Abstract
PURPOSE
The parotidectomy technique still has an elevated paresis and paralysis index, lowering patient life's quality. The correct identification of the facial nerve can prevent nerve damage. Fluorescent dye identifies nerves in experimental studies but only few articles focused its use on facial nerve study in parotidectomies. We aimed to stain the rat facial nerve with fluorescent dye to facilitate visualization and dissection in order to prevent injuries.
METHODS
Forty adult male Wistar rats were submitted to facial injection of saline solution (Gsf-control group, 10) or fluorescent dye solution (Gdye group, 30) followed by parotidectomy preserving the facial nerve, measuring the time for localization and facility of localization (LocTime and LFN). Nerve function was assessed using the Vibrissae Movements (PMV) and Eyelid Closure Motion (PFP) scores.
RESULTS
Nerve localization was faster in Gdye group, with 83% Easy LFN rate. The Gdye group presented with low nerve injury degree and better PMV and PFP scores, with high sensitivity and accuracy.
CONCLUSIONS
This experimental method of facial nerve fluorescence was effective for intraoperative nerve visualization, identification and preservation. The technique may be used in future facial nerve studies, translated to humans, contributing to the optimization of parotid surgery in the near future.
Facial Nerve; Fluorescent Dyes; Parotid Gland; Rats
Introduction
Over the past century there has been little change in the surgical technique for tumor resection in the parotid gland, which is mainly based on anatomical landmarks. Despite recent technical refinements, the rates of complications such as paralysis and facial nerve paresis have not decreased.
The first report of total parotidectomy with conservation of the facial nerve under general anesthesia was performed by M. Codreanu in 189211. Laage-Hellman JE. Facial nerve in parotidectomies. Arch Otolaryngol. 1965;81(5):527-33. PMID: 14275912.,22. Codreanu M. Tumora in regiunea parotidei in dreapta: operat recidivat peste cinci ani :operat a dona oara: vindicarea cu conservarea facialului siaglandei parotide. Spitalul Bucurest. 1892;12:273.. In 1907, Thomas Carwardine was the first to suggest identifying the facial nerve before resecting parotid tumors, noting that the care and time spent would reflect positively on the aesthetic result33. Kidd H. Complete excision of the parotid gland with preservation of the facial nerve. Br Med J. 1950;i:898-991. PMID: 15414338.,44. Carwardine T. Excision of the parotid gland with preservation of the facial nerve: its possibility. Lancet. 1907;170(4387):897.. The development of the current parotid surgery technique was a result of the contributions of Henry Samuel Shucksmith and Hayes Martin, who wrote that the facial nerve trunk should be routinely exposed at the opening of the stylomastoid foramen prior to proceeding with tumor resection. This marked the beginning of the era of the anterograde facial nerve dissection used today.
Since the 2000s, electrophysiological monitoring has been used to prevent injuries of the extratemporal portion of the facial nerve during parotidectomy55. López M, Quer M, León X, Orús C, Recher K, J V. Usefulness of facial nerve monitoring during parotidectomy. Acta Otorrinolaringol Esp. 2001;52:418-21. PMID: 11526649.. However, more recent articles support the notion that intraoperative monitoring of the facial nerve reduces surgery time but has little impact on nerve dysfunction66. Ozturk K, Akyildiz S, Gode S, Turhal G, Gursan G, T K. The effect of partial superficial parotidectomy on amplitude, latency and threshold of facial nerve stimulation. Eur Arch Otorhinolaryngol. 2015;11. [Epub ahead of print]. PMID: 25862067.,77. Liu H, Wen W, Huang H, Liang Y, Tan X, Liu S, Liu C, Hu M. Recurrent pleomorphic adenoma of the parotid gland: intraoperative facial nerve monitoring during parotidectomy. Otolaryngol Head Neck Surg. 2014;151:87-91. PMID: 24671460..
Fluorescent dyes have been used in laboratory research since the 1970s, and are an important tool for research into neural cell differentiation, neural regeneration, cell maturation and cell transplants88. Axelrod D. Carbocyanine dye orientation in red cell membrane studied by microscopic fluorescence polarization. Biophys J. 1979;26:557-74. PMID: 263688.. Fluorescent dyes of the cyanine family were first investigated in 1982 by Simset al.99. Sims PJ, Waggoner AS, Wang CH, Hoffman JF. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974;13:3315-30. PMID: 4842277. and Illertet al.1010. Illert M, Fritz N, Aschoff A, Holländer H. Fluorescent compounds as retrograde tracers compared with horseradish peroxidase (HRP). II A parametric study in the peripheral motor system of the cat. J Neurosci Methods. 1982;6:199-218. PMID: 6183536. through the staining of peripheral nerves in cats, and by Aschoff et al, who studied other fluorescent compounds until obtaining FastDio(r), currently the most effective one1111. Aschoff A, Holländer H. Fluorescent compounds as retrograde tracers compared with horseradish peroxidase (HRP). I. A parametric study in the central visual system of the albino rat. J Neurosci Methods. 1982;6(3):179-97. PMID: 7144234.. It is therefore that staining of the facial nerve facilitates the intraoperative visualization of the facial nerve trunk and its branches. There is, however, lack of literature on this issue.
