Abstract
PURPOSE:
To investigate whether there is any effect resulting from preconditioning with nutraceutical supplementation containing arginine and oil mixes with high ω9:ω6 ratio and low ω6:ω3 ratio containing EPA and DHA, ALA fatty acids on inflammatory mediators, antioxidant and lipid profile modulation in surgical trauma.
METHODS:
Twenty-six men scheduled for radical prostatectomy were randomized into three groups and treated as follows: Group 1 (skim milk, 0% fat), Group 2 (supplement with ω6:ω3 ratio of 8:1 and arginine) and Group 3 (supplement with high ω9:ω6 ratio of 3.2:1 and low ω6:ω3 ratio of 1.4:1 and arginine). Patients received skin milk or supplements twice a day (200 ml) during five days prior to surgery. Peripheral venous blood samples were collected at three different timepoints: five days before surgery (PRE), before anesthesia induction (IND) and on the 2nd postoperative day (POS). Parameters analyzed included inflammatory cytokines (IL-1β, IL-6, IL-8 and TNF-α), antioxidants (catalase), lipid profile and heat shock protein (HSP-27).
RESULTS:
There were no significant differences between groups on inflammatory mediators and antioxidant parameters. However, lipid profile values (Cholesterol, LDL, Triglycerides, VLDL), were significantly different.
CONCLUSION:
Preconditioning with arginine and oil mixes containing high ω9:ω6 ratio and low ω6:ω3 ratio, has no effects on inflammatory mediators and oxidative stress in patients undergoing radical prostatectomy. Reduction of cholesterol, triglycerides, LDL and VLDL profiles may be related to the trauma effect.
Trauma; Prostatectomy; Oils; Inflammation Mediators; Oxidative Stress
Introduction
Trauma and surgery trigger the production of proinflammatory cytokines, and can induce severe alterations in the immune system, which results in increased rates of septic and inflammatory complications postoperatively11. Senkal M, Zumtobel V, Bauer KH, Marpe B, Wolfram G, Frei A, Eickhoff U, Kemen M. Outcome and cost-effectiveness of perioperative enteral immunonutrition in patients undergoing elective upper gastrointestinal tract surgery: a prospective randomized study. Arch Surg. 1999 Dec;134(12):1309-16. PMID: 10593328.. Perioperative supplementation with immunomodulatory diets is safe and effective in reducing postoperative infections, length of hospital stay and reduce the need for antibiotics, increasing cellular and humoral immunity compared to standard nutrition22. Braga M, Gianotti L, Vignali A, Di Carlo V. Immunonutrition in gastric cancer surgical patients. Nutrition. 1998 Nov-Dec;14(11-12):831-5. PMID: 9834924.
3. Gianotti L, Braga M, Fortis C, Soldini L, Vignali A, Colombo S, Radaelli G, Di Carlo V. A prospective, randomized clinical trial on perioperative feeding with an arginine-, omega-3 fatty acid-, and RNA-enriched enteral diet: effect on host response and nutritional status. JPEN J Parenter Enteral Nutr. 1999 Nov-Dec;23(6):314-20. PMID: 10574478.
4. Tepaske R, Velthuis H, Oudemans-van Straaten HM, Heisterkamp SH, van Deventer SJ, Ince C, Eÿsman L, Kesecioglu J. Effect of preoperative oral immune-enhancing nutritional supplement on patients at high risk of infection after cardiac surgery: a randomised placebo-controlled trial. Lancet. 2001;358(9283):696-701. PMID: 11551575
5. Nakamura K, Kariyazono H, Komokata T, Hamada N, Sakata R, Yamada K. Influence of preoperative administration of omega-3 fatty acid-enriched supplement on inflammatory and immune responses in patients undergoing major surgery for cancer. Nutrition. 2005 Jun;21(6):639-49. PMID: 15925286.
6. Waitzberg DL, Saito H, Plank LD, Jamieson GG, Jagannath P, Hwang TL, Mijares JM, Bihari D. Postsurgical infections are reduced with specialized nutrition support. World J Surg. 2006 Aug;30(8):1592-604. PMID: 16794908. - 77. Zheng Y, Li F, Qi B, Luo B, Sun H, Liu S, Wu X. Application of perioperative immunonutrition for gastrointestinal surgery: a meta-analysis of randomized controlled trials. Asia Pac J Clin Nutr. 2007;16 Suppl 1:253-7. PMID:17392114..
Fatty acids are organic molecules composed of atoms of carbon, hydrogen and oxygen. Food ingestion provide most daily needs. On other hand liver, adipose tissue and mammary glands are able to synthesize fatty acids from glucose and amino acids by means of specific enzymatic reactions88. Calder PC. Long-chain n-3 fatty acids and inflammation: potential application in surgical and trauma patients. Braz J Med Biol Res. 2003 Apr;36(4):433-46. PMID: 12700820.. Fatty acids frequently incorporated into membrane phospholipids are the ω-3 eicosapentaenoic (EPA), and docosahexaenoic (DHA) acids, ω-6 arachidonic acid (AA), and ω -9 oleic acid (OA)99. Chan S, McCowen KC, Bistrian B. Medium-chain triglyceride and n-3 polyunsaturated fatty acid-containing emulsions in intravenous nutrition. Curr Opin Clin Nutr Metab Care. 1998 Mar;1(2):163-9. PMID: 10565343.. Polyunsaturated fatty acids (PUFAs) influence immune and inflammatory responses, the synthesis of inflammatory modulators (eicosanoids and resolvins) and signal transduction, with greater or lesser production of inflammatory cytokines1010. Waitzberg DL, Torrinhas RS. Fish oil lipid emulsions and immune response: what clinicians need to know. Nutr Clin Pract. 2009 Aug-Sep;24(4):487-99. doi: 10.1177/0884533609339071.
https://doi.org/10.1177/0884533609339071...
