Abstracts
Leptin, a polypeptide hormone secreted mainly by adipose tissue, plays an important role in feed intake regulation, energy metabolism and reproduction in several species. Its function has been intensively studied in mammals; however, in birds limited information is available. The cDNA sequence for chicken leptin has been reported, and high hepatic expression levels of leptin were associated with fat deposition in selected bird lines. However, controversies still remain concerning to the chicken leptin gene and several authors failed to amplify this gene from genomic DNA or cDNA. In view of this controversy and the importance of this gene, the present study aimed to investigate the leptin gene in a population of birds developed by Embrapa Swine and Poultry Research Center (Brazil). First of all, the sequences of Gallus gallus leptin gene (GenBank AF012727) and Mus musculus (GenBank NM_008493) were aligned with the objective of designing primers in conserved regions among the two species, since 94.6% of similarity is described in the literature in those species. For all four pairs of primers designed, several amplification tests were performed with both DNA and cDNA, but neither unique fragment nor expected band size was ever achieved. The leptin sequence in GenBank does not represent the sequence of the chicken leptin gene.
Gallus gallus; fat deposition; primer design
A leptina, hormônio polipeptídico secretado principalmente pelo tecido adiposo, tem um papel importante na regulação da ingestão de alimentos, metabolismo de energia e reprodução em mamíferos. A função do gene da leptina tem sido intensamente estudada em mamíferos, porém, em aves, ainda é pouco conhecida. O cDNA deste gene foi identificado em galinhas, e a alta expressão hepática e os níveis de leptina no plasma foram associados à alta deposição de gordura presente em linhagens de aves selecionadas. Entretanto, permanecem controvérsias sobre o gene da leptina em galinhas, pois diversos autores não conseguiram amplificar este gene a partir de DNA genômico ou cDNA. Tendo em vista essas controvérsias e a importância desse gene, o presente trabalho teve como objetivo amplificar o gene da leptina numa população de aves desenvolvida pela Embrapa Suínos e Aves (Brasil). Primeiramente, as seqüências do gene da leptina de Gallus gallus (GenBank AF012727) e Mus musculus (GenBank NM_008493) foram alinhadas com o objetivo de desenhar os primers em regiões conservadas nas duas espécies, pois como descrito na literatura, esses genes apresentam 94,6% de similaridade. Para os quatro pares de primers desenhados, foram realizados diversos testes de amplificação utilizando DNA e cDNA, mas não foi obtido um fragmento único ou a banda esperada. A seqüência do gene da leptina depositada no GenBank não representa a seqüência do gene da leptina de galinhas.
Gallus gallus; deposição de gordura; desenho de primers
NOTE
Investigation of Leptin gene in broiler and layer chicken lines
Investigação do gene da Leptina em linhagens de aves de corte e postura
Kerli NinovI; Mônica Corrêa LedurII; Helena Javiel AlvesIII; Millor Fernandes do RosárioIV; Kátia NonesV; Luiz Lehmann CoutinhoVI, * * Corresponding author < llcoutin@esalq.usp.br>
IUSP/ESALQ - Programa de Pós-Graduação em Ciência Animal e Pastagens
IIEmbrapa Suínos e Aves, C.P. 21 - 89700-000 - Concórdia, SC - Brasil
IIIUSP/FZEA - Programa de Pós-Graduação em Zootecnia, Av. Duque de Caxias Norte, 225 - 13635-900 - Pirassununga, SP - Brasil
IVUSP/ESALQ - Programa de Pós-Graduação em Genética e Melhoramento de Plantas
VCrop & Food Research, Private Bag 4704 - Christchurch 8140 - New Zealand
VIUSP/ESALQ - Depto. de Zootecnia, C.P. 09 - 13418-900 - Piracicaba, SP - Brasil
ABSTRACT
Leptin, a polypeptide hormone secreted mainly by adipose tissue, plays an important role in feed intake regulation, energy metabolism and reproduction in several species. Its function has been intensively studied in mammals; however, in birds limited information is available. The cDNA sequence for chicken leptin has been reported, and high hepatic expression levels of leptin were associated with fat deposition in selected bird lines. However, controversies still remain concerning to the chicken leptin gene and several authors failed to amplify this gene from genomic DNA or cDNA. In view of this controversy and the importance of this gene, the present study aimed to investigate the leptin gene in a population of birds developed by Embrapa Swine and Poultry Research Center (Brazil). First of all, the sequences of Gallus gallus leptin gene (GenBank AF012727) and Mus musculus (GenBank NM_008493) were aligned with the objective of designing primers in conserved regions among the two species, since 94.6% of similarity is described in the literature in those species. For all four pairs of primers designed, several amplification tests were performed with both DNA and cDNA, but neither unique fragment nor expected band size was ever achieved. The leptin sequence in GenBank does not represent the sequence of the chicken leptin gene.
