K maintenance in the structure of micas ( Resende et al., 2007Resende, M.; Curi, N.; Rezende, S.B.; Corrêa, G.F. 2007. Pedology: Basis for Distinguishing Environments = Pedologia: Base para Distinção de Ambientes. 5ed. UFLA, Lavras, MG, Brazil (in Portuguese). ). This fact is evidenced by the appearance of mica in the silt fraction of all the studied soils and feldspar in most of them ( Table 4 ). P2 was the only profile to present high base saturation, with an average of 84 % ( Table 3 ). This is due to the high exchangeable Mg2+ and Ca2+ contents from a parent material of sandstone rocks of the Araxá Group, as previously discussed. In general, when considering the macroelements, the Inceptisols are rich in K only, and poor in Ca, Mg, and P. The contents of P and S were very low ( Table 3 ), according to Ribeiro et al. (1999a).
As regards the micronutrients ( Table 3 ), Mn and Fe presented extremely high available contents (Ribeiro et al., 1999b), mainly in P19, due to the ferriferous parent material. The presence of Fe oxides in the soil is essential since they practically control the solubility of this element, which is very much influenced by pH and soil redox potential ( Krauskopf, 1972Krauskopf, K. 1972. Geochemistry of micronutrients. p. 7-40. In: Mortvedt, J.J.; Giordano, P.M.; Lindsay, W.M., eds. Micronutrients in agriculture. Soil Science Society of America, Madison, WI, USA. ).
In profile P22, the available Fe contents were low, in fact in the literature they are considered very low (Ribeiro et al., 1999b) an observation corroborated by the absence of Fe oxides ( Table 4 ). Soils derived from pelitic rocks tend to present higher B contents, as they are more concentrated in sediments of marine origin ( Resende et al., 2007Resende, M.; Curi, N.; Rezende, S.B.; Corrêa, G.F. 2007. Pedology: Basis for Distinguishing Environments = Pedologia: Base para Distinção de Ambientes. 5ed. UFLA, Lavras, MG, Brazil (in Portuguese). ). However, this trend could not be observed since the B contents were in general low or very low in all profiles.
The chemical characteristics of Entisols (P23) involve very low values for both micro and macronutrients, with low base saturation and high Al saturation although the exchangeable and potential acidity contents are also low ( Table 3 ). The acidity of these soils is considered as high (Ribeiro et al., 1999b) and is possibly related to the quartz weathering present in their sand, silt, and clay fractions ( Table 4 ), which release H4SiO4 ( Resende et al., 2007Resende, M.; Curi, N.; Rezende, S.B.; Corrêa, G.F. 2007. Pedology: Basis for Distinguishing Environments = Pedologia: Base para Distinção de Ambientes. 5ed. UFLA, Lavras, MG, Brazil (in Portuguese). ). Due to the sandy texture and low CEC, Entisols exhibit lower retention capacity of inorganic contaminants as compared to Oxisols ( Melo et al., 2011Melo, L.C.A.; Alleoni, L.R.F.; Carvalho, G.; Azevedo, R.A. 2011. Cadmium and barium toxicity effects on growth and antioxidant capacity of soybean (Glycine max L.) plants, grown in two soil types with different physicochemical properties. Journal of Plant Nutrition and Soil Science 174: 847-859. ).