In 2008, Dogru et al.1212. Dogru S VDD, Hansen MR. Retrograde labeling of the rat facial nerve with carbocyanine dyes to enhance intraoperative identification. Ann Otol Rhinol Laringol. 2008;117(10):753-8. PMID: 18998504. used various types of fluorescent dyes with different applications to visualize the facial nerve with a confocal stereomicroscope with epifluorescence and concluded that this technique can facilitate nerve identification. In 2011, Wu et al.1313. Wu AP, Whitney MA, Crisp JL, Friedman B, Tsien RY, Nguyen QT. Improved facial nerve identification with novel fluorescently labeled probe. Laryngoscope. 2011;121:805-10. doi: 10.1002/lary.21411.
https://doi.org/10.1002/lary.21411...
performed a transection of the facial nerve in transgenic mice expressing Yellow Fluorescent Protein (YFP) under the control of a neuron-specific promoter. Intravenous injections of fluorescein in these mice helped to locate the stumps and to perform reanastomosis and did not affect nerve function. Whitney et al.1414. Whitney MA, Crisp JL, Nguyen LT, Friedman B, Gross LA, Steinbach P, Tsien RY, Nguyen QT. Fluorescent peptides highlight peripheral nerves during surgery in mice. Nat Biotech. 2011;29(4):352-6. doi: 10.1038/nbt.1764.
https://doi.org/10.1038/nbt.1764...
studied nerve regeneration in mice by using Cy5-NP41, an intravenous fluorescent dye that binds to peripheral nerve tissue. Although the dye proved to be effective, the authors called for more pharmacokinetic studies. In 2014, Kleijan et al.1515. KleinJan GH, Buckle T, van Willigen DM, van Oosterom MN, Spa SJ, Kloosterboer HE, van Leeunwen FW. Fluorescent lectins for local in vivo visualization of peripheral nerves. Molecules. 2014;8(19):7. doi: 10.3390/molecules19079876.
https://doi.org/10.3390/molecules1907987...
reported on the use of a lecithin-derived fluorescent dyes able to bind to the extracellular matrix of peripheral nerve tissue. They identified WGA-Cys5 as the most promising when applied intramuscularly into the lower limbs of mice to study the sciatic nerve.
Thus, considering the scarcity of studies on the subject, here we used an experimental model that aims to reduce the high rates of postoperative complications in parotid gland surgery through the visualization and preservation of the facial nerve. The primary objective of this work was to facilitate the location and dissection of the facial nerve and its branches during surgery in rats by applying the fluorescent dye carbocyanine (FastDiO(r)) by facial transdermal injection, using a simple microscope with polarized light. The secondary objective was to determine whether there was injury to the facial nerve trunk and its branches after dissection. The results observed in rats may ultimately be translated to humans, and contribute to the optimization of parotid gland surgery in the near future.
Methods
The study and general research project was approved by the Ethics Committee of the Universidade Federal de São Paulo under number 180495, in accord with the International Organization of Medical Sciences-CIOMS.
We used 40 adult Wistar rats weighing between 200 and 300 grams who were housed in individual cages with a 12-hour light-dark cycle and with free access to food and liquids. Animals were randomized into two groups: The control group with saline solution (Gsf group) with 10 animals have received subcutaneous microinjections of 100 microL (0.1 ml) of saline in the right hemiface only with a Hamilton syringe and the dye fluorescent group (Gdye group) with 30 animals who have received subcutaneous microinjections of 100 microL of a buffered solution of 2.5 mg/ml of FastDiO(r) dye (3,3'-dilinoleyloxacarbocyanine perchlorate-Invitrogen, Carlsbad, California, Molecular Probes, 42,364, Sigma-Aldrich(r)) in right hemiface only.
