Abstract
There is growing interest in studies on sanitizers other than chlorine that can maintain the quality of organic products without affecting their phytochemical content. The effects of using chlorinated and ozonized water treatments, as sanitizing procedures, on the post-harvest quality of organic and conventional broccoli (Brassica oleracea L.) cv. Italica was evaluated. The biochemical parameters (chlorophyll, polyphenols, flavonoids, vitamin C and antioxidant capacity) of the broccoli samples were analyzed at day 0 (arrival of the plant from the field, original features), and 1, 4 and 7 days after harvest. The polyamine analysis was performed on arrival of the plant from the field and on the first and seventh days. The cultivation procedure influenced polyphenol, vitamin C and total chlorophyll content, and the highest value was observed in organic broccoli after the fourth day. Flavenoid content was higher in organic broccoli. The use of ozone appears not to have had an influence on the amount of polyphenolic, flavonoids and vitamin C during storage. Total chlorophyll content was less affected by ozonized water than by the chlorine treatment as at the first and fourth days of storage. The highest content of putrescine was found in conventional broccoli, while the highest levels of spermidine and spermine were found in organic broccoli. Antioxidant capacity was highest in organic broccoli after day 4 of storage and was affected by the bioactive compounds analyzed. Methods of cultivation influenced natural antioxidant and chlorophyll contents in broccoli under cold storage.
PLANT PHYSIOLOGY AND BIOCHEMISTRY
Ozonated water and chlorine effects on the antioxidant properties of organic and conventional broccoli during postharvest
Giuseppina Pace Pereira LimaI,* * Corresponding author < gpplima@ibb.unesp.br> ; Tatiana Marquini MachadoI; Luciana Manoel de OliveiraII; Luciana da Silva BorgesI; Valber de Albuquerque PedrosaI; Paola VanzaniIII; Fabio VianelloIV
ISão Paulo State University/Institute of Biosciences Dept. of Chemistry and Biochemistry, C.P. 510 18618-970 Botucatu, SP Brazil
IIFederal Institute of Education, Science and Technology of São Paulo, Av. Prof. Celso Ferreira, 1333, Jd. Europa I 18707-150 Avaré, SP Brazil
IIIUniversity of Padua Dept. of Molecular Medicine, Viale Colombo, 3, CAP 35.131 Padova Italy
IVUniversity of Padua Dept. of Comparative Biomedicine and Food Science
ABSTRACT
There is growing interest in studies on sanitizers other than chlorine that can maintain the quality of organic products without affecting their phytochemical content. The effects of using chlorinated and ozonized water treatments, as sanitizing procedures, on the post-harvest quality of organic and conventional broccoli (Brassica oleracea L.) cv. Italica was evaluated. The biochemical parameters (chlorophyll, polyphenols, flavonoids, vitamin C and antioxidant capacity) of the broccoli samples were analyzed at day 0 (arrival of the plant from the field, original features), and 1, 4 and 7 days after harvest. The polyamine analysis was performed on arrival of the plant from the field and on the first and seventh days. The cultivation procedure influenced polyphenol, vitamin C and total chlorophyll content, and the highest value was observed in organic broccoli after the fourth day. Flavenoid content was higher in organic broccoli. The use of ozone appears not to have had an influence on the amount of polyphenolic, flavonoids and vitamin C during storage. Total chlorophyll content was less affected by ozonized water than by the chlorine treatment as at the first and fourth days of storage. The highest content of putrescine was found in conventional broccoli, while the highest levels of spermidine and spermine were found in organic broccoli. Antioxidant capacity was highest in organic broccoli after day 4 of storage and was affected by the bioactive compounds analyzed. Methods of cultivation influenced natural antioxidant and chlorophyll contents in broccoli under cold storage.
Introduction
Broccoli (Brassica oleracea L. cv. Italica) is a vegetable characterized by high nutritional value, low caloric content and high dietary fiber and ascorbic acid contents, and it contains a range of substances that have been widely considered to be anti-carcinogens and antioxidants (Hasperué et al., 2011).
