Abstract
The influence of silver nanoparticles (Ag-NPs) on the optical properties of Bi2O3-GeO2 glasses was investigated aiming the characterization of their potential for applications in photonic devices. The samples were prepared by the melt-quenching technique starting from high purity oxides (GeO2, Bi2O3 and AgNO3). Heat-treatment during different times was performed to nucleate Ag-NPs. A transmission electron microscope was used to confirm the presence of Ag-NPs and to determine their sizes and composition. The glass network was studied by spontaneous Raman scattering. The optical absorption spectra showed an absorption band at ca. 500 nm associated to the bismuth ions, and a 7-fold enhancement of absorption was observed in the samples with Ag-NPs. Photoluminescence (PL) spectra were obtained by exciting the samples with a 808 nm laser. Maximum enhancements of ca. 100% of the PL band centered at 1300 nm and ca. 70% of the Raman spectrum were observed due to the nucleation of the Ag-NPs. The enhanced optical response of the samples with Ag-NPs is attributed to the growth of the local field on the isolated bismuth ions and clusters that are located in the vicinity of the nanoparticles. The results illustrate the large potential of Bi2O3-GeO2 with Ag-NPs to be used in photonic devices.
Keywords:
heavy metal oxide glass; silver nanoparticle; germanate glass; infrared photonic device; electroluminescent devices
Introduction
Heavy metal oxide (HMO) glasses are attracting large attention because of their linear and nonlinear optical properties that make them ideal candidates for applications such as ultrafast optical switches, optical limiters and active photonic devices.11 Sun, H. T.; Fujii, M.; Sakka, Y.; Bai, Z.; Shirahata, N.; Zhang, L.; Miwa, Y.; Gao, H.; Opt. Lett. 2010 , 35, 1743.
2 Meng, X. G.; Qiu, J. R.; Peng, M. Y.; Chen, D. P.; Zhao, Q. Z.; Jiang, X. W.; Zhu, C. S.; Opt. Express2005 , 13, 1628.
3 Hasegawa, T.; Nagashima, T.; Sugimoto, M.; Opt. Commun.2005 , 250, 411.
4 Kassab, L. R. P.; da Silva, D. S.; de Almeida, R.; de Araújo, C. B.; Appl. Phys. Lett.2009 , 94, 101912.
5 Kassab, L. R. P.; da Silva, D. S.; de Araújo, C. B.; J. Appl. Phys.2010 , 107, 113506.
6 Manzani, D.; de Araújo, C. B.; Boudebs, G.; Messaddeq, Y.; Ribeiro, S. J. L.; J. Phys. Chem. B2013 , 117, 408.
7 Oliveira, T. R.; Falcão-Filho, E. L.; de Araújo, C. B.; da Silva, D. S.; Kassab, L. R. P.; da Silva, D. M.; J. Appl. Phys. 2013 , 114, 073503.
8 de Araújo, C. B.; da Silva, D. S.; de Assumpção, T. A. A.; Kassab, L. R. P.; da Silva, D. M.; The Sci. World J.2013 , 385193.
9 da Silva, D. S.; Naranjo, L. P.; Kassab, L. R. P.; de Araújo, C. B.; Appl. Phys. B2012 , 106, 1015.
10 Rulkov, B.; Ferin, A. A.; Popov, S. V.; Taylor, J. R.; Razdobreev, I.; Bigot, L.; Bouwmans, G.; Opt. Express2007 , 15, 5473.