Mineralogy
The soil mineralogy results ( Table 4 ) indicate the presence of kaolinite, gibbsite, goethite, illite, hematite, hydroxy-interlayered vermiculite, and maghemite, among others. The presence of minerals was confirmed in the sand, silt and clay fraction via interplanar spacing (d). These minerals are in agreement with those observed by other authors for Brazilian soils ( Fontes and Weed, 1991Fontes, M.P.F.; Weed, S.B. 1991. Iron oxides in selected Brazilian Oxisols. I. Mineralogy. Soil Science Society of America Journal 55: 1143-1149. ; Fontes and Carvalho Jr., 2005Fontes, M.P.; Carvalho Jr, I.A. 2005. Color attributes and mineralogical characteristics, evaluated by radiometry, of highly weathered tropical soils. Soil Science Society of America Journal 69: 1162-1172. ; Simas et al., 2005Simas, F.N.; Schaefer, C.E.; Fernandes Filho, E.I.; Chagas, A.C.; Brandao, P.C. 2005. Chemistry, mineralogy and micropedology of highland soils on crystalline rocks of Serra da Mantiqueira, southeastern Brazil. Geoderma 125: 187-201. ; Schaefer et al., 2008Schaefer, C.; Fabris, J.; Ker, J. 2008. Minerals in the clay fraction of Brazilian Latosols (Oxisols): a review. Clay Minerals 43: 137-154. ; Camêlo et al., 2017Camêlo, D.L.; Ker, J.C.; Fontes, M.P.F.; Corrêa, M.M.; Costa, A.C.S.; Melo, V.F. 2017. Pedogenic iron oxides in iron-rich Oxisols developed from mafic rocks. Revista Brasileira de Ciência do Solo 41: e0160379. ; Camêlo et al., 2018Camêlo, D.L.; Ker, J.C.; Fontes, M.P.F.; Costa, A.C.S.; Corrêa, M.M.; Leopold, M. 2018. Mineralogy, magnetic susceptibility and geochemistry of Fe-rich Oxisols developed from several parent materials. Scientia Agricola 75: 410-419. ; Pacheco et al., 2018Pacheco, A.A.; Ker, J.C.; Schaefer, C.E.G.R.; Fontes, M.P.F.; Andrade, F.V.; Martins, E.d.S.; Oliveira, F.S.D. 2018. Mineralogy, micromorphology, and genesis of soils with varying drainage along a hillslope on granitic rocks of the Atlantic Forest Biome, Brazil. Revista Brasileira de Ciência do Solo 42: e0170291. ).
Goethite seems to be the most stable and abundant form of Fe oxide in tropical soils. The factors favoring its formation, to the detriment of hematite, are lower temperatures, higher H2O activity, high organic matter content, and lower Fe content in the parent material ( Schwertmann and Taylor, 1989Schwertmann, U.; Taylor, R.M. 1989. Iron oxides. p. 379-438. In: Dixon, J.B., ed. Minerals in soil environments. Soil Science Society of America, Madison, WI, USA. ). The association between goethite and hematite, a common characteristic in most soils in tropical regions, was very well evidenced in these soils ( Schwertmann, 1985Schwertmann, U. 1985. The effect of pedogenic environments on iron oxide minerals. p. 171-200. In: Stewart, B.A., ed. Advances in Soil Science. Springer, New York, NY, USA. ).
In the most weathered and acidic soils, gibbsite is observed in large quantities, especially in the Oxisols P2 and P12 ( Tables 4 and 5 ). Because of the relatively high soil development, the tendency for the Ultisols was similar for the Fe and Al oxy-hydroxides, in addition to the common presence of kaolinite and illite. However, the absence of significant amounts of gibbsite, which were detected only in thermal analysis ( Table 5 ), shows that the Ultisols, in general, are less developed in relation to the Oxisols, which is in agreement with the theory of soil formation.
According to Schaeffer et al. (2008)Schaefer, C.; Fabris, J.; Ker, J. 2008. Minerals in the clay fraction of Brazilian Latosols (Oxisols): a review. Clay Minerals 43: 137-154. relatively large contents of gibbsite tend to occur in Brazilian Oxisols, where weathering and leaching processes are more intense. According to these authors, the higher presence of gibbsite, together with goethite, may favor the formation of aluminized goethite, which tends to occur mainly in tropical climate soils with a well-defined dry and humid season. These climate characteristics are found in most of the state of Minas Gerais.