. Omega-3 fatty acids act as competitor for the same metabolic pathway of arachidonic acid, inhibiting its oxidation and suppressing the production of eicosanoids. In addition, some of the mediators derived from ω3 include the 5-series leukotrienes and 3-series thromboxanes that produce less inflammation and do not promote tumor growth1010. Waitzberg DL, Torrinhas RS. Fish oil lipid emulsions and immune response: what clinicians need to know. Nutr Clin Pract. 2009 Aug-Sep;24(4):487-99. doi: 10.1177/0884533609339071.
https://doi.org/10.1177/0884533609339071...
- 1111. Calder PC. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2006 Sep;75(3):197-202. PMID: 16828270.. The ω3 is also a substrate for the synthesis of resolvins associated with potent anti-inflammatory and immunoregulatory activity1212. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006 Jun;83(6 Suppl):1505S-1519S. PMID: 16841861..
Arginine is a conditionally essential amino acid in stress situations and acts accelerating the excretion of hormones that promote anabolism after trauma, stimulating cellular immunity and lymphokine generation. Is also a substrate for the production of nitric oxide (NO), which enhances the effects of macrophages and bactericidal activity, and improve surgical wound healing and protect against infection and ischemia-reperfusion injury66. Waitzberg DL, Saito H, Plank LD, Jamieson GG, Jagannath P, Hwang TL, Mijares JM, Bihari D. Postsurgical infections are reduced with specialized nutrition support. World J Surg. 2006 Aug;30(8):1592-604. PMID: 16794908. - 77. Zheng Y, Li F, Qi B, Luo B, Sun H, Liu S, Wu X. Application of perioperative immunonutrition for gastrointestinal surgery: a meta-analysis of randomized controlled trials. Asia Pac J Clin Nutr. 2007;16 Suppl 1:253-7. PMID:17392114. , 1313. Xu J, Zhong Y, Jing D, Wu Z. Preoperative enteral immunonutrition improves postoperative outcome in patients with gastrointestinal cancer. World J Surg. 2006 Jul;30(7):1284-9. PMID: 16830214..
The effect of oral suplementation with ω9, ω3 and arginine has not been assessed in the setting of prostatectomy. This study aimed to verify if oral nutritional supplement using arginine and oil mixes with high ω9:ω6 ratio and low ω6:ω3 ratio containing ω3 acids (ALA, DHA and EPA) has nutraceutical preconditioning effect on the inflammatory mediators, oxidative stress and lipid profile in trauma surgery in a sample of patients undergoing radical prostatectomy.
Methods
This randomized, double-blind, paired clinical study was approved by "Waldemar Alcantara" General Hospital Ethics Committee, Protocol N°. 15/2012 C.E., October 10, 2012 and conduced in compliance with the Helsinki Declaration of 1975, as revised in 2008 (World Medical Association www.wma.net/e/policy/b3.htm) and Resolution 196/96 of the Brazilian National Health Service (http://conselho.saude.gov.br/resolucoes/reso_96.htm).
Written informed consent was obtained from all patients. Twenty six patients undergoing prostatectomy were included in the study. Exclusion criteria were: patients with septic shock; undergoing immunosuppressive therapy; thrombocytopenic and / or continuos use of potent antiplatelet drugs (bisssulfato de clopidogrel, heparin, aspirin); with diabetes mellitus, renal insufficiency, hepatic impairment, heart disease or undergoing angioplasty and stenting; pancreatitis and body mass index - (BMI) <18.5 and >30kg/m2. Randomic allocation of patients to groups G1, G2 or G3 was made by software program (www.lee.dante.br). Patients who met inclusion criteria were treated as follows: Group 1 (n=9) (skim milk with 0% fat), Group 2 (n=8) (supplement with ω6:ω3 ratio 8:1 and arginine) and Group 3 (n=9) (supplement with high ω9:ω6 ratio 3.2:1 and low ω6:ω3 ratio of 1.4:1 and arginine 10g). The supplements were administered to each group in two daily doses of 200ml, within five days prior to surgery.
Peripheral venous blood was collected at three different timepoints: on the fifth day before the administration of the supplement (PRE), before anesthesia induction (IND) and on the 2nd postoperative day (POS). Parameters analyzed included inflammatory (IL-1β, IL-6, IL-8 and TNF-α), antioxidants (catalase), lipid profile and preconditioning (HSP-27).
Surgical procedure
All surgical procedures were performed under anesthesia at the "Waldemar Alcantara" General Hospital (Fortaleza, Ceará) using the same technique for all prostatectomies.
Biochemical analysis
Cytokine assay was performed by Luminex(r) (Milliplex Map Kit - High Sensitivity Human Cytokine Magnetic Bead Kit, Millipore) method. Heat Shock Protein's dosage (HSP - 27) was also performed by Luminex(r) method (Milliplex Map Kit - 5-Plex Heat Shock Protein Magnetic Bead Kit, Millipore). Antioxidants parameters were measured using Milliplex(r) Map Human Panel Oxidative stress Magnetic Bead Panel (H0XSTMAG-18K). Assay kits were purchased from EMD Millipore Corporation(c), Billerica, MA, USA. Lipid profile was assessed by one private laboratory hired for this purpose.