11 Fujimoto, Y.; Nakatsuka, M.; Appl. Phys. Lett.2003 , 82, 3325.
12 Peng, M.; Qin, J.; Chen, D.; Meng, X.; Yang, L.; Jiang X.; Zhu, C.; Opt. Lett.2004 , 29, 1998.
13 Peng, M.; Qiu, J.; Chen, D.; Meng, X.; Zhu, C.; Opt. Lett. 2005 , 30, 2433.
14 Ren, J.; Wu, B.; Jiang, X.; Dong, H.; Zeng, H.; Qin, J.; Appl. Phys. B2007 , 88, 363.
15 Ren, J.; Dong, H.; Zeng, H.; Hu, H.; Zhu, C.; Qin, J.; IEEE Photonics Technol. Lett.2007 , 19, 1395.
16 Arai, Y.; Suzuki, T.; Ohishi, Y.; Morimoto, S.; Khonton, S.; Appl. Phys. Lett.2007 , 90, 261110.
17 Hughes, M. A.; Akada, T.; Suzuki, T.; Ohishi, Y.; Hewak, D. W.; Opt. Express2009 , 17, 19345.-1818 Dianov, C. M.; Dvoyrin, V. V.; Mashinsky, V. M.; Umnikvov, A. A.; Yaskov, M. V.; Guryanov, A. N.; Quantum Electron. 2005 , 35, 1083. In general, besides low optical absorption in the visible and in the near-infrared (NIR) regions, the HMO glasses present high index of refraction (ca. 2.0), low cutoff phonon energies (ca. 800 cm-1), large polarizability, large resistance to moisture and good thermal stability that allow the fabrication of optical fibers. Particularly, the glasses containing bismuth oxide (Bi2O3) deserve large attention because of the broadband photoluminescence (PL) from 1000 to 1600 nm, due to bismuth (Bi) ions.1212 Peng, M.; Qin, J.; Chen, D.; Meng, X.; Yang, L.; Jiang X.; Zhu, C.; Opt. Lett.2004 , 29, 1998.
13 Peng, M.; Qiu, J.; Chen, D.; Meng, X.; Zhu, C.; Opt. Lett. 2005 , 30, 2433.
14 Ren, J.; Wu, B.; Jiang, X.; Dong, H.; Zeng, H.; Qin, J.; Appl. Phys. B2007 , 88, 363.
15 Ren, J.; Dong, H.; Zeng, H.; Hu, H.; Zhu, C.; Qin, J.; IEEE Photonics Technol. Lett.2007 , 19, 1395.
16 Arai, Y.; Suzuki, T.; Ohishi, Y.; Morimoto, S.; Khonton, S.; Appl. Phys. Lett.2007 , 90, 261110.-1717 Hughes, M. A.; Akada, T.; Suzuki, T.; Ohishi, Y.; Hewak, D. W.; Opt. Express2009 , 17, 19345. Ren et al.,1414 Ren, J.; Wu, B.; Jiang, X.; Dong, H.; Zeng, H.; Qin, J.; Appl. Phys. B2007 , 88, 363.,1515 Ren, J.; Dong, H.; Zeng, H.; Hu, H.; Zhu, C.; Qin, J.; IEEE Photonics Technol. Lett.2007 , 19, 1395. for instance, reported efficient optical amplification in the region 1272-1348 nm using Bi-doped magnesium-germanate,1414 Ren, J.; Wu, B.; Jiang, X.; Dong, H.; Zeng, H.; Qin, J.; Appl. Phys. B2007 , 88, 363. and Bi-doped germano-silicate glasses,1515 Ren, J.; Dong, H.; Zeng, H.; Hu, H.; Zhu, C.; Qin, J.; IEEE Photonics Technol. Lett.2007 , 19, 1395. and Dianov et al.1818 Dianov, C. M.; Dvoyrin, V. V.; Mashinsky, V. M.; Umnikvov, A. A.; Yaskov, M. V.; Guryanov, A. N.; Quantum Electron. 2005 , 35, 1083. demonstrated laser action between 1150 and 1300 nm in a Bi-doped aluminosilicate fiber.1818 Dianov, C. M.; Dvoyrin, V. V.; Mashinsky, V. M.; Umnikvov, A. A.; Yaskov, M. V.; Guryanov, A. N.; Quantum Electron. 2005 , 35, 1083. The NIR broadband emission was also observed in a Bi-doped soda-lime silicate,1616 Arai, Y.; Suzuki, T.; Ohishi, Y.; Morimoto, S.; Khonton, S.; Appl. Phys. Lett.2007 , 90, 261110. and chalcogenide glass.1717 Hughes, M. A.; Akada, T.; Suzuki, T.; Ohishi, Y.; Hewak, D. W.; Opt. Express2009 , 17, 19345. Therefore new possibilities for optical communications using optical fibers containing bismuth in comparison with the results already reported with rare-earth ions doped fibers are envisaged.
In the present work we investigate the influence of silver nanoparticles (Ag-NPs) on the optical properties of Bi2O3-GeO2 glasses. The main motivation for this work was to obtain a composite material with enhanced optical properties in comparison with the well-known Bi2O3-GeO2 glass. The results showed a 7-fold enhanced absorption in the visible range, PL growth of ca. 100% at 1300 nm as well as a ca. 70% enhancement of the spontaneous Raman scattering cross-section due to the presence of the Ag-NPs. In the Experimental section we describe the methods and techniques used for fabrication and characterization of the glasses. In the Results and Discussion section we report on the structural and optical properties of the samples. Finally, in the Conclusions, we present a summary of the results.