Key words:Gallus gallus, fat deposition, primer design
RESUMO
A leptina, hormônio polipeptídico secretado principalmente pelo tecido adiposo, tem um papel importante na regulação da ingestão de alimentos, metabolismo de energia e reprodução em mamíferos. A função do gene da leptina tem sido intensamente estudada em mamíferos, porém, em aves, ainda é pouco conhecida. O cDNA deste gene foi identificado em galinhas, e a alta expressão hepática e os níveis de leptina no plasma foram associados à alta deposição de gordura presente em linhagens de aves selecionadas. Entretanto, permanecem controvérsias sobre o gene da leptina em galinhas, pois diversos autores não conseguiram amplificar este gene a partir de DNA genômico ou cDNA. Tendo em vista essas controvérsias e a importância desse gene, o presente trabalho teve como objetivo amplificar o gene da leptina numa população de aves desenvolvida pela Embrapa Suínos e Aves (Brasil). Primeiramente, as seqüências do gene da leptina de Gallus gallus (GenBank AF012727) e Mus musculus (GenBank NM_008493) foram alinhadas com o objetivo de desenhar os primers em regiões conservadas nas duas espécies, pois como descrito na literatura, esses genes apresentam 94,6% de similaridade. Para os quatro pares de primers desenhados, foram realizados diversos testes de amplificação utilizando DNA e cDNA, mas não foi obtido um fragmento único ou a banda esperada. A seqüência do gene da leptina depositada no GenBank não representa a seqüência do gene da leptina de galinhas.
Palavras-chave: Gallus gallus, deposição de gordura, desenho de primers
INTRODUCTION
One of the challenges of the poultry industry is to improve chicken's carcass quality and reduce fat content, without prejudicial effects on the genetic gains already obtained. Leptin (LEP), a polypeptidic hormone secreted mainly by adipose tissue, plays an important role in feed intake regulation, energy metabolism and reproduction (Zhang et al., 1994). Thus, it represents an excellent candidate gene for polymorphism investigation and association with economic traits in livestock species.
The exon-intron organization of this gene is conserved among mouse, human and bovine, presenting three exons and two introns (Taniguchi et al., 2002). In bovine, polymorphisms on LEP gene has been associated with body fat, feed intake and milk yield (Buchanan et al., 2002, Liefers et al., 2002, Nkrumah et al., 2005). In swine, Robert et al. (1998) observed LEP gene polymorphisms and mRNA levels associated with carcass composition and subcutaneous fat thickness, respectively.
Unlike mammals, little is known about the avian LEP gene function. In chicken, only its coding sequence was identified and sequenced by Taouis et al. (1998). Hepatic expression of leptin was detected exclusively in chickens by these authors. This particularity can be attributed to the avian lipid metabolism, in which the liver is the primary site of lipogenesis. Dridi et al. (2005) found higher rates of leptin hepatic expression and plasma levels in bird lines selected for high fat deposition.
However, controversies still remain upon the chicken leptin gene. Friedman-Einat et al. (1999) failed to reproduce the results published by Taouis et al. (1998), although using the same set of primers. Pitel et al. (2000) concluded that the leptin gene was not yet mapped in chickens, unlike previously published data from the same authors (Pitel et al., 1999).
Considering the controversies regarding the existence of the leptin gene in chicken and the chicken leptin cDNA sequences available in gene bank, the strategy employed in this study was to use two different chicken lines and several primers in different regions of the available sequences.