Statistical analysis
The study data were entered in Microsoft Excel for Windows version 2007. GraphPad Prism 5.0 (GraphPad Software, San Diego, California, USA, www.graphpad.com) was used for statistical analysis and graphics design. All data was tested for distribution (Kolmogorov-Smirnov test). Non-parametric data were analyzed using Kruskal-Wallis/Dunn tests. Results were presented as mean±SD. The level of statistical significance was set at 5% (p<0.05).
Results
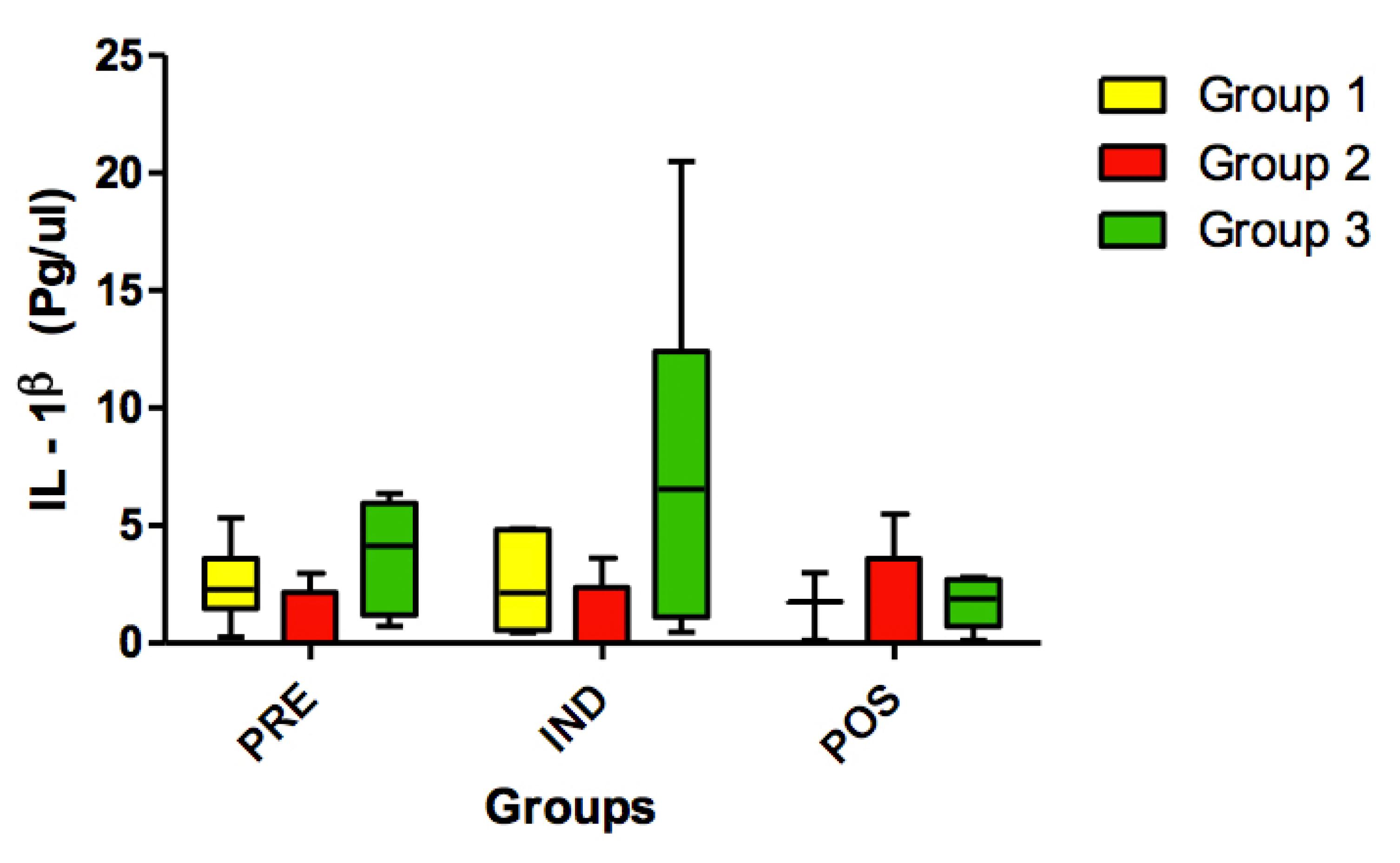
No statistically significant differences (p>0.05) were found for IL-1β (Figure 1), IL-6 (Figure 2), IL-8 (Figure 3), TNF-α (Figure 4) and HSP-27 (Figure 5) variables.
Concentration of IL-1ß (pg/ul) in all groups (G1, G2 and G3) at 3 timepoints: before administration of the supplement (PRE) before anesthesia induction (IND) and postoperative (POS). p>0.05. (G1 vs. G2, G1 vs. G3, G2 vs. G3). Kruskal-Wallis test /Dunn (p>0.05).
Concentration of IL-6 (pg/ul) in all groups (G1, G2 and G3) at 3 timepoints: before administration of the supplement (PRE) before anesthesia induction (IND) and postoperative (POS). p>0.05. (G1 vs. G2, G1 vs. G3, G2 vs. G3). Kruskal-Wallis test /Dunn (p>0.05).
Concentration of IL-8 (pg/ul) in all groups (G1, G2 and G3) at 3 timepoints: before administration of the supplement (PRE) before anesthesia induction (IND) and postoperative (POS). p>0.05. (G1 vs. G2, G1 vs. G3, G2 vs. G3). Kruskal-Wallis test /Dunn (p>0.05).
Concentration de TNF - á (pg/ul) in all groups (G1, G2 and G3) at 3 timepoints: before administration of the supplement (PRE) before anesthesia induction (IND) and postoperative (POS). p>0.05. (G1 vs. G2, G1 vs. G3, G2 vs. G3). Kruskal-Wallis test /Dunn (p>0.05).