The microinjection technique for rats consists of injection into the facial muscles of the right hemiface, about 2.0 cm anterior to the tragus at the junction point (P) of an imaginary line joining the middle third of the tragus to the oral rhyme (TR Line) and the imaginary bisecting line of the angle formed between the nasal dorsum and the rat nasolabial folds (bisector) (Figure 1). The location of this line was confirmed by palpating the needle under the skin, slightly parallel and shallow relative to the facial muscles. This was done following anesthesia with an intraperitoneal injection of 10% ketamine hydrochloride (50 mg/kg) and 2% xylazine hydrochloride (50 mg/kg) (day 0). Facial nerve function was evaluated following the injection, with complete anesthetic recovery.
P: Point of microinjection; TR Line: Imaginary line joining the middle third of the tragus to the oral rhyme (TR Line); Bisecting Line: Imaginary bisecting line of the angle formed between the nasal dorsum and the rat nasolabial folds (bisector).
The first reading of facial nerve function was conducted on day zero by two independent observers (A and B), and neither of which knew which group each rat belonged to. After two days, the second reading was conducted in both groups. Facial nerve function was measured by the method proposed by Borin et al.2323. Borin A, Toledo RN, Faria SD, Testa JR, OLC. Behavioral and histologic experimental model of facial nerve regeneration in rats. Braz J Otorhinolaryngol. 2006;72:775-84. PMID: 17308830., which consists of scoring the rat's Vibrissae Movements (PMV) and Eyelid Closure (PFP) on a scale of 0 to 5 (Charts 1 and 2).
Surgical protocol of the localization and dissection of the facial nerve
On the second day after injection, the rats were anesthetized and submitted to surgery to locate the extratemporal portion of the facial nerve trunk in both groups. To avoid that the operator's fatigue could influence the outcome of the procedure, we randomly selected five rats per day to undergo surgery. The procedures in all 40 rats took eight days in total. Importantly, the day of the initial injection was always set according to the day of the surgery.
The surgery was performed by the same operator in a laboratory environment, in both groups (Gdye and Gsf), with an aseptic technique and adequate surgical material, using a simple microscope (Surgical Loupe (Seiler(r), St Louis-MO, 3.0x magnification, focal length of 420mm) and Cree LED Ultraviolet(r)polarized Light Source (365-410nm) - UniqueFire WF-502B brand), which is long-lasting and adapted for frontal use.
Measuring the time to locate the facial nerve (LocTime) began after exposing the parotid on the right and dissecting the mandibular and buccal branches of the facial nerve by following their paths anteriorly with the resection of the parotid, up to the trunk's entry into the skull base (Figure 2). Next, we dissected the temporal and zygomatic branches of the facial nerve as above, using a simple microscope and ultraviolet polarized light. When reaching the skull base, we assigned an LFN (localization of the facial nerve) score, which indicates the difficulty of exposure and dissection (Chart 3).
- Exposition of the dissection on the right side with the mandibular and buccal fluorescent colored branches of the facial nerve.
BBr: Buccal branch; MBr: Mandibular branch.
Rats in both groups were kept alive and their facial nerve function was assessed once again at the end of anesthesia by the two observers (A and B). They were observed and scored for four weeks, following an algorithm-based schedule (Chart 4). Data generated by the observers was submitted for statistical analysis using the following tests: Anova, General linear model, Kappa concordance index, and the equality test of two proportions. Significance was set at p<0.05.
Results
In order to facilitate the analysis, the results were divided as expected outcomes in:
A-Time and Difficulty of localization of the facial nerve;
B- Assessment of Facial Nerve Functions;
C- Performance characterization of the fluorescent dye;
We observed that no animals were lost and no complications occurred during any of the procedures. All facial nerve branches and trunks were found in both groups, and all parotid glands were resected uneventfully.
Time and difficulty of localization of the facial nerve
To investigate the potential benefit of using fluorescence staining of the facial nerve for its visualization and dissection during surgery, we conducted different analysis comparing rats which had their nerves stained with FastDiO(r) to a mock-stained control group (Gdye and Gsf groups).
First, we analyzed the time necessary to find the facial nerves and the difficulty of the procedure. Table 1shows the facial nerve localization time (LocTime) for both groups. Compared to the Gsf group, the Gdye group had significantly lower mean and median times (Figure 3), indicating that the nerve localization during surgery was faster when the dye was used. As expected, when assessing the localization of the facial nerve (LFN) scores, which indicate the difficulty of exposure and dissection during the parotidectomy, we observed that 83% of the Gdye group surgeries were considered easy while this number dropped to 60% in the Gsf group. This difference, however, was not statistically significant (Table 2).