Experimental
The samples investigated were fabricated using the melt-quenching method with the starting composition (in mol%): 89.6GeO2-9.6Bi2O3-0.8AgNO3. The reagents were melted in an alumina crucible for 1 h at 1200 ºC, quenched in a preheated brass mold, annealed at 420 ºC during 1 h (to avoid internal stress) and cooled to room temperature inside the furnace. After this procedure, the samples were polished and cut in order to have parallel faces. The samples prepared with AgNO3 were heat-treated (HT) during 1, 12 and 24 h at 420 ºC to thermally reduce the Ag+ ions to Ag0 and to nucleate the Ag-NPs. The melting temperature of 1200 ºC was chosen based on our previous experiments that showed higher NIR luminescence when compared to the emission from the samples melted at 1100 ºC. Samples with composition (in mol%): 86GeO2-14Bi2O3 and 80.4GeO2-19.6Bi2O3 were also prepared, without Ag-NPs, and melted at 1100 ºC and 1200 ºC, for comparison with the optical response of the glass containing Ag-NPs.
A 200 kV transmission electron microscope (TEM) was used to determine the sizes and shapes of the Ag-NPs. Energy dispersive X-ray spectroscopy was performed during the TEM analysis in order to confirm the presence of Ge, Bi and Ag-NPs. Electron diffraction measurements were performed to determine the crystalline structure of Bi NPs according to JCPDS-ICDD data base.1919 Sepulveda-Guzman, S.; Elizondo-Villarreal, N.; Ferrer, D.; Castro, A. T.; Gao, X.; Zhou, J. P.; Yacaman, M. J.; Nanotechnology2007 , 18, 335604 For these measurements the samples were milled, mixed with distilled water, and partially decanted. The floating part was taken by using a metallic screen and analyzed by TEM.
The linear optical absorption spectra were measured with a spectrophotometer operating from 350 to 1600 nm. The PL measurements were made by exciting the samples with a 808 nm continuous-wave diode laser (maximum power: 200 mW). A spectrometer, attached to a photomultiplier and a computer, was used to analyze the PL signals. Spontaneous Raman scattering measurements using a laser operating at 532 nm were performed to study the glass network. All measurements were made with the samples at room temperature.
Results and Discussion
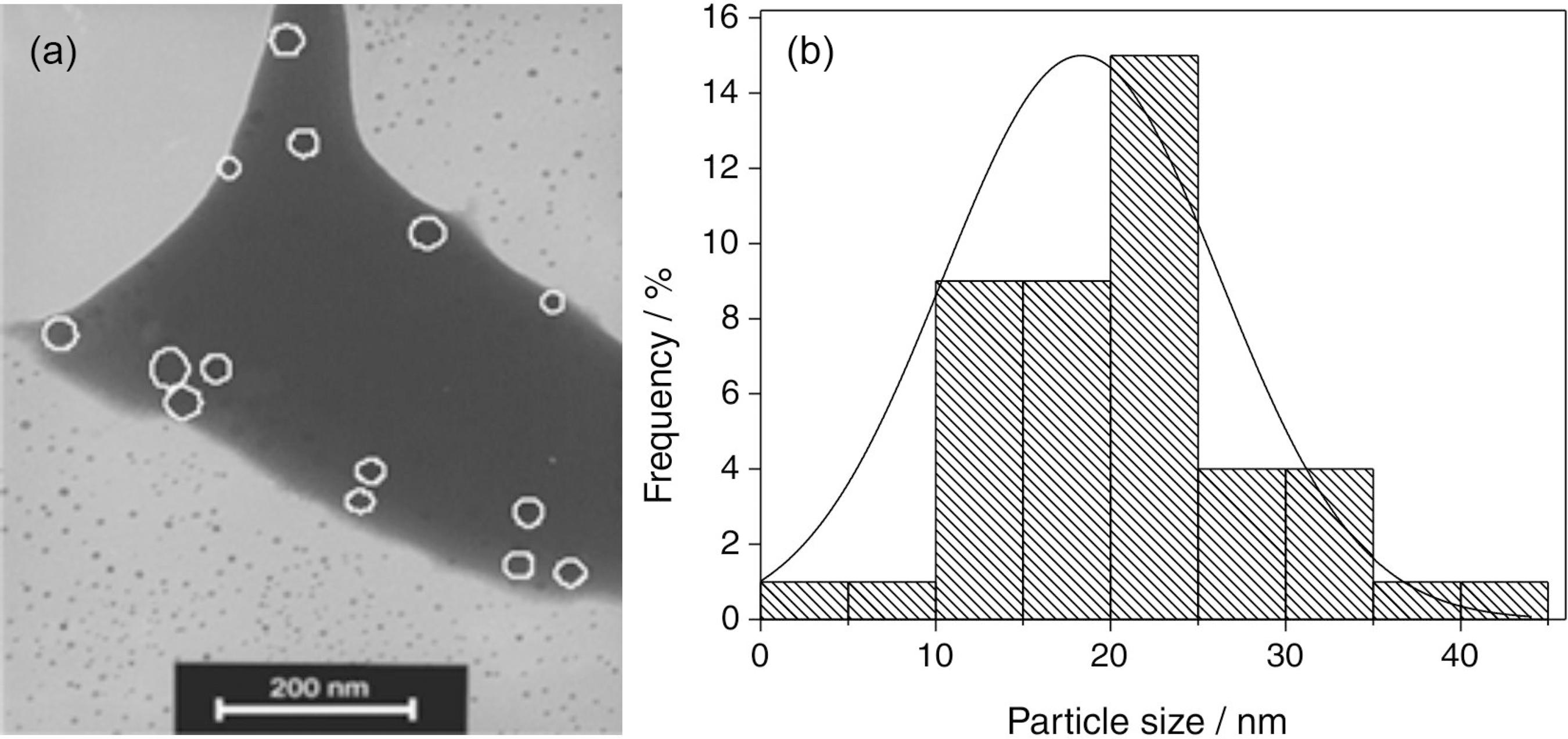
The TEM images obtained for samples HT during 1 h showed the presence of Ag-NPs with average diameter of ca. 20 nm. One representative TEM image is shown in Figure 1a and the size distribution of the NPs is shown in Figure 1b.