Broccoli is a source of phenolic compounds (flavonoids) and contains significant amounts of other important phytochemicals, such as ascorbic acid, carotenoids and glucosinolates (Naguib et al., 2012). It is harvested when the flowers are still immature with the sepals completely around the flower. The immature organs require a continuous supply of water, nutrients and hormones to maintain homeostasis. After harvesting, these types of organs suffer severe stress that leads to the appearance of senescence symptoms (Hasperué et al., 2011). Several studies have shown that this plant, when kept under refrigeration, maintains the nutritional properties of its phytochemical compounds, such as carotenoids, chlorophylls and vitamin C and a higher antioxidant capacity. All of these characteristics are important because they contribute to the reduction of organism damage by reactive oxygen species (ROS) (Naguib et al., 2012). The content of these compounds in plants may be increased if plants are grown organically. However, recent studies have questioned this assertion.
Organically-grown vegetables are attractive because of several factors, such as better flavor, a long postharvest life and a low chemical residue content, that lead consumers to choose this type of vegetable.
Chlorine is generally used to clean vegetables. However, it is not recommended because of its ability to form toxic products, particularly in organically cultivated produce. Recently, safety concerns have increased about the reaction of chlorine with organic waste products, resulting in the formation of potentially mutagenic or carcinogenic substances such as trihalomethanes and chloramines, and their impact on human health and environmental safety (Beltrán et al., 2005). Thus, interest in studies of sanitizers other than chlorine that will maintain the quality of organic products without affecting their phytochemical content is on the increase. Ozonized water has the potential to be used as an alternative to chlorine as a sanitizer (Beltrán et al., 2005). However, the self-decomposition of ozone is accompanied by the formation of reactive species, such as hydroxyl (OH), hydroxy-peroxyl (H2O) and superoxide anion (O2) radicals (Hoigné and Bader, 1983).
This study aimed to compare the influence of ozone treatment with chlorine on the chemical composition, which is related to the antioxidant content, of organic and conventional broccoli.
Materials and Methods
Plant material and storage conditions
Organic broccoli (Brassica oleracea L. var. Italica, cv. Ramoso Santana) was obtained from producers certifi ed by the Brazilian IBD (Agricultural and Food Inspections and Certifications). Broccoli under conventional cultivation was acquired from the same geographical area and at the same harvesting time. The geographic coordinates of these locations are approximately 22º 44' 50" S and 48º 34' 00" W, with an average altitude of 765 meters. The climate is subtropical, with humid summers and dry winters. Average annual rainfall is 1,534 mm, with an average for the wettest month (Jan) of 242 mm and 38 mm for the driest months (July and Aug). The average annual temperature is 21 ºC.
For the sake of comparison, all plants were acquired from the same cultivar and were at the same physiological phase. Plants were obtained 75 days after the planting of conventional seeds harvested in July, in the early hours of the morning and were immediately transported to the laboratory.
After the broccoli had been harvested and washed to remove most of the impurities, it was subjected to sanitizing treatments with chlorine or double ozonation. In the treatment with chlorine, broccoli was immersed in water containing 0.1 % sodium hypochlorite for 10 min. The treatment with ozone was conducted for 5 and 10 min, by immersing the broccoli in a 186-L plastic tank coupled to an ozone generator containing a centrifugal pump, which circulates the liquid inside the tank. The control consisted of immersion of broccoli in tap water for 5 min.
After the treatments, the broccoli was sorted again, gathered in bundles and stored at 5 ± 1 ºC and 93 ± 1 % relative humidity for seven days. The broccoli samples were analyzed as soon as they were harvested from the field (Day 0), after sanitation treatment (Day 1), and after storage at low temperature (Days 4 and 7). For each plant, the material (fresh matter) was immediately analyzed for vitamin C. For the other analyses, the broccoli samples were powdered in liquid nitrogen and stored at -80 ºC for later determination of total flavonoids, total chlorophyll, polyamines and antioxidant capacity. The phenol content was determined from the dry matter. The analyses of polyphenols content were analyzed after the sample had been dried in an oven with air circulating at 45 ºC until constant weight had been reached.