MATERIAL AND METHODS
Experimental Lines
Two chicken lines, a broiler (TT) and a layer (CC) were used for this study. TT is a broiler male line developed by the Embrapa Poultry Breeding Program, and has been under within line selection for improving body weight, feed conversion, retail cut yield, breast meat weight, viability, fertility, hatchability, and reducing abdominal fat. The CC is a White Leghorn pure line that has been selected for improving egg production, egg weight, feed conversion, hatchability, sexual maturity, fertility, viability, egg quality and reducing body weight. A detailed description of the lines was reported by Figueiredo et al. (2003a; 2003b). Blood samples were collected at slaughter in tubes containing EDTA and immediately frozen at -70ºC for further DNA analyses.
DNA extraction
Genomic DNA was extracted with the DNAzol® reagent (Invitrogen) following manufacture's protocol. DNA concentration was assessed by spectrophotometer at OD260nm and DNA purity was assessed by OD260nm:OD280nm ratio and the quality by electrophoresis on 1% agarose gel stained with ethidium bromide.
RNA extraction
Total RNA was isolated from adipose and hepatic tissue of a 21 day-old chicken using Trizol Reagent (Invitrogen) and following the manufacture's protocol. RNA concentration was assessed by spectrophotometer at OD260nm, RNA purity was assessed by OD260nm:OD280nm ratio and quality by electrophoresis on 1% agarose gel.
The cDNA synthesis was performed using the SuperScript First-Strand Synthesis System for RT-PCR Kit (Invitrogen). Reverse transcription took place at 42°C for 50 minutes, with further enzyme inactivation at 70°C for 15 minutes. To remove the RNA from the hybrid cDNA:RNA molecule an enzymatic digestion was performed with two units of RNase H for 20 minutes at 37°C.
Primers design
Three set of primers were designed using Primer3 primer design software (Rozen & Skaletzky, 2000 - www.frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). These primers were designed based on the chicken sequence leptin mRNA (GenBank - AF012727) and the fourth set of primers was selected from the paper published by Dridi et al. (2005). The primers were analyzed on NetPrimer (http://www.premierbiosoft.com/netprimer/netprlaunch/netprlaunch.html), in order to avoid secondary structures, such as hairpins and loops, and primer dimer. To assess the homology between mRNA sequence of Gallus gallus (GenBank AF012727) and Mus musculus (GenBank NM_008493) sequence alignment was conducted using Multalin software (Corpet, 1988 http://prodes.toulouse.inra.fr/multalin/multalin.html).
PCR
Different methodologies were used for PCR optimization. PCRs were performed in the thermocycler PTC-200 (MJ Research). The PCR Optimizer Kit (Invitrogen) was used and is constituted of buffers with different pH and magnesium concentrations: Buffer A 5X (MgCl2 7.5 mM, pH 8.5), B 5X (MgCl2 10 mM, pH 8.5), C 5X (MgCl2 12.5 mM, pH 8.5), E 5X (MgCl2 7.5 mM, pH 9.0), and J 5X (MgCl2 10 mM, pH 9.5). All buffers share the same concentrations of Tris-HCl (300 mM) and ammonium sulfate (75 mM). For each PCR reaction, 3 µL of genomic DNA (20 ng µL-1) was added to 22 µL of the reaction mix: 5.0 µL of one buffer 5X (A, B, C, E and J), 1 µL of dNTP (10 mM), 2.5 µL of each primer (2.5 pmols µL-1), 0.3 µL (1U) of Platinum Taq DNA Polymerase High Fidelity (Invitrogen), in a final volume of 25 µL. Besides the tests with pH and magnesium concentrations, tests with amplification conditions were performed with the objective of settling down the best condition for each one of the primer pairs. All tests were performed with 30 cycles of amplification. Initial denaturation temperature oscillated from 92 to 95°C, and time went from 1 to 3 minutes. Annealing temperature ranged from 45 to 70°C, and time went from 1 to 3 minutes. Extension temperature was 72°C, with time varying from 0.5 to 2 minutes. Final extension was 72°C for 10 minutes for all conditions.