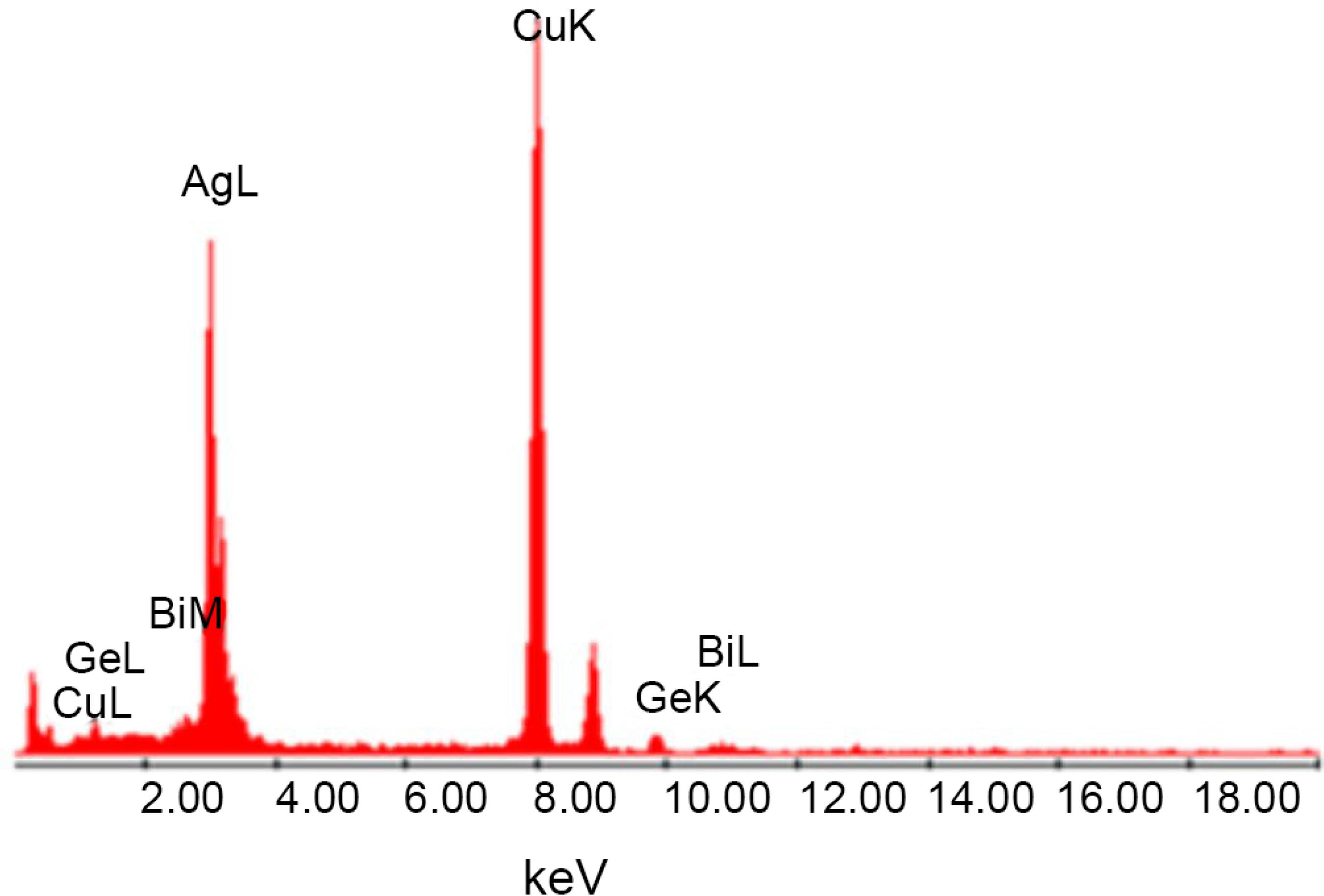
Figure 2 shows energy dispersive spectroscopy (EDS) results that confirm the presence of Ge, Bi and Ag in the samples. The features corresponding to the Cu K line are due to the grid where the sample is placed for the TEM measurements.