Content of total phenols
The analysis of total phenols was performed in accordance with the Folin-Ciocalteu spectrophotometric method (Singleton and Rossi Jr, 1965). The sample material, dried and powdered, was weighed and placed into centrifuge tubes containing 50 % acetone in water. The samples were then incubated in an ultrasonic bath for 20 minutes and centrifuged at 6,000 × g (Hettich Zentrifugen, Mikro220R) for 10 minutes. The supernatants were re-extracted and combined. FolinCiocalteu reagent was added; after 3 min at 25 ºC, a saturated solution of Na2CO3 was added, and the reaction mixture was then incubated for 1 h. The absorbance was measured at 760 nm.
Content of total flavonoids
For the flavonoid content analysis, the extracts were prepared in accordance with the method described by Popova et al. (2004), with adjustments. Briefly, fresh material samples were powdered in liquid nitrogen, weighed and mixed with 10 % (w/v) acidified methanol. The samples were subsequently placed in an ultrasonic bath for 30 minutes, and a 5 % aluminum chloride solution was added. The samples were then centrifuged for 20 minutes at 10,000 × g. Finally, the samples were filtered, and the absorbance was measured at 425 nm.
Content of vitamin C
The determination of vitamin C was conducted according to Terada et al. (1978) with minor modifications. Fresh samples (250 mg) were powdered in liquid nitrogen and homogenized in 0.5 % oxalic acid (3 mL) for 20 s. Then they were centrifuged at 6000 × g for 20 min at 4 ºC. An aliquot of the supernatant (1 mL) was combined with a 0.25 % aqueous solution of 2,6-dichlorophenolindophenol (2,6 DCPIP) (150 µL), 4.5 M sulfuric acid (1 mL), 2,4-dinitrophenylhydrazine (2,4-DNPH) (2 g in 100 mL water) and 10 % thiourea in 50 % ethanol (50 µL). The mixture was homogenized and boiled for 15 min, and after cooling, 85 % sulfuric acid (5 mL) was added. Spectrophotometric measurements were taken at 520 nm. The results were compared to a calibration curve derived from data from ascorbic acid (100 µg mL1) in 0.5 % oxalic acid.
Content of total chlorophylls
Extraction of chlorophylls was conducted according to the method validated by Nagata and Yamashita (1992), based on the different molar absorptivity coeffi cient of chlorophyll pigments in an extraction solution composed of acetone-hexane in a 4:6 ratio. The samples were powdered in liquid nitrogen and homogenized in a mini-Turrax in acetone (4 mL) and hexane (6 mL) for 1 min. The extraction was performed on samples protected from light. Then, sample absorbances were measured at 663 nm for chlorophyll a and at 645 nm for chlorophyll b (UV-Vis spectrophotometer. The absorbance values were converted to mg × 100 g1 fresh weight on the basis of the following equations:
Total chlorophyll was obtained from the sum of Eq. 1 and Eq. 2.
Antioxidant activity: DPPH free radical scavenging activity
Antioxidant activity was determined according to the methodology of Brand-Williams et al. (1995) as modified by Rossetto et al. (2009). The DPPH solution was freshly prepared in 99.8 % ethanol (10 mg in 50 mL). Fresh broccoli samples (1.0 g) were extracted in 99.8 % ethanol (10 mL) and centrifuged at 6,000 × g for 10 min at 5 ºC. The supernatant aliquots (0.5 mL) were combined with 99.8 % ethanol (3 mL). After homogenization, a DPPH solution (300 µL) was added to the test tubes, and the samples were then stored in the dark for 60 minutes. A negative control was prepared with 0.3 mM DPPH in ethanol, to observe the DPPH radical decay against the sample antioxidant capacity. The readings were performed at 517 nm and converted to antioxidant capacity percentage by the following equation: % reduced DPPH = [(Abs control Abs sample)/Abs control]/100. A calibration curve was prepared with Trolox (20, 40, 80, 120 and 160 µmol), and the results were expressed as µmol Trolox equivalent g1 sample (TEAC).