Expression of HSP-27 (MFI) in all groups (G1, G2 and G3) at 3 timepoints: before administration of the supplement (PRE) before anesthesia induction (IND) and postoperative (POS). p>0.05. (G1 vs. G2, G1 vs. G3, G2 vs. G3). Kruskal-Wallis test /Dunn (p>0.05).
Statistically significant differences (p<0.05) were found for triglyceride (PRE vs. POS timepoints) (Figure 6), total cholesterol (Figure 7) (PRE vs. IND vs. POS) and LDL cholesterol (LDL-c) (Figure 8) variables (PRE vs. IND vs. POS).
Concentration of triglycerides (mg/dl) in all groups (G1, G2 and G3) at 3 timepoints: before administration of the supplement (PRE) before anesthesia induction (IND) and postoperative (POS). p>0.05. (G1 vs. G2, G1 vs. G3, G2 vs. G3). Kruskal-Wallis test /Dunn (p<0.05).
Total cholesterol (mg/dl) values in all groups (G1, G2 and G3) in 3 timepoints: before administration of the supplement (PRE) before anesthesia induction (IND) and postoperative (POS). p>0.05. (G1 vs. G2, G1 vs. G3, G2 vs. G3). Kruskal-Wallis test /Dunn (p<0.05).
Concentration of LDL-c (mg/dl) in all groups (G1, G2 and G3) in 3 timepoints: before administration of the supplement (PRE) before anesthesia induction (IND) and postoperative (POS). p>0.05. (G1 vs. G2, G1 vs. G3, G2 vs. G3). Kruskal-Wallis test /Dunn (p<0.05).
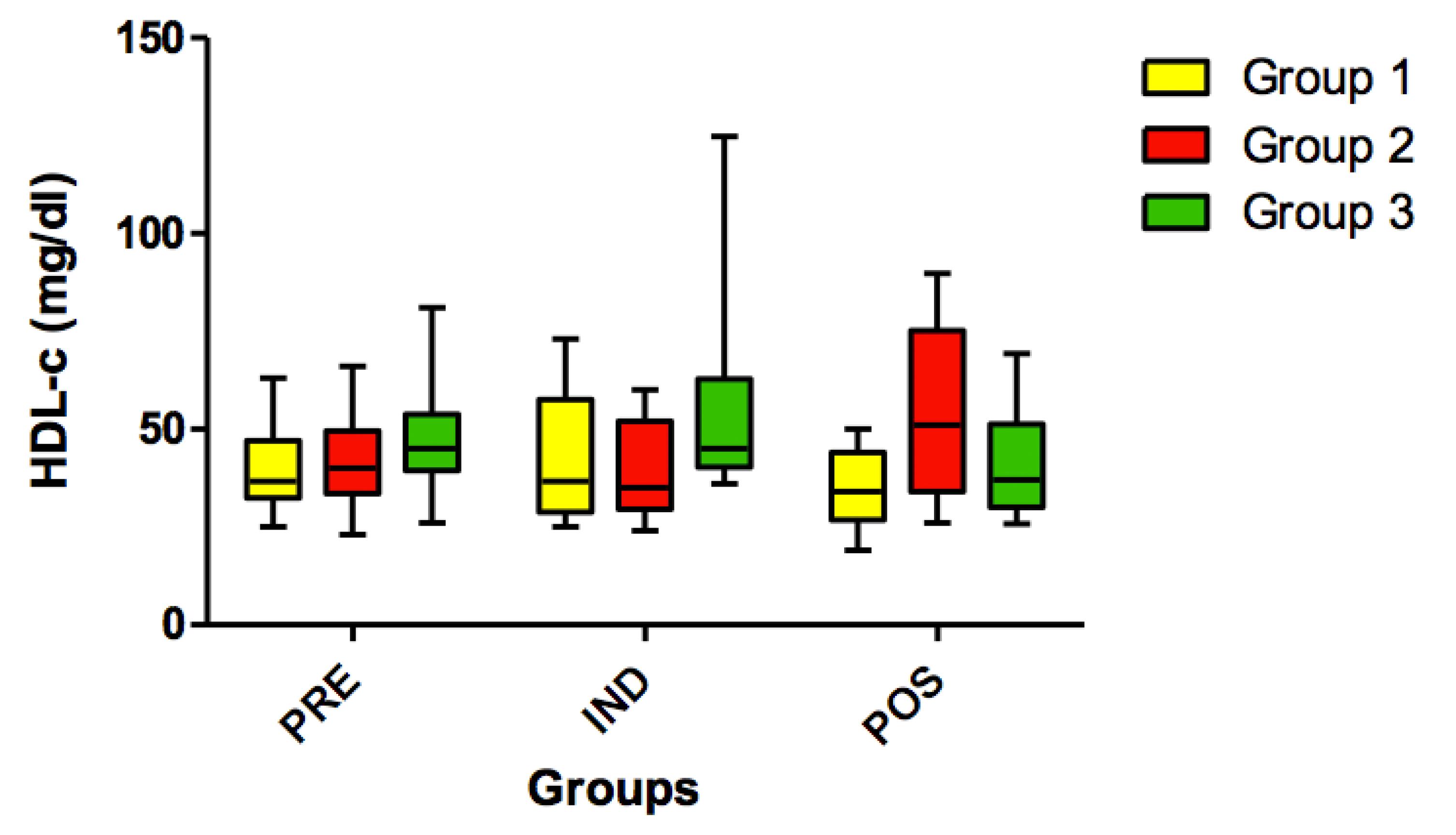
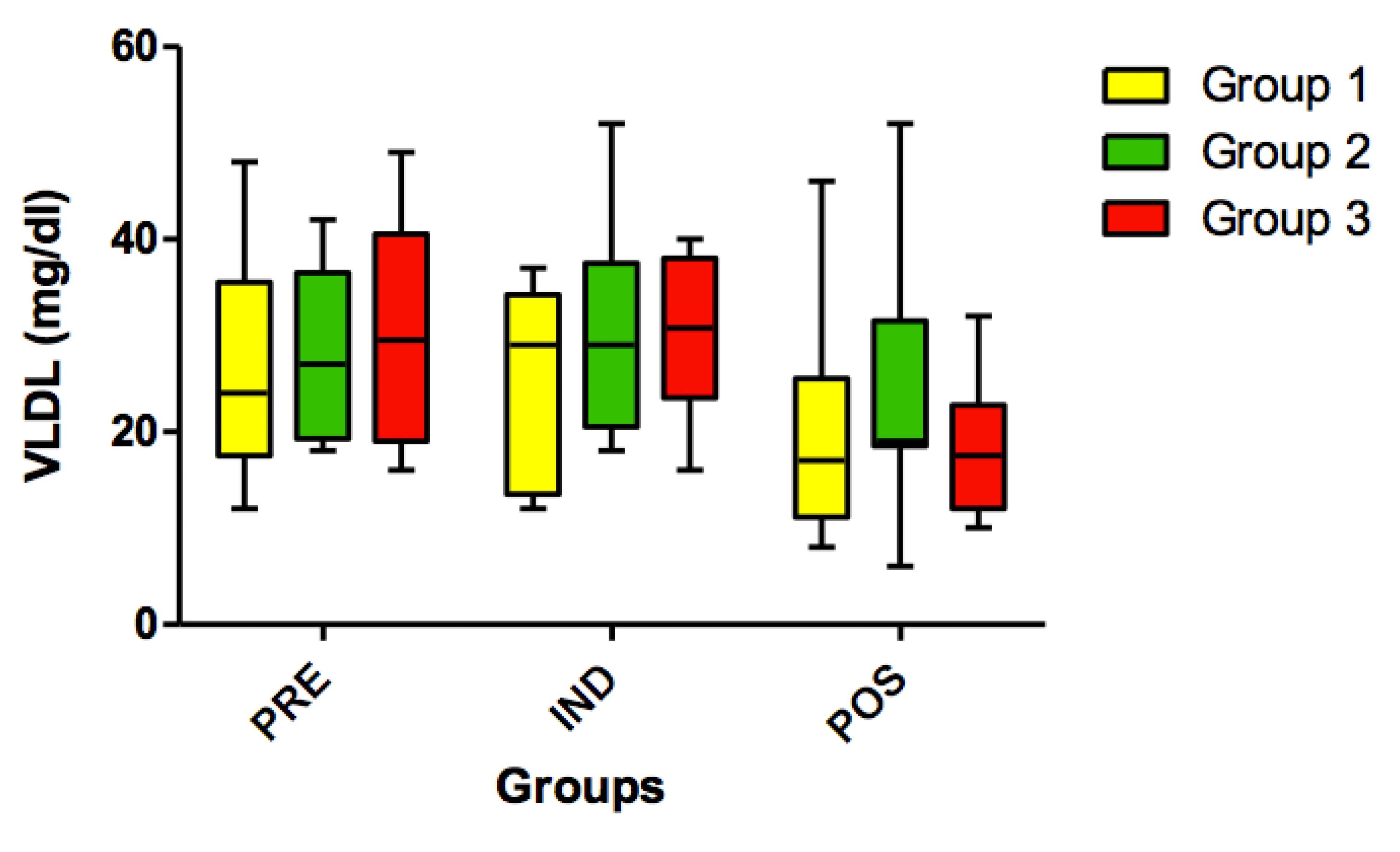
No statistically significant differences (p>0.05) were found for HDL cholesterol (Figure 9) and for VLDL variables in all timepoints (PRE vs. IND vs. POS) (Figure 10).
Concentration of HDL-c (mg/dl) in all groups (G1, G2 and G3) in the 3 time-points: before administration of the supplement (PRE) before anesthesia induction (IND) and postoperative (POS). p>0.05. (G1 vs. G2, G1 vs. G3, G2 vs. G3). Kruskal-Wallis test /Dunn (p>0.05).
Concentration VLDL (mg/dl) in all groups (G1, G2 and G3) in all 3 timepoints: before administration of the supplement (PRE) before anesthesia induction (IND) and postoperative (POS). p>0.05. (G1 vs. G2, G1 vs. G3, G2 vs. G3). Kruskal-Wallis test /Dunn (p<0.05).