(a) TEM image of 89.6GeO2-9.6Bi2O3-0.8AgNO3 (mol%) glass with Ag-NPs, heat treated during 1 hour at 420 ºC; (b) size distribution of the Ag-NPs.
EDS spectrum of the 89.6GeO2-9.6Bi2O3-0.8AgNO3 (mol%) glass sample heat-treated during 1 hour at 420 ºC.
Figure 3a shows a TEM image of Bi NPs that present rhomboedral structure in agreement with literature.1919 Sepulveda-Guzman, S.; Elizondo-Villarreal, N.; Ferrer, D.; Castro, A. T.; Gao, X.; Zhou, J. P.; Yacaman, M. J.; Nanotechnology2007 , 18, 335604 This measurement was made with samples without Ag-NPs. The inset of Figure 3a shows the pattern that was used to calculate the diffraction indices (104 and 110) of the Bi structure according to the JCPDS-ICDD data base.2020 JCPDS International Centre for Diffraction Data 2003 PDF 85-1330; PCPDFWIN v. 24. The Bi NPs size distribution is presented in Figure 3b and shows average dimensions of ca. 9 nm. We recall that the formation of Bi NPs and Bi clusters due to electron beam irradiation in a TEM was previously reported.1919 Sepulveda-Guzman, S.; Elizondo-Villarreal, N.; Ferrer, D.; Castro, A. T.; Gao, X.; Zhou, J. P.; Yacaman, M. J.; Nanotechnology2007 , 18, 335604 In the present study this effect was observed several minutes after starting the irradiation; motion of Bi NPs was noted where the electron beam was focused as well as a tendency of coalescence.
(a) TEM image of 89.6GeO2-9.6Bi2O3-0.8AgNO3 (mol%) glass without Ag-NPs. The Bi particles observed were formed several minutes after the exposition to the electron beam irradiation. The inset shows the diffraction pattern that confirms the rhombohedral structure of bismuth; (b) size distribution of the Bi particles.
The absorption spectra of the samples prepared with and without Ag-NPs are shown in Figure 4. Notice that the sample without Ag-NPs presents a band centered at ca. 500 nm related to divalent bismuth ions (Bi2+), as reported for other Bi-doped glasses.2121 Guo, X.; Li, H. J.; Su, L. B.; Yu, P. S.; Zhao, H. Y.; Liu, J. F.; Xu, J.; Laser Phys. 2011 , 21, 901.,2222 Miller, A. E.; Nassau, K.; Lyons, K. B.; Lines, M. E.; J. Non- Cryst. Solids1988 , 99, 289. The spectra of the samples with AgNO3 in the starting composition present a large broadband in the blue-orange region with an amplitude that increases with the increase of the heat-treatment time. This behavior is due to the localized surface plasmons band associated to the Ag-NPs, but it is also possible that the Bi2+ located in the vicinity of the Ag-NPs are also contributing due to the enhancement of their absorption transitions centered at ca. 500 nm. The 7-fold increase observed in the absorption band is an evidence of the large volume fraction of the Ag-NPs. The inset in Figure 4 illustrates the behavior of the samples without Ag-NPs, heat-treated during 1, 12 and 24 hours. It can be seen a small decrease of the Bi2+ absorption band as reported for other bismuth doped glasses;2121 Guo, X.; Li, H. J.; Su, L. B.; Yu, P. S.; Zhao, H. Y.; Liu, J. F.; Xu, J.; Laser Phys. 2011 , 21, 901.,2323 Jiang, X.; Su, L.; Guo, X.; Tang, H.; Fan, X.; Zhan, Y.; Wang, Q.; Zheng, L.; Li, H.; Xu, J.; Opt. Lett. 2012 , 37, 4260. this change is negligible in comparison with the large increase observed for the samples with Ag-NPs.
Room temperature absorption spectra of the 89.6GeO2-9.6Bi2O3-0.8AgNO3 (mol%) glass. The inset shows the behavior of the sample without Ag-NPs for different heat-treatment times. Sample heat-treated during: 1 h (solid circles); 12 h (triangles); 24 h (asterisks). The spectrum of the sample without Ag-NPs is shown for reference (squares). Samples thickness: 2.0 mm.