Determination of polyamine content
The polyamine content (putrescine, PUT; spermidine, SPD; spermine; SPM) was determined in accordance with the method described by Flores and Galston (1982) and modified by Lima et al. (2008).
Experimental Design
The experimental design was completely randomized, with two types of cultivation (organic and conventional), and after the sanitizing effect (water, chlorine, ozone 5 and ozone 10). The samples (for each repetition, n = 5) were assayed in triplicate. The analyses were conducted after the arrival of the samples from the field and prior to sanitation treatment (Day 0). Another sampling was performed soon after the sanitation treatment, named "Day 1". The plants were then stored in a cold chamber at 5 ± 1 ºC. Additional analyses were performed on the fourth and seventh day of storage for the proposed determinations.
Data were subjected to an ANOVA, and the measurements were compared using the CoStat (6.4) software program and, a two-way completely randomized statistical processing was carried out. The separation of the medium was performed with the Tukey HSD test p< 0.05. Differences between the averages were determined for individual days (0, 1, 4 and 7, for cultivation effects, and 1, 4 and 7 for sanitizing treatment effects) for chlorophyll, polyphenol, flavonoid, vitamin C and antioxidant activity. The polyamine content was compared within the individual day.
Results and Discussion
Regarding the characterization of broccoli on day 0 (immediately after harvest), and on day 1 (after sanitizing treatments), the vegetables grown under conventional cultivation showed the highest content of phenols when compared to those cultivated organically (Table 1). On the others days analysed, organic broccoli had higher contents of polyphenols as compared to those grown conventionally. No differences (p < 0.05) were observed between treatments using sanitizers in relation to phenolic compounds during the storage time (Table 2).
The values determined for total phenols were higher than those presented by Costa et al. (2006) for postharvested broccoli stored at 20 ºC. The differences were primarily due to the different storage temperature, which must have induced senescence and altered the content of the phytochemicals. Costa et al. (2006) reported a total phenol content of 0.69 g kg1 on the day of sampling and 1.26 g kg1 at the end of the experiment. The values were higher in the present study, but no increase was observed that could be attributed to the time of storage.
A clear trend of higher levels of flavonoids in organic broccoli was noted on day 4 and day 7 (Table 1). No effects due to sanitation treatments and storage time on the flavonoids content in broccoli were observed on the first day (Table 2). Flavonoids are usually more concentrated in organic than conventionally grown vegetables (Winter and Davis, 2006; Lima and Vianello, 2011), and a similar behavior was noticed in the present study on days 4 and 7. The polyphenol content in plants is influenced by culture practice, growing conditions and harvest time. Organic vegetables tend to show a higher content of phenolics as compared with conventionally cultivated plants (Lima and Vianello, 2011). A fundamental difference between organic and conventional production systems is in the management of soil fertility, which can influence the nutritional composition and can subsequently influence the synthesis of secondary plant metabolites (Vallverdú-Queralt et al., 2012).
Conventional broccoli tended to have a lower content of soluble phenols and total flavonoids on days 4 and 7 in storage at low temperature. These results suggest that organic fertilizers may affect induction in the synthesis of phenolic compounds. Because of the elimination of pesticides that reduce predators, organic farming leads to an increase of plant phenolic compounds, including flavonoids (Winter and Davis, 2006). This occurred in the present study, regardless of the sanitization procedure used. Organic crops tend to alter the shikimic acid pathway leading to a modification of their polyphenol content (Sousa et al., 2008). Naguib et al. (2012) reported a 151 % increase in phenol content in "Italica" organic broccoli and noted the influence of growing conditions on the increase of secondary metabolite compounds.
The use of ozone does not influence the phenolic compounds amount during storage (p < 0.05). A similar behavior can be seen when analyzing the levels of flavonoids, unlike some other approaches. Alothman et al. (2010) performed sanitation studies on pineapple (Ananas comosus L.) and banana (Musa spp.) and reported that the level of phenolic compounds tended to increase when subjected to ozone treatment; this effect was attributed to the activation of phenylalanine ammonia lyase (PAL), an enzyme related to the production of phenolic compounds.