To investigate a possible association between time and difficulty, we compared the LFN scores to the LocTime between groups and observed a significant difference in the mean LocTime between the Easy and Difficult LFN scores when analyzing both groups together and separately, p<0.001 (Table 3, Figure 4). These results indicate that the more difficult the dissection is, the more time is needed, and that this relationship is independent of the use of the fluorescent dye.
Assessment of facial nerve functions
To assess possible injuries to the facial nerve trunk and its branches after dissection, we analyzed facial nerve functions by scoring the rat's Vibrissae Movements (PMV) and Eyelid Closure (PFP) by two independent observers. Firstly, we observed that all the kappa concordance indexes were statistically significant and classified as Good to Ideal, in both PMV (kappa 0.98 - Ideal) and PFP (Kappa 0.71 - Good) and on all days (Table 4, Figure 5). The high Kappa index confirmed that the facial nerve function could correctly be measured by the PMV and PFP scores.
Each bars represents an observation time in days.
To assess PMV and PFP by group and time (days 2, 7, 14 and 28), we used a general linear model to measure the effects of the main variables and their interactions. We observed a significant difference between PMV and PFP scores when we compared the groups Gdye and Gsf, and the different times (Table 5). The PMV e PFP scores were significantly higher in the Gdye group, suggesting that the lesions of the facial nerves were milder and the recovery was faster in this group (Figures 6 and 7). Interestingly, when we looked at the variable time and all paired interactions, we observed that the PMV and PFP scores varied significantly over time for both groups (Table 6). These variations are likely related to the manipulation of the facial nerve that may result in a decrease in its function (the score decreases with the severity of the lesion) and its recovery with time. Importantly, there were no cases of permanent paralysis in the rats examined.
Table 7 shows the variations in PMV and PFP scores by group and by observation time. Regarding the interaction of PMV (Figure 8) and of PFP (Figure 9) scores between groups by observation time, we observed a significant variation across the different time points. Specifically, for both PMV and PFP scores, we noticed a drastic drop on day 2 followed by a gradual return to the baseline scores during the following weeks. Of note, the variations were lower for the Gdye group, which probably relates to a less intense nerve manipulation and, consequently, less damage in the fluorescent dye facial nerve.
Performance characterization of the fluorescent dye
Finally, we measured the performance of the fluorescent dye in order to classify its function. According to our analysis, FastDiO(r) demonstrated high sensitivity (83%), and accuracy (72%). However, it showed low specificity (40%).
Discussion
The surgical technique for locating and preserving the facial nerve has changed very little in the last century and the current technique is a variation of the works of Shucksmith et al.16 16. Shucksmith HS, Boyle TM, WKJ W. The surgery of parotid tumours; exposure of main trunk of facial nerve. Br Med J. 1951;2(4735):830-1. PMID: 14869735.and Martin1717. Martin H. The operative removal of tumors of the parotid salivary gland. Surgery. 1952;31(5):670-82. PMID: 14922115. based primarily on anatomical landmarks. Despite the emergence of technical refinements in recent decades, rates of complication such as paralysis and paresis of the facial nerve remain high, ranging from 10% to 70% for patients with benign and malignant neoplasms undergoing parotidectomy1818. Dunn EJ, Kent T, Himes JIC. Parotid neoplasms: a report of 250 cases and review of the literature. Ann Surg. 1976;184(83):500-5. PMID: 1015893.,1919. Garcia-Losarcas N, Gonzales-Hidalgo M, Franco-Castedo CJPB. Estimulacion electrica del nervio facial com funcion pronostica em la cirurgia de parótida. Ver Neurol. 2009;49:119-22. PMID: 19621305..
With the advent of intraoperative monitoring of the facial nerve, the operative time for resection of parotid gland tumors has decreased, but not enough to make the procedure safer to the nerve function. Even in cases of temporary paralysis of the facial nerve, the patient's quality of life is negatively affected.
Guntinas-Lichius et al.2020. Guntinas-Lichius O, Straesser A, Streppel M. Quality of life after facial nerve repair. Laryngoscope. 2007;117:421-6. PMID: 17334302.reported worse quality of life in patients submitted to facial nerve reconstruction, despite good reconstruction success rates, while Ciuman et al.2121. Ciuman RR, Oels W, Jaussi R, Dost P. Outcome, general, and symptom-specific quality of life after various types of parotid resection. Laryngoscope. 2012;122:1254-61. doi: 10.1002/lary.23318.
https://doi.org/10.1002/lary.23318...
showed that there was a global decrease in quality of life in patients submitted to different resection techniques of the parotid gland. Thus, alternative methods to facilitate visualization of the facial nerve trunk and its branches, from the start to the end of surgery, coupled with the improvement of the surgical technique and intraoperative monitoring, could reduce paralysis and paresis rates.