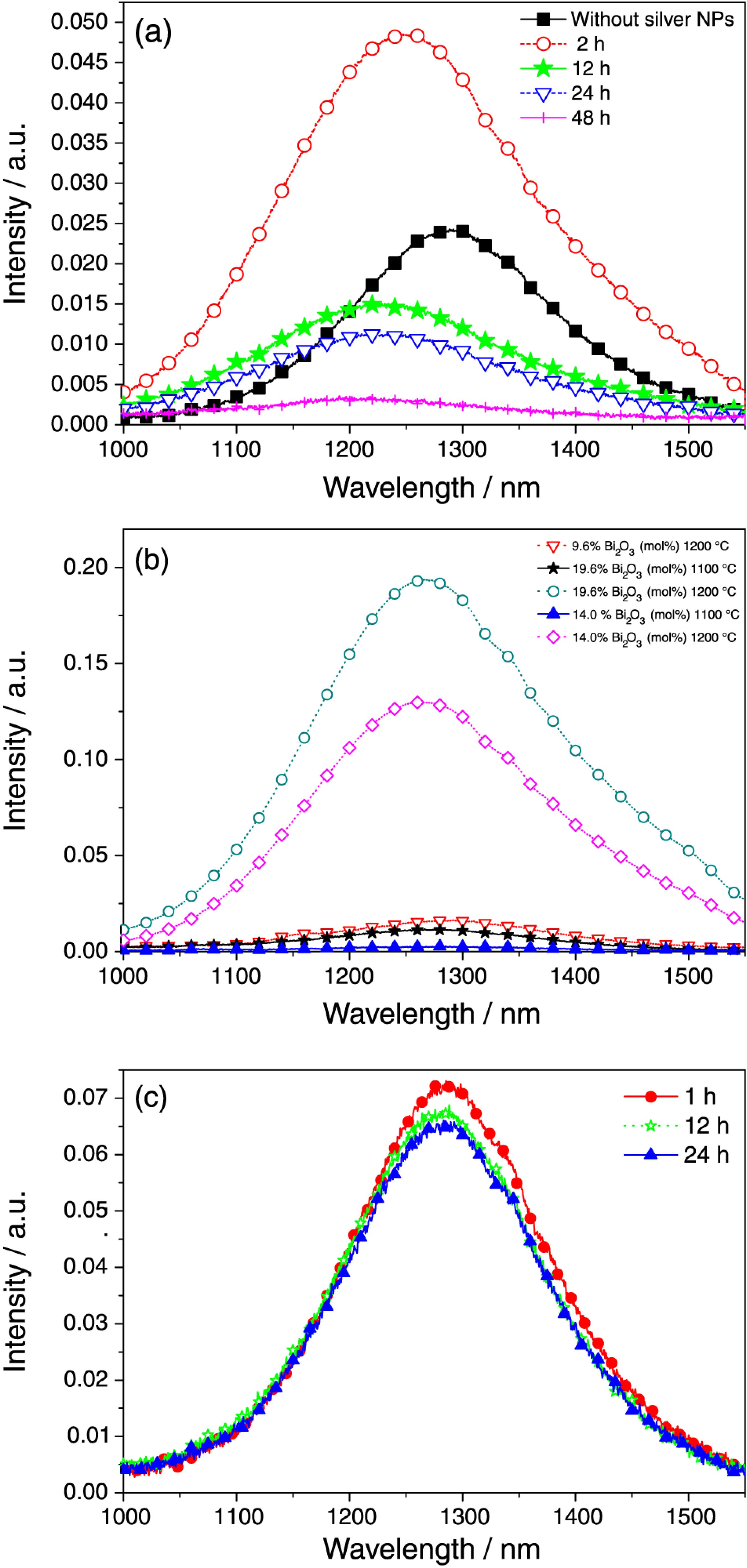
Figure 5 presents the PL spectra of the samples excited at 808 nm. The strong band from 1000 to 1600 nm, centered at 1300 nm, is attributed to the Bi+ luminescence centers. A red-shift of the NIR band in the samples with Ag-NPs can be observed in Figure 5a. For the sample HT during 1 h the NIR band shifted to ca. 1250 nm, whereas for longer heat treatment times the band shifted to ca. 1220 nm. This effect is probably related to changes of the local environment of bismuth in the glass structure, due to the nucleation of Ag-NPs. Analogous behavior was observed in previous work2323 Jiang, X.; Su, L.; Guo, X.; Tang, H.; Fan, X.; Zhan, Y.; Wang, Q.; Zheng, L.; Li, H.; Xu, J.; Opt. Lett. 2012 , 37, 4260. where a shift of the NIR band was attributed to changes in the glass structure.