As occurred in the analysis of polyphenols and flavonoids, vitamin C content was higher in conventional broccoli on the day of arrival of the plants from the field and after sanitizing treatment (Day 1). At 4 and 7 days of storage, there was an inversion, ie, the highest levels of vitamin C were found in plants from organic cultivation (Table 1). Other researchers have not reported differences in vitamin C content between organic and conventional carrots, tomatoes and potatoes (Hoefkens et al., 2010).
Treatment with ozone or chlorine did not induce variations in vitamin C content in organic broccoli on the first day, as compared with the conventional method. However, on the last day of storage, the use of ozone promoted a significant decrease in vitamin C content in broccoli samples (Table 2). Despite this effect of ozone on broccoli, the safety of ozone as a sanitizer may encourage its use as a sanitation treatment even though a similar vitamin C reduction was found in rocket leaves (Martínez- Sánchez et al., 2006) and lettuce (Beltrán et al., 2005).
Yellowing is the most visible deterioration in broccoli and usually occurs with the progress of chlorophyll degradation. Conventional broccoli had higher total chlorophyll levels at harvest, after the application of the treatment. As observed for the other biochemical characteristics analyzed, total chlorophyll content was higher in organic broccoli, showing a possibility of increased life after harvest over conventional broccoli (Table 1).
Sample sanitation with ozone 5 did not induce a reduction of the total chlorophyll content in broccoli on the first day when compared with ozone 10 or chlorine treatments (Table 2). An opposite effect was observed in broccoli sanitized with ozone 5 on day 4 of storage, which also had higher levels of total chlorophyll. On day 7 of storage no difference was found in broccoli treated with sanitizers. Chlorine (the treatment producing a lower loss in chlorophyll content on the seventh day) is not accepted as a sanitizer in organically grown vegetables. Thus, ozone may be considered a promising alternative in the sanitization of organic products, and treatment with ozone may result in the maintenance of the green vegetable color, one of the quality parameters of postharvested broccoli.
During the post-harvest period, the surface of broccoli loses its green color which reduces the commercial value of the product (Hasperué et al., 2011). The use of ozone on organic and conventional broccoli did not affect the color (verified by examining visual quality) and had no negative effect on sample chlorophyll content (Table 2).
Our results with regard to total chlorophyll content provide evidence that organic broccoli presents a lower rate of deterioration, even if samples were subjected to sanitization with ozone, a promoter of radical species formation (Hoigné and Bader, 1983). In fact, broccoli deterioration is directly correlated with chlorophyll and weight loss (Aimlar-or et al., 2010). Although many researchers conclude that some sanitizers induce a degradation of chlorophylls, the results pertaining to organic broccoli did not show any negative effect attributable to ozone treatment.
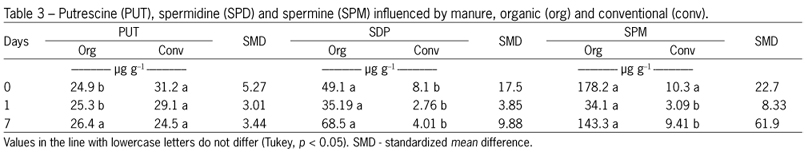
Antioxidant activity was measured by the DPPH method, and shows the influence of the levels of phytochemicals analyzed (Table 1). There is a tendency to higher levels of polyphenols, flavonoids, vitamin C after day 4 of storage, and the same behavior was observed when analyzing DPPH levels. Other factors that have influenced the antioxidant activity are the polyamines spermidine and spermine (Table 3), which occur at higher levels in organically grown vegetables.