To facilitate intraoperative visualization, dissection and preservation of the facial nerve trunk during surgery, we investigated the usefulness and benefits of staining the rat facial nerve with fluorescent neuronal tracers. Our literature search revealed that very few studies have used fluorescent dyes to stain the facial nerve with the aim of identifying the nerve during parotid surgeries. In fact, we found only four such experimental studies and none was conducted in humans.
Differently from Dogru et al.30, we stained the facial nerve of all rats through the microinjection of FastDiO(r) dye using a simple microscope and ultraviolet polarized light as the light source in frontal focus instead of the confocal light of the microscope. The same study also tested several fluorescent dyes with different application techniques: crystals and solution and staining visualization with a confocal microscope1212. Dogru S VDD, Hansen MR. Retrograde labeling of the rat facial nerve with carbocyanine dyes to enhance intraoperative identification. Ann Otol Rhinol Laringol. 2008;117(10):753-8. PMID: 18998504.. In our work, nerve branch function was evaluated through the PMV and PFP scores rather than conduction studies. For this, rats were kept alive and observed for 28 days. Unlike Dogru et al., frontal ultraviolet polarized light was effective at identifying all the branches of the facial nerve, which suggests that confocal microscopy is not necessary for this task. Also, the number of animals studied in our model was greater30compared to that study (six rats in the FastDiO(r) group).
In Wu et al.1313. Wu AP, Whitney MA, Crisp JL, Friedman B, Tsien RY, Nguyen QT. Improved facial nerve identification with novel fluorescently labeled probe. Laryngoscope. 2011;121:805-10. doi: 10.1002/lary.21411.
https://doi.org/10.1002/lary.21411...
study, the authors intravenously injected a new fluorescein (F-NP41) with affinity for neuronal tissue using fluorescence under a confocal light microscope to locate the severed stumps and perform anastomosis. The authors succeeded in locating the nerve and obtained functional results equal to those of controls. However, unlike our model, they used transgenic mice expressing YFP in neural cells to enhance the visualization of regenerating nerve stumps (which may have facilitated staining), used a confocal microscope for visualization, and did not target the facial nerve.
According to Wei et al.2222. Wei Y, Gong K, Ao Q, Wang A, Gong Y, Zuo H, Zhang Y, Wang J, Wang G. Lentiviral vectors enveloped with rabies virus glycoprotein can be used as a novel retrograde tracer to assess nerve recovery in rat sciatic nerve injury models. Cell Tissue Res. 2014;355:255-66. doi: 10.1007/s00441-013-1756-x.
https://doi.org/10.1007/s00441-013-1756-...
, the retrograde nerve staining technique is currently the gold standard for the assessment of recovery of injured peripheral nerves and is an important tool to estimate and understand the benefits of treatment strategies. We therefore anticipated that fluorescent staining could facilitate nerve visualization during surgery. However, little was known about the possible injuries and damages to the nerves, which could affect their function during and after surgery. Thus, we questioned whether retrograde fluorescence staining of the facial nerve and its branches would facilitate their location and help in their safe dissection in an experimental model of parotidectomy.
We chose to use Wistar rats because the anatomy of their facial nerve has been relatively well-studied and is quite similar to that of humans. Also, they are a phylogenetically simpler mammal model, and are easy to purchase, handle, maintain and care for. The injections were performed after animals were randomized into each of the groups: Control Group (Gsf) and the Dye fluorescent group (Gdye), for a total of 40 animals and 40 nerves analyzed. The microinjections were always applied to the right side of the rat and compared to the opposite (not manipulated and normal) side as standardized by Borin et al.2323. Borin A, Toledo RN, Faria SD, Testa JR, OLC. Behavioral and histologic experimental model of facial nerve regeneration in rats. Braz J Otorhinolaryngol. 2006;72:775-84. PMID: 17308830. in order to better correlate the real function of the manipulated facial nerve with the nerve function scores (PMV and PFP) and submitted to the same observation time in both groups for the evaluation of facial nerve function scores in each day to analyze the possible changes in the nerve function as the recovery occurred. In addition, our study was strengthened by the randomization of the rats and the blinding of the two observers.