The presence of illite in all samples of the clay fraction and mica in all samples of the silt and sand fractions stood out in the Inceptisols ( Table 4 ). Illite interplanar spacing (1.00 nm) has the characteristic of not changing in treatments with Mg or K, with or without heating ( Whittig and Allardice, 1986Whittig, L.D.; Allardice, W.R. 1986. X-ray diffraction techniques. p. 331-362. In: Klute, A., ed. Methods of soil analysis: physical and mineralogical methods. American Society of Agronomy, Madison, WI, USA. ).
The P7 and P9 profiles presented in their mineralogical constitution hydroxy-interlayered vermiculite (HIV) and P20 presented vermiculite (Vm) in addition to HIV. Interplanar spacing (d) 1.43 nm (HIV) and 1.0 nm (mica/illite) were similar in the samples taken of Fe oxides and in those with the treatment of K at ambient temperature. When heated to 550 °C, the entire HIV has its Al polymers destroyed and collapses at a (d) of 1.0 nm ( Whittig and Allardice, 1986Whittig, L.D.; Allardice, W.R. 1986. X-ray diffraction techniques. p. 331-362. In: Klute, A., ed. Methods of soil analysis: physical and mineralogical methods. American Society of Agronomy, Madison, WI, USA. ). However, in the profile P20, the 1.0 nm has already undergone an increase in intensity in the treatment with K at ambient temperature, indicating that part of the material is composed of Vm and another part remains at 1.43 nm, which is HIV. At 550 °C, all the material collapses at 1.0 nm on the destruction of the interlayer material.
The crystalline forms of Fe associated with soil clay fraction (Fed), quantified by dithionite-citrate analysis ( Coffin, 1963Coffin, D. 1963. A method for the determination of free iron in soils and clays. Canadian Journal of Soil Science 43: 7-17. ), and those with lower crystallinity (Feo), obtained from a single extraction with ammonium oxalate ( McKeague and Day, 1966McKeague, J.; Day, J. 1966. Dithionite-and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Canadian Journal of Soil Science 46: 13-22. ) are shown in Table 5 . The contents of Fe2O3, extracted by dithionite (Fed), were variable. The highest values (around 10 dag kg−1) were found in two Oxisols (P9 and L10). On the other hand, high values obtained from the Fe-oxalate (Feo) extraction, corresponded to profile P6.
The ratio Feo/Fed can be a proxy for the soil developed degree, being smaller in weathering soils ( Cornell and Schwertmann, 2003Cornell, R.M.; Schwertmann, U. 2003. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. 2ed. Wiley, Weinheim, Germany. ). In general, the values of Feo/Fed showed a predominance of well crystallized Fe forms for the Ultisols and Oxisols, and poorly crystallized Entisols and Inceptisols ( Table 4 ). The Feo/Fed ratios were higher in the Bi horizon of two Inceptisols (P19 and P20). These results were also in agreement with those reported by Pacheco et al. (2018)Pacheco, A.A.; Ker, J.C.; Schaefer, C.E.G.R.; Fontes, M.P.F.; Andrade, F.V.; Martins, E.d.S.; Oliveira, F.S.D. 2018. Mineralogy, micromorphology, and genesis of soils with varying drainage along a hillslope on granitic rocks of the Atlantic Forest Biome, Brazil. Revista Brasileira de Ciência do Solo 42: e0170291. , in which the authors found a predominance of well crystallized Fe in more weathered soils, such as Oxisols. Highly weathered tropical soils have very low amounts of poorly crystallized Fe oxides ( Fontes and Weed, 1991Fontes, M.P.F.; Weed, S.B. 1991. Iron oxides in selected Brazilian Oxisols. I. Mineralogy. Soil Science Society of America Journal 55: 1143-1149. ).