RT-PCR
To verify the integrity of the cDNA, a PCR was made to amplify the constitutive b-actin gene (Primer F: AATGAGAGGTTCAGGTGTCC and Primer R: ATCACA GGGGTGTGGGTGTT). Tests to establish the best amplification conditions by RT-PCR were performed.
For each reaction, 2 µL of cDNA (20 ng µL-1) was added to 23 µL of reaction mix composed by the following: 1.0 µL 10 X Buffer (50 mM KCl, 10 mM Tris-HCl pH 9.0), 1 µL MgSO4 (50 mM), 1 µL dNTP (10 mM), 2.5 µL of each primer (2.5 pmols µL-1), 0.25 µL (1U) of Taq DNA polymerase (Invitrogen), in a final volume of 25 µL. Different amplification conditions were evaluated. Initial denaturation temperature was 95°C, and time oscillated between 1 to 3 minutes. Annealing temperature ranged from 45 to 65°C for 1 minute. Extension temperature was 72°C, with time varying from 1 to 2 minutes. Final extension was 72°C for 10 minutes for all conditions.
RESULTS AND DISCUSSION
Primer design
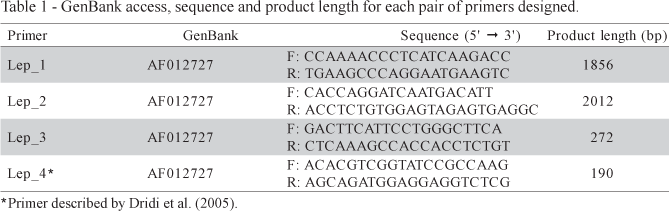
The GenBank access number, sequence and product length of primers designed are in Table 1. Solely the putative sequence of the Gallus gallus leptin mRNA, as described by Taouis et al. (1998) is found on GenBank database. Considering that Friedman-Einant et al. (1999) and Pitel et al. (2000) could not reproduce the results of Taouis et al. (1998), we aligned the mRNA sequence of Gallus gallus (GenBank AF012727) and Mus musculus (GenBank NM_008493) in order to assess conserved regions of the gene, since Taouis et al. (1998) described 94.6 % of similarity among theses sequences (Figure 1). The high similarity of chicken and mouse leptin is questionable. Such similarity is not in agreement with the phylogenetics relationships between nucleotide sequence of leptin in mammals and also in other mammalian genes (Friedman-Einant et al., 1999 and Doyon et al., 2001). Comparing the sequences of prolactin and interferon (which, like leptin, belong to the cytokine family) within mammals, a similarity less than the one between the leptin sequence of chicken and mouse, of 94.6%, was found.
Based upon the LEP gene sequence of mice described by He et al. (1995), it is supposed that there exists an intron with approximately 1730 bp in the sequence of the chicken LEP gene found in the GenBank. Consequently, primers Lep_1 (185 bp) and Lep_2 (2012bp) were designed in a region flanking intron 2. The Lep_3 (272 bp) and Lep_4 (190 bp) were designed in the exon 3. Figure 2 shows the primer sequences and the regions flanked by them.
PCR
Several attempts to amplify the leptin gene using a primer described by Dridi et al. (2005) and three primers designed from the putative sequence described by Taouis et al. (1998) were made. All four primers pairs were submitted to several amplification conditions; however, it was not possible to identify a unique and specific band of the expected size, which should have amplified fragments of 1856, 2012, 272 and 190 bp for Lep_1, Lep_2, Lep_3 and Lep_4, respectively. Representative results of some of the conditions used are in Figure 3.
Our results agree with those of Friedman-Einat et al. (1999), who attempting to amplify the leptin gene and using the results from Taouis et al. (1998) as reference designed 14 pairs of primers in several regions of the presumed leptin sequence of Gallus gallus. Four of their primers were exactly the same as those previously published by Taouis et al. (1998). The experiments were performed with cDNA from hepatic, pancreatic, and adipose tissue of mice and several avian species. They observed amplification of avian cDNA, but always in an unspecific pattern and not showing the expected fragment length. Sequencing of those fragments showed no similarity with the known sequence. The expected fragment was only obtained from mice samples. In addition, these authors also used the hybridization strategies Northern and Southern Blot, but only the control gene GAPDH was detected.