The present study suggests that the use of ozone, even if it is a recognized oxidizing agent, does not alter the antioxidant capacity of broccoli until the fourth day of analysis (Table 2). Several authors have shown that plants from organic farming possess higher antioxidant activity than those grown conventionally. Other researchers have confirmed our findings, including a study on tomato (Lycopersicon esculentum Mill.) juice from organic and conventional vegetables, where antioxidant activity (measured by the DPPH method) was higher in organic tomato juice (Vallverdú-Queralt et al., 2012). In this study, the polyphenolic, flavonoid and vitamin C levels observed in organic broccoli (Tables 1 and 2) and the higher content of spermidine and spermine (polyamines related to cell division) (Tables 3 and 4) may explain the results.
Polyamines represent another class of substances that may contribute to an increase in antioxidant capacity, which is directly related to aging. Interestingly, higher levels of spermidine and spermine were found in organic broccoli samples, during the period of storage (Table 3). The total polyamine content, which is the sum of the putrescine, spermidine and spermine contents, was always higher in organic vegetables. In plants, polyamines exhibit anti-senescent activity, most likely by competing with ethylene via the same common precursor S-adenosylmethionine (SAM) (Pandey et al., 2000). These substances are involved in the regulation of many cellular processes, including DNA replication, transcription, translation, cell proliferation, modulation of enzymatic activities, electrolytic balance and stability of the cell membrane (Igarashi and Kashiwagi, 2010). Thus, because broccoli is a vegetable that activates ethylene synthesis during senescence, the level of polyamines observed in the present study could explain the higher longevity of organic broccoli, as well as the chlorophyll content. These results suggest that polyamine content can influence the maintenance of plant cell membranes; however, more in-depth biochemical studies should be conducted.
Higher levels of putrescine can be found in sanitation treatments on the first day, while this effect disappears after seven days of cold storage. The levels of spermidine and spermine were not altered by the treatments applied using these sanitizers (Table 4). The organic cultivation system, in addition to promoting ecosystem sustainability and less environmental impact, results in the accumulation of higher concentrations of functional substances in plants. These substances include phenolic compounds and polyamines, such as spermidine and spermine, which may positively influence the health of the human population. Chemical compounds, such as vitamins and polyphenols, contribute to the antioxidant activity of an organism.
Conclusions
The sanitation treatment with ozone did not infl uence the contents of polyamines, polyphenolic compounds (included flavonoid), and vitamin C, as well as the maintenance of total chlorophyll levels. This last feature is extremely important because the yellowing of broccoli is an important quality factor for producers and consumers and at the same time indicates the onset of vegetable senescence.
Received June 27, 2013
Accepted November 22, 2013
Edited by: Paulo Cesar Sentelhas
- Aimlar-or, S.; Kaewsuksaeng, S.; Shigyo, M.; Yamauchi, N. 2010. Impact of UV-B irradiation on chlorophyll degradation and chlorophyll-degrading enzyme activities in stores broccoli (Brassica oleracea L. Italica Group) florets. Food Chemistry 120: 645-652.
- Alothman, M.; Kaur, B.; Fazilah, A.; Bhat, R.; Karim, A.A. 2010. Ozone-induced changes of antioxidant capacity of freshcut tropical fruits. Innovative Food Science and Emerging Technologies 11: 666-671.
- Beltrán, D.; Selma, M.V.; Marín, A.; Gil, I.M. 2005. Ozonated water extends the shelf life of fresh-cut lettuce. Journal of Agricultural and Food Chemistry 53: 5654-5663.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. 1995. The phenolic constituents of Prunus domestica I. The quantitative analysis of phenolic constituents. Lebensmittel-Wissenschaft & Technologie 28: 25-30.
- Costa, L.; Vicente, A.R.; Civello, P.M.; Chaves, A.R.; Martínez, G.A. 2006. UV-C treatment delays postharvest senescence in broccoli florets. Postharvest Biology and Technology 39: 204- 210.
- Flores, H.E.; Galston, A.W. 1982. Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiology 69: 701-706.
- Hasperué, J.H.; Chaves, A.R.; Martínez, G.A. 2011. End of day harvest delays postharvest senescence of broccoli florets. Postharvest Biology and Technology 59: 64-70.