The literature highlights the effect of organic matter on the inhibition of Fe crystallization, due to the strong complexation of poorly crystallized Fe oxides by organic compounds ( Schwertmann, 1966Schwertmann, U. 1966. Inhibitory effect of soil organic matter on the crystallization of amorphous ferric hydroxide. Nature 212: 645-646. ; Simas et al., 2005Simas, F.N.; Schaefer, C.E.; Fernandes Filho, E.I.; Chagas, A.C.; Brandao, P.C. 2005. Chemistry, mineralogy and micropedology of highland soils on crystalline rocks of Serra da Mantiqueira, southeastern Brazil. Geoderma 125: 187-201. ). However, for most of the studied soils, a lower proportion of poorly crystallized material was found in the surface horizon, demonstrating little influence of the organic matter on the increased Feo.
The presence of silica in the system inhibits the formation of Fe oxides with higher crystallinity ( Schwertmann and Taylor, 1989Schwertmann, U.; Taylor, R.M. 1989. Iron oxides. p. 379-438. In: Dixon, J.B., ed. Minerals in soil environments. Soil Science Society of America, Madison, WI, USA. ). For this reason, the profile P4, which presented high contents of kaolinite (78 % in the Bw horizon), also presented the highest Feo/Fed ratio in the subsurface horizon ( Table 5 ).
Point of zero charge of soils
The results show high correlation between PZC and the exchangeable Al contents and the Feo/Fed ratio, both in the A and B horizons of the soil classes ( Table 6 ).
Correlation between physical, chemical and mineralogical attributes and the ZPSE and estimated ZPC of A and B horizons of the studied soils.
The negative correlation found between exchangeable Al and PZC can be of great importance as regards management practices in soil classes with very low PZC values (consequently high exchangeable Al). Simas et al. (2005)Simas, F.N.; Schaefer, C.E.; Fernandes Filho, E.I.; Chagas, A.C.; Brandao, P.C. 2005. Chemistry, mineralogy and micropedology of highland soils on crystalline rocks of Serra da Mantiqueira, southeastern Brazil. Geoderma 125: 187-201. reported that liming is not recommended when the Al3+ content in soils requires extremely high CaCO3 doses, since the practice may not be economically viable or may accelerate the mineralization of organic compounds.
An examination of the relationship between Feo/Fed and the PZC of the evaluated soil classes in this study, reaffirms the role of mineralogy in the PZC of soils. In highly weathered soils in the state of São Paulo (Alleoni and Camargo, 1994), PZC showed a positive correlation with gibbsite and ΔpH. Negative correlation between PZC and the soil weathering index was also observed by these authors. Since the ratio of Feo/Fed can be used to estimate the soil weathering degree ( Cornell and Schwertmann, 2003Cornell, R.M.; Schwertmann, U. 2003. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. 2ed. Wiley, Weinheim, Germany. ), the negative correlation observed between PZC and Feo/Fed in this study corroborates the result observed by Alleoni and Camargo (1994). However, in the main classes of soils in the state of Minas Gerais, the correlation between PZC and gibbsite was not observed.
Although the effect of organic matter on PZSE is known in the literature ( Hendershot and Lavkulich, 1979Hendershot, W.; Lavkulich, L. 1979. The effect of sodium chloride saturation and organic matter removal on the value of zero point of charge. Soil Science 128: 136-141. ) to cause its decrease, a negative statistical correlation was not established in this study probably due to the differentiation between the organic matter from distinct localities and vegetation cover, conferring diverse effects and a direct relationship is less evident.
A comparison between both methods (PZC and PZSE) was undertaken, which showed positive correlation between them in the A horizon (r = 0.89) while in the B horizon (r = 0.04) correlation was not significant at p < 0.01. The t-test ( Table 7 ) did not indicate a significant difference between methods, as well as between soil classes and their A and B horizons. A similar analysis was performed in relation to soil classes and no differentiation was observed between them.