Pitel et al. (1999) published the mapping of chicken leptin gene on chromosome 7, based upon the sequence described by Taouis et al. (1998). Nevertheless, Pitel et al. (2000), after sequencing the PCR product obtained from primers based on the sequence described by Taouis et al. (1998), detected that the resulting sequence did not correspond neither to that published, nor to any sequence accounted to the chicken genome in GenBank. Once the mapping of leptin gene would require amplification of a genomic fragment, and because this was not a possible task using the published sequence, Pitel et al. (2000) stated that the leptin gene was not yet mapped, unlike previously published by their own group.
RT-PCR
In order to insure that we had good quality RNA and cDNA, RT-PCR was first performed with b-actin primers. The expected 409 bp fragment was obtained (Figure 4a). For the amplification, primer Lep_3 was chosen because of its location within a coding region. After confirming the cDNA integrity, temperature gradient tests were performed in order to optimize PCR conditions of Lep_3 primer. The gradient ranged from 45 to 65°C, but no amplification of the expected 272 bp fragment was observed (Figure 4b).
After several attempts to amplify the leptin gene using both DNA and cDNA, the expected fragment was not detected. These results corroborate the results found by Friedman-Einant et al. (1999), Pitel et al. (2000) and Carre et al. (2006), which also were not successful in amplifying this gene. Searches for the supposed chicken leptin sequence using the Basic Local Alignment Search Tool (BLAST) were made against the chicken genome (GenBank - http://www.ncbi.nlm.nih.gov/genome/seq/BlastGen/BlastGen.cgi?taxid=9031), and the sequence was not found.
CONCLUSION
The amplification of the chicken leptin gene from a broiler and a layer line used in this study was not possible. Several attempts of amplification of DNA and cDNA using primers designed based on the sequence published were made, but an expected and unique fragment was not obtained. Such evidences suggest that the leptin sequence in GenBank does not represent the sequence of the chicken leptin gene.
Considering the important role played by leptin in diverse metabolic pathways, more studies must be carried out in order to overcome the controversial literature and to provide new horizons for the leptin exploration and understanding.
ACKNOWLEDGEMENTS
To Jorge Luiz Ferreira de Andrade for technical assistence with the pictures. To CNPq for the research productivity scholarship to L.L. Coutinho. This study was supported by grants from FAPESP, EMBRAPA and PRODETAB.
Received December 11, 2006
Accepted September 06, 2007
- BUCHANAN, F.C.; FITZSIMMONS. C.J.; VAN KESSEL, A.G.; THUE, T.D.; INKELMAN-SIM, D.C.; SCHMUTZ , S.M. Association of a missense mutation in the bovine leptin gene with carcass fat content and leptin mRNA levels. Genetics Selection Evolution, v.34, p.105-116, 2002.
- CARRE, W.; WANG, X.; PORTER, T.E.; NYS, Y.; TANG, J.; BERNBER, G.E.; MORGAN, R.; BURNSIDE, J.; AGGREY, S.E.; SIMON, J.; COGBURN, L.A. Chicken genomics resource: sequencing and annotation of 35,407 ESTs from single and multiple tissue cDNA libraries and CAP3 assembly of a chicken gene index. Physiological Genomics, v.16, p.514-524, 2006.
- CORPET, F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Research, v.16, p.10881-10890, 1988.
- DOYON, C.; DROUIN, G.; TRUDEAU, V.L.; MOON, T.W. Molecular evolution of leptin. General and Comparative Endocrinology, v.124, p.188-198, 2001.
- DRIDI, S.; BUYSE, J.; DECUYPERE, E.; TAOUIS, M. Potential role of leptin in increase of fatty acid synthase gene expression in chicken liver. Domestic Animal Endocrinology, v.29, p.646-660, 2005.