To address our first objective, which was to investigate if our fluorescence staining method facilitates the localization and dissection of the facial nerve and its branches during surgery in rats, we first analyzed the differences between facial nerve localization times between groups (Gdye and Gsf) (Table 1). We observed shorter localization time for the stained nerve group, with a lower median and significant p-value (p = 0.001) (Figure 3). This shows that staining facilitated the visualization of the trunk and branches of the facial nerve. Secondly, we analyzed the ease of nerve dissection by creating the facial nerve localization and dissection score (LFN) with two variables (Easy and Difficult), as shown in Table 2. There was no statistical difference between groups (p = 0.126), but the Gdye group had an Easy rate of 83.3% against 60% in the Gsf group. Within each group, LFN differed between Easy and Difficult (p<0.001) (Table 3 and Figure 4). Therefore, responding to the primary objective, we conclude that the fluorescent dye facilitated nerve visualization. However, it did not significantly facilitate dissection.
In turn, Tables 4 to 7 reflect our secondary objective, which was to assess whether the experimental fluorescent staining and dissection damage the facial nerve in rats. As shown in Table 4 and Figure 5, we obtained good to high kappa concordance indexes for all PMV (0.98) and PFP (0.71) score readings on all observed days (p<0.001 for both). Here we have revalidated the functional assessment test of the facial nerve by the scale established by Borin et al.2323. Borin A, Toledo RN, Faria SD, Testa JR, OLC. Behavioral and histologic experimental model of facial nerve regeneration in rats. Braz J Otorhinolaryngol. 2006;72:775-84. PMID: 17308830.. In addition, Table 5 shows that there was a significant difference in the PMV and PFP scores between groups (p<0.001) over the observation period (p<0.001; observation time x group interaction, p = 0.007). The Gdye group obtained significantly better PMV and PFP scores when we analyzed the variables group and time and their interaction (all ps <0.001). Therefore, the fluorescent dye facilitated the visualization of the nerve and its buccal, mandibular, orbicularis and frontal branches, with fewer functional changes in the Gdye group.
In Figures 6 and 7, one can see significantly better PMV and PFP scores in the Gdye group when compared to the Gsf group (p<0.001) without causing permanent injury to the nerve, suggesting that staining with the dye may have facilitated nerve dissection. In Table 6 we note that the changes in both scores occurred soon after surgery (day 2) and remained so until day 7. Starting on day 14, scores returned to the initial baseline (5 points) in all animals (Figures 8 and 9). This means that there were alterations in PMV and PFP scores due to the nerve dissection during surgery in both groups, resulting in nerve paresis that was not related to the dye (as previously described), but to the surgery itself (p <0.001).
Periodic monitoring showed recovery of nerve function, mostly from day 7, with p = 0.064 for PMV and p = 0.674 for PFP, and all reached normality by 14 days after surgery. There was no permanent paralysis and cases of temporary paresis resolved within 14 days (Figures 8 and 9).
Table 7 shows that there was a statistically significant difference (p<0.001) between PMV and PFP scores in the Gdye group between day 2 and the other days of observation. There was also a significant difference in PMV and PFP between the Gdye and Gsf groups (p<0.001). The data suggest that localization of the facial nerve (trunk and branches) was better with the use of the fluorescent dye. Despite the presence of nerve injuries in the Gdye group, these were less severe and completely normalized by day 14.
Importantly, we found that there were no facial nerve injuries before surgery due to the injection or dye. Also, all cases of facial nerve injury observed after surgery were temporary, returning to normal by day 14. All animals recovered completely by day 28, corresponding to the greater ease of dissecting the stained nerve.
Finally, the fluorescence dye used showed 83% sensitivity, 40% specificity and 72% accuracy. The high sensitivity suggests that the method allows for good identification of the nerve, facilitating visualization and nerve preservation during surgery. This is why the dye group had more Easy scores. However, the method is not very specific, as the group without dye had a similar distribution of Easy and Difficult scores. The high accuracy score indicates that using this method gives a 72% probability of reaching the correct diagnosis, i.e., to locate the stained nerve.
Thus, responding to our secondary objective, we conclude that dissection of the facial nerve with fluorescent dye causes only temporary paresis of the nerve, with functional recovery within 14 days after surgery. Our results show that fluorescent staining and dissection of the facial nerve are experimentally feasible and do not cause injury to the nerve, neither through the injection nor staining of the dye. Furthermore, stained nerves are visualized significantly better than unstained ones.
Conclusions
Experimental fluorescent staining of the facial nerve in rats using light microscopy was effective for intraoperative visualization. The method showed high sensitivity and accuracy and allowed the identification and preservation of the facial nerve and its branches during surgical dissection, with cases of temporary paresis only. Therefore, the staining and visualization approach proposed here can be safely applied in nerve studies, and specifically those targeting the facial nerve.