The importance is related to the proposition of methodologies for other studies. Due to the degree of simplification that the equation of Keng and Uehara (1974)Keng, J.C.W.; Uehara, G. 1974. Chemistry, mineralogy and taxonomy of Oxisols and Uttisols. Proceedings of Soil and Crop Sciences Society 33: 119-126. provides, its use should be considered when the focus of the study does not need a higher degree of refinement.
Among the soil classes, the results showed that the Inceptisols presented the lowest PZC values ( Table 4 ), denoting their incipient character and higher density of negative charges, while more oxidic soils presented the highest PZC values (up to a maximum of 5.9).
Most of the studied soils presented pH values ( Table 3 ) higher than the ZPZC values ( Table 5 ), evidencing their electronegative character despite being highly weathered and oxidic. The only exception is the B horizon of profile P11, whose pH in water was higher than its PZC, thus presenting an electropositive character. Once the pH < PZC, the surface of the colloids is positively charged, favoring adsorption of the anionic species ( Fontes, 2012Fontes, M.P. 2012. Behavior of heavy metals in soils: individual and multiple competitive adsorption. p. 77-118. In: Selim, H.M., ed. Competitive sorption and transport of heavy metals in soils and geological media. CRC Press, Boca Raton, FL, USA. ).
Conclusions
The evaluated soils of Minas Gerais differed substantially in terms of texture and fertility, even within the same soil class. Soils presented textures ranging from loamy sand to very clayey with profiles varying from dystrophic to eutrophic, which suggests the participation of the parent material.
Most of these soils presented an advanced stage of weathering with a typical mineralogical composition of highly weathered soils composed of kaolinite, gibbsite, goethite, hematite and illite. They also showed traces of hydroxy-interlayered vermiculite and maghemite.
A positive correlation was observed between the point of zero salt effect (PZSE) and the equation developed by Keng and Uehara (1974)Keng, J.C.W.; Uehara, G. 1974. Chemistry, mineralogy and taxonomy of Oxisols and Uttisols. Proceedings of Soil and Crop Sciences Society 33: 119-126. for estimating the point of zero charge (PZC) of soils in the A horizon.
Significantly high negative correlation ( p < 0.01) was observed between PZC and the exchangeable Al contents and the Feo/Fed ratio, both in the A and B horizons of the soil classes, evidencing the influence of chemicals and mineralogy on the PZC of soils.
Due to the diversity of soils in the state of Minas Gerais, the results obtained reinforce the importance of very detailed descriptions of soil attributes with a view to improving both usage and soil management.
Acknowledgments
The authors are grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico - Brasil (CNPq) for the financial support. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
References
- Almeida, C.C.; Fontes, M.P.F.; Dias, A.C.; Pereira, T.C.T.; Ker, J.C. 2021. Adsorption and desorption of arsenic and its immobilization in soils. Scientia Agricola 78: e20180386.
- Alvarez, V.V.H.; Fonseca, D.M. 1990. Definition of phosphorus levels to determine the maximum phosphate adsorption capacity and the response curves for greenhouse experiments. Revista Brasileira de Ciência do Solo 14: 49-55 (in Portuguese, with abstract in English).
- Camargo, L.A.; Marques Júnior, J.; Pereira, G.T.; Horvat, R.A. 2008. Spatial variability of mineralogical attributes of an oxisol under different relief forms. I. Clay fraction mineralogy. Revista Brasileira de Ciência do Solo 32: 2269-2277 (in Portuguese, with abstract in English).
- Camêlo, D.L.; Ker, J.C.; Fontes, M.P.F.; Corrêa, M.M.; Costa, A.C.S.; Melo, V.F. 2017. Pedogenic iron oxides in iron-rich Oxisols developed from mafic rocks. Revista Brasileira de Ciência do Solo 41: e0160379.
- Camêlo, D.L.; Ker, J.C.; Fontes, M.P.F.; Costa, A.C.S.; Corrêa, M.M.; Leopold, M. 2018. Mineralogy, magnetic susceptibility and geochemistry of Fe-rich Oxisols developed from several parent materials. Scientia Agricola 75: 410-419.