- FIGUEIREDO, E.A.P.; ROSA, P.S.; SCHEUERMANN, G.N.; JAENISCH, F.R.F.; SCHMIDT, G.S.; LEDUR, M.; BRENTANO, L.; COSTA, C.A.F. Genetic gain in body weight feed conversion and carcass traits in White Plymouth Rock broiler strain Embrapa 021. In: WORLD CONFERENCE ON ANIMAL PRODUCTION, 9.; REUNIÃO DA ASSOCIACION LATINOAMERICANA DE PRODUÇÃO ANIMAL, 18., Porto Alegre, 2003. Proceedings Porto Alegre: WAAP; ALPA; SBZ; UFRGS, 2003a. 1 CD-ROM.
- FIGUEIREDO, E.A.P.; SCHMIDT, G.S.; LEDUR, M.C.; AVILA, V.S.; BRUM, P.A.R.; FIORENTIN, L.; JAENISCH, F.R.F. Genetic gain in egg production and egg weight in White Leghorn Embrapa 011. In: WORLD CONFERENCE ON ANIMAL PRODUCTION, 9.; REUNIÃO DA ASSOCIACION LATINOAMERICANA DE PRODUÇÃO ANIMAL, 18., Porto Alegre, 2003. Proceedings Porto Alegre: WAAP; ALPA; SBZ; UFRGS, 2003b. 1 CD-ROM.
- FRIEDMAN-EINAT, M.T.; BOSWELL, G.; HOREV, G.; GIRISHVARMA, I.C.; DUNN TALBOT, R.T.; SHARP, P.J.The chicken leptin gene: Has it been cloned? General and Comparative Endocrinology, v.115, p.354-363, 1999.
- HE, Y.; CHEN, H.; QUON, M.J.; REITMAN, M. The Mouse obese Gene. Journal of Biological Chemistry, v.270, p.28887-28891, 1995.
- LIEFERS, S.C.; TE PAS, M.F.W.; VEERKAMP, R.F.; VAN DER LENDE, T. Associations between leptin gene polymorphisms and production, live weight, energy balance, feed intake and fertility in Holstein heifers. Journal of Dairy Science, v.85, p.1633-1638, 2002.
- NKRUMAH, J.D.; LI, C.; YU, J.; HANSEN, C.; KEISLER, D.H.; MOORE, S.S. Polymorphisms in the bovine leptin promoter associated with serum leptin concentration, growth, feed intake, feeding behavior, and measures of carcass merit. Journal of Animal Science, v.83, p.20-28, 2005.
- PITEL, F.; MONBRUN, C.; GELLIN, J.; VIGNAL, A. Mapping of the LEP (OB) gene to a chicken microchromosome. Animal Genetics, v.30, p.73-74, 1999.
- PITEL, F.; MONBRUN, C.; GELLIN, J.; VIGNAL, A. The chicken LEP (OB) gene has not been mapped. Animal Genetics, v.31, p.281, 2000.
- ROBERT, C.; PALIN, M.F.; COULOMBE, N.; ROBERGE, C.; SILVERSIDES, F.G.; BENKEL, B.F.; MCKAY, R.M.; PELLETIER, G. Backfat thickness in pigs is positively associated with leptin mRNA levels. Canadian Journal of Animal Science, v.78, p.473-482, 1998.
- ROZEN, S.; SKALETZKY, H.J. Primer 3 on the WWW for general users and for biologist programmers Totowa, NJ: Humana Press, 2000. 568p.
- TANIGUCHI, Y.; ITOH, T.; YAMADA, T.; SASAKI, Y. Genomic structure and promoter analysis of the bovine leptin gene. IUBMB Life, v.53, p.131-135, 2002.
- TAOUIS, M.; CHEN, J.W.; DAVIAUD, C.; DUPONT, J.; DEROUET, M.; SIMON, J. Cloning the chicken leptin gene. Gene, v.208, p.239-242, 1998.
- ZHANG, Y.; PROENÇA, R.; MAFFEI, M.; BARONE, M.; LEOPOLD, L.; FRIEDMAN, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature, v.372, p.425-432, 1994.
Publication Dates
-
Publication in this collection
31 Mar 2008 -
Date of issue
Apr 2008
History
-
Accepted
06 Sept 2007 -
Received
11 Dec 2006