References
-
1Laage-Hellman JE. Facial nerve in parotidectomies. Arch Otolaryngol. 1965;81(5):527-33. PMID: 14275912.
-
2Codreanu M. Tumora in regiunea parotidei in dreapta: operat recidivat peste cinci ani :operat a dona oara: vindicarea cu conservarea facialului siaglandei parotide. Spitalul Bucurest. 1892;12:273.
-
3Kidd H. Complete excision of the parotid gland with preservation of the facial nerve. Br Med J. 1950;i:898-991. PMID: 15414338.
-
4Carwardine T. Excision of the parotid gland with preservation of the facial nerve: its possibility. Lancet. 1907;170(4387):897.
-
5López M, Quer M, León X, Orús C, Recher K, J V. Usefulness of facial nerve monitoring during parotidectomy. Acta Otorrinolaringol Esp. 2001;52:418-21. PMID: 11526649.
-
6Ozturk K, Akyildiz S, Gode S, Turhal G, Gursan G, T K. The effect of partial superficial parotidectomy on amplitude, latency and threshold of facial nerve stimulation. Eur Arch Otorhinolaryngol. 2015;11. [Epub ahead of print]. PMID: 25862067.
-
7Liu H, Wen W, Huang H, Liang Y, Tan X, Liu S, Liu C, Hu M. Recurrent pleomorphic adenoma of the parotid gland: intraoperative facial nerve monitoring during parotidectomy. Otolaryngol Head Neck Surg. 2014;151:87-91. PMID: 24671460.
-
8Axelrod D. Carbocyanine dye orientation in red cell membrane studied by microscopic fluorescence polarization. Biophys J. 1979;26:557-74. PMID: 263688.
-
9Sims PJ, Waggoner AS, Wang CH, Hoffman JF. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974;13:3315-30. PMID: 4842277.
-
10Illert M, Fritz N, Aschoff A, Holländer H. Fluorescent compounds as retrograde tracers compared with horseradish peroxidase (HRP). II A parametric study in the peripheral motor system of the cat. J Neurosci Methods. 1982;6:199-218. PMID: 6183536.
-
11Aschoff A, Holländer H. Fluorescent compounds as retrograde tracers compared with horseradish peroxidase (HRP). I. A parametric study in the central visual system of the albino rat. J Neurosci Methods. 1982;6(3):179-97. PMID: 7144234.
-
12Dogru S VDD, Hansen MR. Retrograde labeling of the rat facial nerve with carbocyanine dyes to enhance intraoperative identification. Ann Otol Rhinol Laringol. 2008;117(10):753-8. PMID: 18998504.
-
13Wu AP, Whitney MA, Crisp JL, Friedman B, Tsien RY, Nguyen QT. Improved facial nerve identification with novel fluorescently labeled probe. Laryngoscope. 2011;121:805-10. doi: 10.1002/lary.21411.
» https://doi.org/10.1002/lary.21411 -
14Whitney MA, Crisp JL, Nguyen LT, Friedman B, Gross LA, Steinbach P, Tsien RY, Nguyen QT. Fluorescent peptides highlight peripheral nerves during surgery in mice. Nat Biotech. 2011;29(4):352-6. doi: 10.1038/nbt.1764.
» https://doi.org/10.1038/nbt.1764 -
15KleinJan GH, Buckle T, van Willigen DM, van Oosterom MN, Spa SJ, Kloosterboer HE, van Leeunwen FW. Fluorescent lectins for local in vivo visualization of peripheral nerves. Molecules. 2014;8(19):7. doi: 10.3390/molecules19079876.
» https://doi.org/10.3390/molecules19079876 -
16Shucksmith HS, Boyle TM, WKJ W. The surgery of parotid tumours; exposure of main trunk of facial nerve. Br Med J. 1951;2(4735):830-1. PMID: 14869735.
-
17Martin H. The operative removal of tumors of the parotid salivary gland. Surgery. 1952;31(5):670-82. PMID: 14922115.
-
18Dunn EJ, Kent T, Himes JIC. Parotid neoplasms: a report of 250 cases and review of the literature. Ann Surg. 1976;184(83):500-5. PMID: 1015893.
-
19Garcia-Losarcas N, Gonzales-Hidalgo M, Franco-Castedo CJPB. Estimulacion electrica del nervio facial com funcion pronostica em la cirurgia de parótida. Ver Neurol. 2009;49:119-22. PMID: 19621305.
-
20Guntinas-Lichius O, Straesser A, Streppel M. Quality of life after facial nerve repair. Laryngoscope. 2007;117:421-6. PMID: 17334302.