- Coffin, D. 1963. A method for the determination of free iron in soils and clays. Canadian Journal of Soil Science 43: 7-17.
- Cornell, R.M.; Schwertmann, U. 2003. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. 2ed. Wiley, Weinheim, Germany.
- Empresa Brasileira de Pesquisa Agropecuária [EMBRAPA]. 2013. Brazilian System of Soil Classification = Sistema Brasileiro de Classificação de Solos. Embrapa, Brasília, DF, Brazil (in Portuguese).
- Empresa Brasileira de Pesquisa Agropecuária [EMBRAPA]. 2017. Manual of Soil Analysis Methods = Manual de Métodos de Análise de Solo. Embrapa Solos, Rio de Janeiro, RJ, Brazil (in Portuguese).
- Fontes, M.P. 2012. Behavior of heavy metals in soils: individual and multiple competitive adsorption. p. 77-118. In: Selim, H.M., ed. Competitive sorption and transport of heavy metals in soils and geological media. CRC Press, Boca Raton, FL, USA.
- Fontes, M.P.; Carvalho Jr, I.A. 2005. Color attributes and mineralogical characteristics, evaluated by radiometry, of highly weathered tropical soils. Soil Science Society of America Journal 69: 1162-1172.
- Fontes, M.P.F.; Alleoni, L.R.F. 2006. Electrochemical attributes and availability of nutrients, toxic elements, and heavy metals in tropical soils. Scientia Agricola 63: 589-608.
- Fontes, M.P.F.; Camargo, O.A.; Sposito, G. 2001. Electrochemistry of colloidal particles and its relationship with the mineralogy of highly weathered soils. Scientia Agricola 58: 627-646.
- Fontes, M.P.F.; Weed, S.B. 1991. Iron oxides in selected Brazilian Oxisols. I. Mineralogy. Soil Science Society of America Journal 55: 1143-1149.
- Fontes, M.P.F.; Weed, S.B. 1996. Phosphate adsorption by clays from Brazilian Oxisols: relationships with specific surface area and mineralogy. Geoderma 72: 37-51.
- Hendershot, W.; Lavkulich, L. 1979. The effect of sodium chloride saturation and organic matter removal on the value of zero point of charge. Soil Science 128: 136-141.
- Keng, J.C.W.; Uehara, G. 1974. Chemistry, mineralogy and taxonomy of Oxisols and Uttisols. Proceedings of Soil and Crop Sciences Society 33: 119-126.
- Krauskopf, K. 1972. Geochemistry of micronutrients. p. 7-40. In: Mortvedt, J.J.; Giordano, P.M.; Lindsay, W.M., eds. Micronutrients in agriculture. Soil Science Society of America, Madison, WI, USA.
- Matos, A.; Fontes, M.; Costa, L.; Martinez, M. 2001. Mobility of heavy metals as related to soil chemical and mineralogical characteristics of brazilian soils. Environmental Pollution 111: 429-435.
- McKeague, J.; Day, J. 1966. Dithionite-and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Canadian Journal of Soil Science 46: 13-22.
- Mekaru, T.; Uehara, G. 1972. Anion adsorption in ferruginous tropical soils. Soil Science Society of America Journal 36: 296-300.
- Mello, J.; Roy, W.; Talbott, J.; Stucki, J. 2006. Mineralogy and arsenic mobility in arsenic-rich Brazilian soils and sediments. Journal of Soils and Sediments 6: 9-19.
- Melo, L.C.A.; Alleoni, L.R.F.; Carvalho, G.; Azevedo, R.A. 2011. Cadmium and barium toxicity effects on growth and antioxidant capacity of soybean (Glycine max L.) plants, grown in two soil types with different physicochemical properties. Journal of Plant Nutrition and Soil Science 174: 847-859.