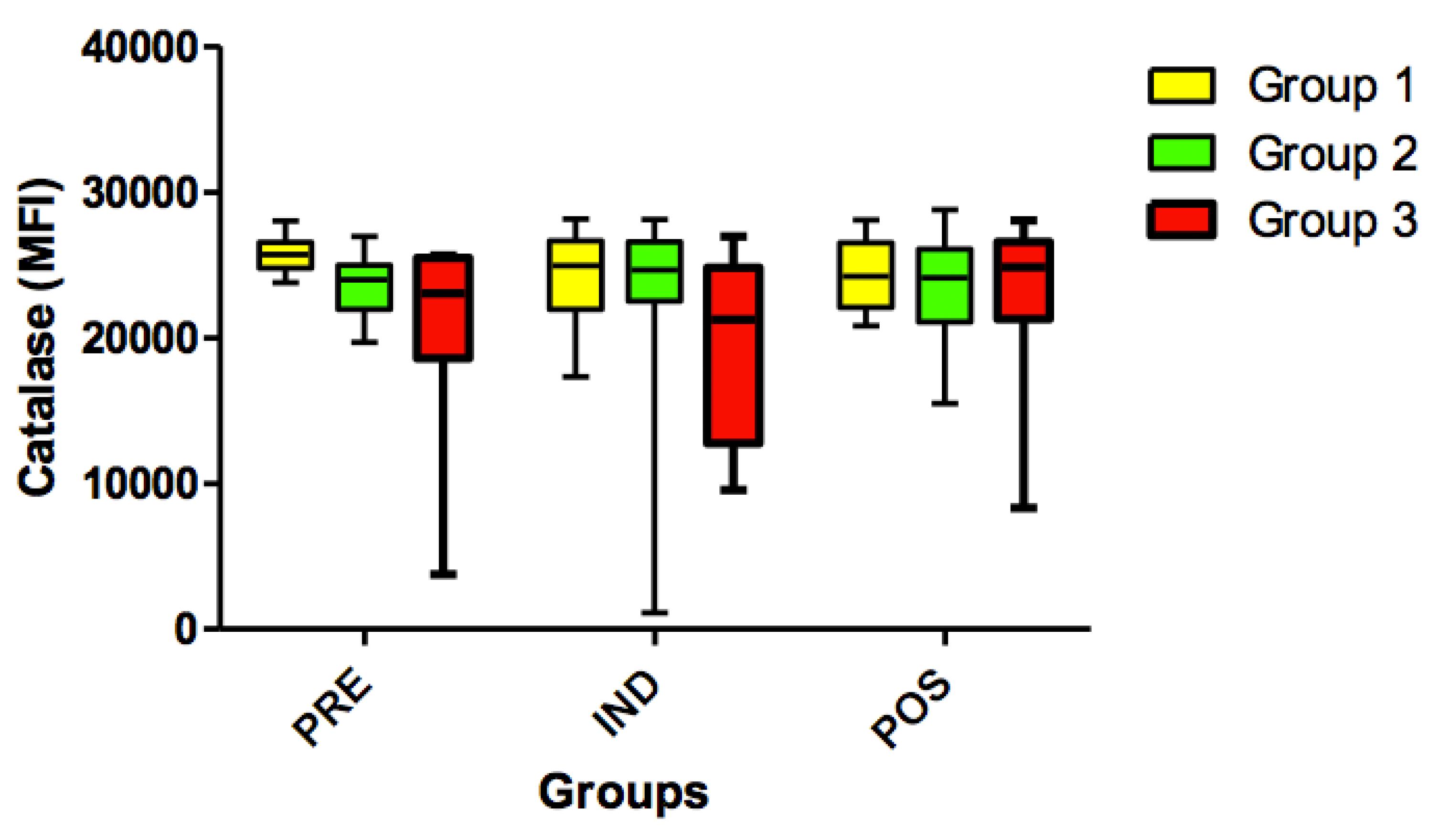
Also, no significant differences (p> 0.05) were found for the catalase variable when comparing all timepoints (PRE vs. IND vs. POS) (Figure 11).
Expression of catalase in all groups (G1, G2 and G3) in the 3 time-points: before administration of the supplement (PRE) before anesthesia induction (IND) and postoperative (POS). p> 0.05. (G1 vs. G2, G1 vs. G3, G2 vs. G3). Kruskal-Wallis test /Dunn (p>0.05).
Discussion
Published studies using cell cultures from animals or humans have shown that EPA and DHA may cause decreased production of IL-1β, TNF-α and IL-61414. Kelley DS. Modulation of human immune and inflammatory responses by dietary fatty acids. Nutrition. 2001 Jul-Aug;17(7-8):669-73. PMID: 11448594. - 1515. Calder PC. Dietary modification of inflammation with lipids. Proc Nutr Soc. 2002 Aug;61(3):345-58. PubMed PMID: 12296294.. However, this results were not repeated in the present study. No statistically significant difference was observed in IL-1β, IL-6, IL-8 and TNF-α between G1 (skim milk with 0% fat), G2 (arginine group: supplement with ω6:ω3 ratio of 8:1 and arginine) and G3 (oil mixes + arginine group: supplement with ω9:ω6 ratio of 3.2:1 and ω6:ω3 ratio of 1.4:1 and arginine), none of the time-points (PRE), (IND) and (POS).
Clinical trials using immunomodulatory formulas containing ω3 (3.3g/day), arginine (1.2g/day) and nucleotides (1.2g/day) for seven consecutive days before and after surgery in cancer patients has showed that the immune response seen in patients receiving supplemented diets occurred later (after the 2nd post-operative day)22. Braga M, Gianotti L, Vignali A, Di Carlo V. Immunonutrition in gastric cancer surgical patients. Nutrition. 1998 Nov-Dec;14(11-12):831-5. PMID: 9834924. - 33. Gianotti L, Braga M, Fortis C, Soldini L, Vignali A, Colombo S, Radaelli G, Di Carlo V. A prospective, randomized clinical trial on perioperative feeding with an arginine-, omega-3 fatty acid-, and RNA-enriched enteral diet: effect on host response and nutritional status. JPEN J Parenter Enteral Nutr. 1999 Nov-Dec;23(6):314-20. PMID: 10574478. , 55. Nakamura K, Kariyazono H, Komokata T, Hamada N, Sakata R, Yamada K. Influence of preoperative administration of omega-3 fatty acid-enriched supplement on inflammatory and immune responses in patients undergoing major surgery for cancer. Nutrition. 2005 Jun;21(6):639-49. PMID: 15925286.. These findings could can explain the different results obtained in the present study. Although several of these observations match the effects of ω3 found in studies of cell culture, animals and healthy subjects, and could be used as evidence of the effectiveness of ω3 PUFA in trauma in post-operative timepoints, the complex nature of the formula prevents a clear interpretation. The effects can be assigned to any of the specified nutrients (arginine, RNA, ω3), or by the combination of these nutrients1616. Calder PC. Long-chain n-3 fatty acids and inflammation: potential application in surgical and trauma patients. Braz J Med Biol Res. 2003 Apr;36(4):433-46. PMID: 12700820..