-
21Ciuman RR, Oels W, Jaussi R, Dost P. Outcome, general, and symptom-specific quality of life after various types of parotid resection. Laryngoscope. 2012;122:1254-61. doi: 10.1002/lary.23318.
» https://doi.org/10.1002/lary.23318 -
22Wei Y, Gong K, Ao Q, Wang A, Gong Y, Zuo H, Zhang Y, Wang J, Wang G. Lentiviral vectors enveloped with rabies virus glycoprotein can be used as a novel retrograde tracer to assess nerve recovery in rat sciatic nerve injury models. Cell Tissue Res. 2014;355:255-66. doi: 10.1007/s00441-013-1756-x.
» https://doi.org/10.1007/s00441-013-1756-x -
23Borin A, Toledo RN, Faria SD, Testa JR, OLC. Behavioral and histologic experimental model of facial nerve regeneration in rats. Braz J Otorhinolaryngol. 2006;72:775-84. PMID: 17308830.
-
Financial source: CAPES
-
1
Research performed at Head and Neck Division, Otorhinolaryngology and Head and Neck Department and the Neurophysiology Laboratory, Physiology Department, Universidade Federal de São Paulo (UNIFESP), Brazil. Part of PhD degree thesis, Postgraduate Program in Medicine-Otorhinolaryngology, UNIFESP. Tutor: Onivaldo Cervantes.
Publication Dates
-
Publication in this collection
Feb 2016
History
-
Received
05 Oct 2015 -
Reviewed
11 Dec 2015 -
Accepted
12 Jan 2016








 Y-axis: LocTime: localization time of the facial nerve in minutes. Bars represent the different groups, Gcor: Fluorescent dye group, Gsem: Group without dye. p=0.001.
Y-axis: LocTime: localization time of the facial nerve in minutes. Bars represent the different groups, Gcor: Fluorescent dye group, Gsem: Group without dye. p=0.001.
 Y-axis: mean PFL scores, LFN: Score for the localization of the facial nerve, LocTime: Localization Time of the facial nerve, Gdye: Fluorescent dye group, Gsf: Group without dye, Both: Both groups.
Y-axis: mean PFL scores, LFN: Score for the localization of the facial nerve, LocTime: Localization Time of the facial nerve, Gdye: Fluorescent dye group, Gsf: Group without dye, Both: Both groups.
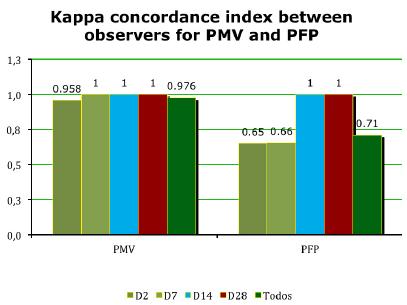 Y-axis: Kappa concordance index, PMV: Vibrissae movement score, PFP: Eyelid closure score
Y-axis: Kappa concordance index, PMV: Vibrissae movement score, PFP: Eyelid closure score
 Y-axis: Total score of PMV, X-axis: Gdye and Gsf groups, PMV: Vibrissae movement score, Gdye: Fluorescent dye group, Gsf: Group without dye.
Y-axis: Total score of PMV, X-axis: Gdye and Gsf groups, PMV: Vibrissae movement score, Gdye: Fluorescent dye group, Gsf: Group without dye.
 Y-axis: Total score of PFP, X-axis: Gdye and Gsf groups, PFP: Eyelid closure score, Gdye: Fluorescent dye group, Gsf: Group without dye.
Y-axis: Total score of PFP, X-axis: Gdye and Gsf groups, PFP: Eyelid closure score, Gdye: Fluorescent dye group, Gsf: Group without dye.
 Y-axis: Total score of PMV, X-axis: Observation Time (days), PMV: Vibrissae movement score, D0-D28: Observation time in days, Gdye: Fluorescent dye group, Gsf: Group without dye.
Y-axis: Total score of PMV, X-axis: Observation Time (days), PMV: Vibrissae movement score, D0-D28: Observation time in days, Gdye: Fluorescent dye group, Gsf: Group without dye.
 Y-axis: Total score of PFP, X-axis: Observation Time (days), PFP: Eyelid closure score, D0-D28: Time points in days, Gdye: Fluorescent dye group, Gsf: Group without dye.
Y-axis: Total score of PFP, X-axis: Observation Time (days), PFP: Eyelid closure score, D0-D28: Time points in days, Gdye: Fluorescent dye group, Gsf: Group without dye.