(a) Phot o luminescence (PL) spec tra of the 89.6GeO2-9.6Bi2O3-0.8AgNO3 (mol%) glass excited at 808 nm, for different heat-treatment intervals. The sample without Ag-NPs is shown for reference; (b) results of 90.4GeO2-9.6Bi2O3, 86GeO2-14 Bi2O3 and 80.4GeO2-19.6Bi2O3 glasses (mol%), without Ag-NPs, melted at 1100 and 1200 ºC; (c) behavior of the PL spectra for the sample without Ag-NPs for three heat-treatment time intervals.
Figure 5b shows the results for GeO2-Bi2O3 glasses prepared without Ag-NPs, melted at 1100 and 1200 ºC. Notice that the samples melted at 1200 ºC present larger NIR emission that indicates a larger number of Bi+ centers. This was the reason for the choice of the 1200 ºC melting temperature in the present work.
Figure 5a exhibits ca. 100% enhancement of the PL intensity at ca. 1300 nm when a comparison between the sample HT during 1 h and the sample without Ag-NPs is made. The PL enhancement is attributed to the local field growth in the vicinity of the Ag-NPs. For the samples HT during longer times quenching was observed probably due to the larger number of Ag-NPs nucleated and the reduction of the distances between the Ag-NPs and the luminescence centers. The PL quenching should not be attributed to the oxidation of Bi+ because the change observed in the samples without Ag-NPs HT during 12 and 24 h is negligible, as illustrated by Figure 5c.
Figure 6 presents the Raman spectra for the samples with and without Ag-NPs. The shoulder in the region from 200 to 300 cm-1 may be attributed to the asymmetric motion of Bi atoms that are simultaneously half-bridged and half-nonbridged to oxygen (O–Bi-O).2424 Yu, Q.; Chen, F.: Xu, T.; Dai, S.; Zhang, Q.; J. Non-Cryst. Solids2013 , 378, 254.,2525 Baia, L.; Kiefer, W.; Simon, S.; Ruman; Rep. Phys. 2004 , 56, 430. The band at ca. 430 cm-1 is assigned to the symmetric stretching of bridged oxygen in bisecting the Ge-O-Ge plane in predominantly six-membered rings.2626 Durben, D. J.; Wolf, G. H.; Phys. Rev. B1991 , 43, 2355. The Raman band centered at 815 cm-1 is related to the asymmetric vibration of the nonbridging oxide structures, Ge-O− and B-O. The Raman signal due to the GeO− vibration is expected to be dominant in the present case.2323 Jiang, X.; Su, L.; Guo, X.; Tang, H.; Fan, X.; Zhan, Y.; Wang, Q.; Zheng, L.; Li, H.; Xu, J.; Opt. Lett. 2012 , 37, 4260. For the samples with Ag-NPs, the maximum enhancement of ca. 70% was observed due to the enhanced local electromagnetic field in the interface between the Ag-NPs and the dielectric medium, the so-called surface enhanced Raman scattering (SERS) effect.2727 Dousti, M. R.; Sahar, M. R.; Amjad, R. J.; Ghoshal, S. K.; Awang, A.; J. Lumin.2013 , 143, 368.
Raman spectra of the glass 89.6GeO2-9.6Bi2O3-0.8AgNO3 (mol%) heat-treated during 1, 12 and 24 h, at 420 ºC. The spectrum for the sample without Ag-NPs is shown for reference (laser wavelength: 532 nm).
Conclusions
This is the first report that shows the influence of silver nanoparticles on the absorption spectrum, near-infrared luminescence and Raman vibrational modes of the Bi2O3-GeO2 glass. The results show that this glass containing Ag-NPs has large potential to be used for color filters in the blue-orange spectral region as well as for devices requiring intense and large bandwidth luminescence in the 1200-1600 nm region. The 7-fold increase observed for the linear absorption band in the blue-orange region and the ca. 100% enhancement in the NIR luminescence demonstrate the important role of Ag-NPs for the optical performance of this material. Surface enhanced Raman scattering due to the influence of the Ag-NPs was also observed.
-
FAPESP has sponsored the publication of this article.
Acknowledgments
We acknowledge financial support by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo a Ciência e Tecnologia do Estado de Pernambuco (FACEPE). The work was performed in the framework of the National Institute of Photonics (INCT de Fotônica) project. We acknowledge the Nanotechnology National Laboratory (LNNano), CNPEMCampinas/Brazil, for the HR-TEM measurements, and Laboratório de Micoreletrônica/EPUSP, for the Raman measurements.
References
-
1Sun, H. T.; Fujii, M.; Sakka, Y.; Bai, Z.; Shirahata, N.; Zhang, L.; Miwa, Y.; Gao, H.; Opt. Lett 2010 , 35, 1743.