- Hoefkens, C.; Sioen, I.; Baert, K.; Meulenaer, B.D.; Hensuw, S.D.; Vandekinderen, I.; Devlieghere, F.; Opsomer, A.; Verbeke, W.; Camp, J.V. 2010. Consuming organic versus conventional vegetables: the effect on nutrient and contaminant intakes. Food and Chemical Toxicology 48: 3058-3066.
- Hoigné, J.; Bader, H. 1983. Rate constants of reactions of ozone with organic and inorganic compounds in water. II. Dissociating organic compounds. Water Research 17: 185-194.
- Igarashi, K.; Kashiwagi, K. 2010. Characteristics of cellular polyamine transport in prokaryotes and eukaryotes. Plant Physiology and Biochemistry 48: 506-512.
- Lima, G.P.P.; Rocha, S.A.; Takaki, M.; Ramos, P.R.R.; Ono, E.O. 2008. Comparison of polyamine, phenol and flavonoid contents in plants grown under conventional and organic methods. International Journal of Food Science and Technology 43: 1838- 1843.
- Lima, G.P.P.; Vianello, F. 2011. Review on the main differences between organic and conventional plant-based foods. International Journal of Food Science and Technology 46: 1-13.
- Martínez-Sánchez, A.; Allende, A.; Bennett, R.N.; Ferreres, F.; Gil, M.I. 2006. Microbial, nutritional and sensory quality of rocket leaves as affected by different sanitizers. Postharvest Biology and Technology 42: 86-97.
- Nagata, M.; Yamashita, I. 1992. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. Journal of the Japanese Society for Food Science and Technology 39: 925-928.
- Naguib, A.El-M.M.; El-Baz, F.K.; Salama, Z.A.; Hanaa, H.A.E.B.; Ali, H.F.; Gaafar, A.A. 2012. Enhancement of phenolics, flavonoids and glucosinolates of Broccoli (Brassica oleracea, var. Italica) as antioxidants in response to organic and bio-organic fertilizers. Journal of the Saudi Society of Agricultural Sciences 11: 135-142.
- Pandey, A.; Ranade, S.A.; Nagar, P.K.; Kumar, N. 2000. Role of polyamines and ethylene as modulators of plant senescence. Journal of Bioscience 25: 291-299.
- Popova, M.; Bankova, V.; Butoyska, D.; Petkov, V.; Nikolova- Damyanova, B.; Sabatini, A.G.; Marcazzan, G.L.; Bogdanov, S. 2004. Validated methods for the quantification of biologically active constituents of poplar-type propolis. Phytochemical Analysis 15: 235-240.
- Rossetto, M.R.M.; Vianello, F.; Rocha, S.A.; Lima, G.P.P. 2009. Antioxidant substances and pesticide in parts of beet organic and conventional manure. African Journal of Plant Science 3: 245-253.
- Singleton, V.L.; Rossi Jr, J.A. 1965. Colorimetry of total phenolics with phosphomolybidic-phosphotungstic acid reagents. American Journal of Enology and Viticulticulture 16: 144-158.
- Sousa, A.; Ferreira, I.C.F.R.; Barros, L.; Bento, A.; Pereira, J.A. 2008. Effect of solvent and extraction temperatures on the antioxidant potential of traditional stoned table olives "alcaparras". LWT - Food Science and Technology 41: 739-745.
- Terada, M.; Watanabe, Y.; Kunitoma, M.; Hayashi, E. 1978. Differential rapid analysis of ascorbic acid and ascorbic acid 2-sulfate by dinitrophenilhydrazine method. Annals of Biochemistry 84: 604-608.
- Vallverdú-Queralt, A.; Medina-Remón, A.; Casals-Ribes, I.; Lamuela-Raventos, R.M. 2012. Is there any difference between the phenolic content of organic and conventional tomato juices? Food Chemistry 130: 222-227.
- Winter, C.F.; Davis, S.F. 2006. Organic foods. Journal of Food Science 71: 117-124.
Publication Dates
-
Publication in this collection
14 Apr 2014 -
Date of issue
Apr 2014
History
-
Received
27 June 2013 -
Accepted
22 Nov 2013