Other studies also failed to demonstrate effects of ω3 PUFAs in the production of proinflammatory cytokines in humans. It is not clear for what reason these discrepancies are in the literature, but it is likely that technical factors contribute to this. Another factor which has recently been identified is genetic polymorphism affecting the production of cytokines. It was found that the effect of fish oil on cytokine production by human mononuclear cells was dependent on the nature of the polymorphism (308 TNF-α and +252 TNF-β), raising the possibility of identifying those that are most and least likely to experiment specific anti-inflammatory effects of fish oil1212. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006 Jun;83(6 Suppl):1505S-1519S. PMID: 16841861. , 2020. Moloney TC, Hoban DB, Barry FP, Howard L, Dowd E. Kinetics of thermally induced heat shock protein 27 and 70 expression by bone marrow-derived mesenchymal stem cells. Protein Sci. 2012 Jun;21(6):904-9. doi: 10.1002/pro.2077.
https://doi.org/10.1002/pro.2077...
.
Regarding the HSP-27, whose expression is induced in response to a variety of environmental and cellular stresses, previous observations showed that pretreatment with ω3 induced an increased expression of heat shock proteins1717. McGuinness J, Neilan TG, Sharkasi A, Bouchier-Hayes D, Redmond JM. Myocardial protection using an omega-3 fatty acid infusion: quantification and mechanism of action. J Thorac Cardiovasc Surg. 2006 Jul;132(1):72-9. PMID: 16798305. - 1818. Lee CY, Sit WH, Fan ST, Man K, Jor IW, Wong LL, Wan ML, Tan-Un KC, Wan JM. The cell cycle effects of docosahexaenoic acid on human metastatic hepatocellular carcinoma proliferation. Int J Oncol. 2010 Apr;36(4):991-8. PMID:20198345.. The increased expression of these proteins in experimental models for sepsis, shock, critical illness and injury reduces the organ injury, attenuates the pro-inflammatory response and improves survival1919. Ziegler TR, Ogden LG, Singleton KD, Luo M, Fernandez-Estivariz C, Griffith DP, Galloway JR, Wischmeyer PE. Parenteral glutamine increases serum heat shock protein 70 in critically ill patients. Intensive Care Med. 2005 Aug;31(8):1079. PMID: 15973519. - 2020. Moloney TC, Hoban DB, Barry FP, Howard L, Dowd E. Kinetics of thermally induced heat shock protein 27 and 70 expression by bone marrow-derived mesenchymal stem cells. Protein Sci. 2012 Jun;21(6):904-9. doi: 10.1002/pro.2077.
https://doi.org/10.1002/pro.2077...
. However, there was no statistically significant difference between groups in none of timepoints (PRE), (IND) or (POS) studied here.
Zuliani et al.2121. Zuliani G, Galvani M, Leitersdorf E, Volpato S, Cavalieri M, Fellin R. The role of polyunsaturated fatty acids (PUFA) in the treatment of dyslipidemias. Curr Pharm Des. 2009;15(36):4087-93. PMID: 20041810. suggest that ω3 act on lipid profile. The plasma cholesterol and triglyceride levels are reduced with the use of fish oil in normolipidemic and hyperlipidemic patients2121. Zuliani G, Galvani M, Leitersdorf E, Volpato S, Cavalieri M, Fellin R. The role of polyunsaturated fatty acids (PUFA) in the treatment of dyslipidemias. Curr Pharm Des. 2009;15(36):4087-93. PMID: 20041810.. In this trial, the levels of total cholesterol showed significant reductions in the preoperative time-point (PRE) compared with the postoperative (POS), and between anesthetic induction (IND) and postoperative (POS) time-points in all patients (G1, G2 and G3).
In the analysis of triglycerides, there was no significant reduction from the anesthetic induction (IND), to postoperative (POS) in G1 and G2 groups. On the other hand the results disclosed significant differences in G3 patients (PRE vs. POS and IND vs. POS). Denke2222. Denke MA. Dietary prescriptions to control dyslipidemias. Circulation. 2002 Jan 15;105(2):132-5. PMID: 11790687. studies suggest that increased serum levels of HDL are mainly related to physical activity. No significant differences were observed in this study.
LDL, VLDL, total cholesterol and triglycerides levels were reduced in all groups and timepoints, suggesting that the trauma effect occurred due to the recruitment of leukocytes to form lipid membranes to mitigate the inflammatory response and tissue regeneration. Therefore, it cannot be said that there was a preconditioning effect of arginine and oil mixes.
Catalase is an endogenous antioxidant enzyme that catalyzes the breakdown of intracellular and extracellular peroxide (H2O2) and hydrogen and plays a key role in protecting cells against reactive oxygen species2323. Gupta A, Butts B, Kwei KA, Dvorakova K, Stratton SP, Briehl MM, Bowden GT. Attenuation of catalase activity in the malignant phenotype plays a functional role in an in vitro model for tumor progression. Cancer Lett. 2001 Nov 28;173(2):115-25. PMID: 11597785.. In this trial, catalase variable showed no statistically significant difference between groups and time-points.