-
2Meng, X. G.; Qiu, J. R.; Peng, M. Y.; Chen, D. P.; Zhao, Q. Z.; Jiang, X. W.; Zhu, C. S.; Opt. Express2005 , 13, 1628.
-
3Hasegawa, T.; Nagashima, T.; Sugimoto, M.; Opt. Commun.2005 , 250, 411.
-
4Kassab, L. R. P.; da Silva, D. S.; de Almeida, R.; de Araújo, C. B.; Appl. Phys. Lett.2009 , 94, 101912.
-
5Kassab, L. R. P.; da Silva, D. S.; de Araújo, C. B.; J. Appl. Phys.2010 , 107, 113506.
-
6Manzani, D.; de Araújo, C. B.; Boudebs, G.; Messaddeq, Y.; Ribeiro, S. J. L.; J. Phys. Chem. B2013 , 117, 408.
-
7Oliveira, T. R.; Falcão-Filho, E. L.; de Araújo, C. B.; da Silva, D. S.; Kassab, L. R. P.; da Silva, D. M.; J. Appl. Phys 2013 , 114, 073503.
-
8de Araújo, C. B.; da Silva, D. S.; de Assumpção, T. A. A.; Kassab, L. R. P.; da Silva, D. M.; The Sci. World J.2013 , 385193.
-
9da Silva, D. S.; Naranjo, L. P.; Kassab, L. R. P.; de Araújo, C. B.; Appl. Phys. B2012 , 106, 1015.
-
10Rulkov, B.; Ferin, A. A.; Popov, S. V.; Taylor, J. R.; Razdobreev, I.; Bigot, L.; Bouwmans, G.; Opt. Express2007 , 15, 5473.
-
11Fujimoto, Y.; Nakatsuka, M.; Appl. Phys. Lett.2003 , 82, 3325.
-
12Peng, M.; Qin, J.; Chen, D.; Meng, X.; Yang, L.; Jiang X.; Zhu, C.; Opt. Lett.2004 , 29, 1998.
-
13Peng, M.; Qiu, J.; Chen, D.; Meng, X.; Zhu, C.; Opt. Lett 2005 , 30, 2433.
-
14Ren, J.; Wu, B.; Jiang, X.; Dong, H.; Zeng, H.; Qin, J.; Appl. Phys. B2007 , 88, 363.
-
15Ren, J.; Dong, H.; Zeng, H.; Hu, H.; Zhu, C.; Qin, J.; IEEE Photonics Technol. Lett.2007 , 19, 1395.
-
16Arai, Y.; Suzuki, T.; Ohishi, Y.; Morimoto, S.; Khonton, S.; Appl. Phys. Lett.2007 , 90, 261110.
-
17Hughes, M. A.; Akada, T.; Suzuki, T.; Ohishi, Y.; Hewak, D. W.; Opt. Express2009 , 17, 19345.
-
18Dianov, C. M.; Dvoyrin, V. V.; Mashinsky, V. M.; Umnikvov, A. A.; Yaskov, M. V.; Guryanov, A. N.; Quantum Electron 2005 , 35, 1083.
-
19Sepulveda-Guzman, S.; Elizondo-Villarreal, N.; Ferrer, D.; Castro, A. T.; Gao, X.; Zhou, J. P.; Yacaman, M. J.; Nanotechnology2007 , 18, 335604
-
20JCPDS International Centre for Diffraction Data 2003 PDF 85-1330; PCPDFWIN v. 24.
-
21Guo, X.; Li, H. J.; Su, L. B.; Yu, P. S.; Zhao, H. Y.; Liu, J. F.; Xu, J.; Laser Phys 2011 , 21, 901.
-
22Miller, A. E.; Nassau, K.; Lyons, K. B.; Lines, M. E.; J. Non- Cryst. Solids1988 , 99, 289.
-
23Jiang, X.; Su, L.; Guo, X.; Tang, H.; Fan, X.; Zhan, Y.; Wang, Q.; Zheng, L.; Li, H.; Xu, J.; Opt. Lett 2012 , 37, 4260.
-
24Yu, Q.; Chen, F.: Xu, T.; Dai, S.; Zhang, Q.; J. Non-Cryst. Solids2013 , 378, 254.
-
25Baia, L.; Kiefer, W.; Simon, S.; Ruman; Rep. Phys 2004 , 56, 430.
-
26Durben, D. J.; Wolf, G. H.; Phys. Rev. B1991 , 43, 2355.
-
27Dousti, M. R.; Sahar, M. R.; Amjad, R. J.; Ghoshal, S. K.; Awang, A.; J. Lumin.2013 , 143, 368.
Publication Dates
-
Publication in this collection
Dec 2015
History
-
Received
27 June 2015 -
Accepted
31 July 2015