- Ministério das Minas e Energia. 1982. Project RadamBrazil: Geology, Geomorphology, Pedology, Vegetation, Potential Land Use = Projeto RadamBrasil: Geologia, Geomorfologia, Pedologia, Vegetação e Uso Potencial da Terra. Ministério das Minas e Energia, Departamento Nacional de Produção Mineral, Rio de Janeiro, RJ, Brazil (in Portuguese).
- Pacheco, A.A.; Ker, J.C.; Schaefer, C.E.G.R.; Fontes, M.P.F.; Andrade, F.V.; Martins, E.d.S.; Oliveira, F.S.D. 2018. Mineralogy, micromorphology, and genesis of soils with varying drainage along a hillslope on granitic rocks of the Atlantic Forest Biome, Brazil. Revista Brasileira de Ciência do Solo 42: e0170291.
- Raij, B.V.; Peech, M. 1972. Eletrochemical properties of some Brazilian soils. Soil Science Society of America Proceedings 36: 587-593.
- Resende, M.; Curi, N.; Rezende, S.B.; Corrêa, G.F. 2007. Pedology: Basis for Distinguishing Environments = Pedologia: Base para Distinção de Ambientes. 5ed. UFLA, Lavras, MG, Brazil (in Portuguese).
- Ribeiro, A.C.; Guimarães, P.T.G.; Alvarez, V.H.V. 1999a. Recommendations for the use of correctives and fertilizers in Minas Gerais: 5thApproach = Recomendações para uso de corretivos e fertilizantes em Minas Gerais. 5ª Aproximação. Comissão de Fertilidade do Solo do Estado de Minas Gerais, Viçosa, MG, Brazil (in Portuguese).
- Ribeiro, A.C., Guimarães, P.T.G.; Alvarez, V.H.V. 1999b. Recommendations for the Use of Correctives and Fertilizers in Minas Gerais: 5thApproach = Recomendações para Uso de Corretivos e Fertilizantes em Minas Gerais: 5ª Aproximação. 5ed. Comissão de Fertilidade do Solo do Estado de Minas Gerais, Viçosa, MG, Brasil (in Portuguese).
- Schaefer, C.; Fabris, J.; Ker, J. 2008. Minerals in the clay fraction of Brazilian Latosols (Oxisols): a review. Clay Minerals 43: 137-154.
- Schwertmann, U. 1966. Inhibitory effect of soil organic matter on the crystallization of amorphous ferric hydroxide. Nature 212: 645-646.
- Schwertmann, U. 1985. The effect of pedogenic environments on iron oxide minerals. p. 171-200. In: Stewart, B.A., ed. Advances in Soil Science. Springer, New York, NY, USA.
- Schwertmann, U.; Taylor, R.M. 1989. Iron oxides. p. 379-438. In: Dixon, J.B., ed. Minerals in soil environments. Soil Science Society of America, Madison, WI, USA.
- Simas, F.N.; Schaefer, C.E.; Fernandes Filho, E.I.; Chagas, A.C.; Brandao, P.C. 2005. Chemistry, mineralogy and micropedology of highland soils on crystalline rocks of Serra da Mantiqueira, southeastern Brazil. Geoderma 125: 187-201.
- Soil Survey Staff. 2014. Keys to Soil Taxonomy. USDA-Natural Resources Conservation Service, Washington, DC, USA.
- Whittig, L.D.; Allardice, W.R. 1986. X-ray diffraction techniques. p. 331-362. In: Klute, A., ed. Methods of soil analysis: physical and mineralogical methods. American Society of Agronomy, Madison, WI, USA.
- Yeomans, J.C.; Bremner, J.M. 1988. A rapid and precise method for routine determination of organic carbon in soil. Communications in Soil Science and Plant Analysis 19: 1467-1476.
Edited by
Publication Dates
-
Publication in this collection
16 Oct 2020 -
Date of issue
2021
History
-
Received
03 Mar 2020 -
Accepted
12 June 2020