In the present study, although none of the patients presented complications, diet-related or not (fever, diarrhea, vomiting or shock), the potential benefits cited in the literature associated with immunomodulators use were not found. It is believed that this may be due to the small sample size (26 patients distributed in three groups). Other reasons include the short duration of preoperative immunomodulator administration (only five days), and short-term follow up of patients, since the discharge, and therefore the last blood collection was taken on the 2nd postoperative day, while several studies have analyzed inflammatory parameters after one post-surgical week. Still to be considered, in the studies that showed a reduction in serum levels of cytokines in surgery, it is that they used a similar amount of ω3, but only in the form of EPA and DHA. The type of ω3 may have been an influence, and ALA could have negatively contributed to the oil mixture, thereby interfering with the results.
Conclusions
Oral nutritional supplementation using arginine and oil mixes with a high ω9:ω6 ratio and low ω6:ω3 containing ω3 acids (ALA, EPA and DHA), has no effect as nutraceutical pre-conditioning on the inflammatory mediators and oxidative stress in patients undergoing radical prostatectomy. Reduction of lipid profile variables (cholesterol, triglycerides, LDL, VLDL) are related to the trauma effect.
References
-
1Senkal M, Zumtobel V, Bauer KH, Marpe B, Wolfram G, Frei A, Eickhoff U, Kemen M. Outcome and cost-effectiveness of perioperative enteral immunonutrition in patients undergoing elective upper gastrointestinal tract surgery: a prospective randomized study. Arch Surg. 1999 Dec;134(12):1309-16. PMID: 10593328.
-
2Braga M, Gianotti L, Vignali A, Di Carlo V. Immunonutrition in gastric cancer surgical patients. Nutrition. 1998 Nov-Dec;14(11-12):831-5. PMID: 9834924.
-
3Gianotti L, Braga M, Fortis C, Soldini L, Vignali A, Colombo S, Radaelli G, Di Carlo V. A prospective, randomized clinical trial on perioperative feeding with an arginine-, omega-3 fatty acid-, and RNA-enriched enteral diet: effect on host response and nutritional status. JPEN J Parenter Enteral Nutr. 1999 Nov-Dec;23(6):314-20. PMID: 10574478.
-
4Tepaske R, Velthuis H, Oudemans-van Straaten HM, Heisterkamp SH, van Deventer SJ, Ince C, Eÿsman L, Kesecioglu J. Effect of preoperative oral immune-enhancing nutritional supplement on patients at high risk of infection after cardiac surgery: a randomised placebo-controlled trial. Lancet. 2001;358(9283):696-701. PMID: 11551575
-
5Nakamura K, Kariyazono H, Komokata T, Hamada N, Sakata R, Yamada K. Influence of preoperative administration of omega-3 fatty acid-enriched supplement on inflammatory and immune responses in patients undergoing major surgery for cancer. Nutrition. 2005 Jun;21(6):639-49. PMID: 15925286.
-
6Waitzberg DL, Saito H, Plank LD, Jamieson GG, Jagannath P, Hwang TL, Mijares JM, Bihari D. Postsurgical infections are reduced with specialized nutrition support. World J Surg. 2006 Aug;30(8):1592-604. PMID: 16794908.
-
7Zheng Y, Li F, Qi B, Luo B, Sun H, Liu S, Wu X. Application of perioperative immunonutrition for gastrointestinal surgery: a meta-analysis of randomized controlled trials. Asia Pac J Clin Nutr. 2007;16 Suppl 1:253-7. PMID:17392114.
-
8Calder PC. Long-chain n-3 fatty acids and inflammation: potential application in surgical and trauma patients. Braz J Med Biol Res. 2003 Apr;36(4):433-46. PMID: 12700820.
-
9Chan S, McCowen KC, Bistrian B. Medium-chain triglyceride and n-3 polyunsaturated fatty acid-containing emulsions in intravenous nutrition. Curr Opin Clin Nutr Metab Care. 1998 Mar;1(2):163-9. PMID: 10565343.
-
10Waitzberg DL, Torrinhas RS. Fish oil lipid emulsions and immune response: what clinicians need to know. Nutr Clin Pract. 2009 Aug-Sep;24(4):487-99. doi: 10.1177/0884533609339071.
» https://doi.org/10.1177/0884533609339071 -
11Calder PC. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2006 Sep;75(3):197-202. PMID: 16828270.
-
12Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006 Jun;83(6 Suppl):1505S-1519S. PMID: 16841861.
-
13Xu J, Zhong Y, Jing D, Wu Z. Preoperative enteral immunonutrition improves postoperative outcome in patients with gastrointestinal cancer. World J Surg. 2006 Jul;30(7):1284-9. PMID: 16830214.
-
14Kelley DS. Modulation of human immune and inflammatory responses by dietary fatty acids. Nutrition. 2001 Jul-Aug;17(7-8):669-73. PMID: 11448594.
-
15Calder PC. Dietary modification of inflammation with lipids. Proc Nutr Soc. 2002 Aug;61(3):345-58. PubMed PMID: 12296294.
-
16Calder PC. Long-chain n-3 fatty acids and inflammation: potential application in surgical and trauma patients. Braz J Med Biol Res. 2003 Apr;36(4):433-46. PMID: 12700820.
-
17McGuinness J, Neilan TG, Sharkasi A, Bouchier-Hayes D, Redmond JM. Myocardial protection using an omega-3 fatty acid infusion: quantification and mechanism of action. J Thorac Cardiovasc Surg. 2006 Jul;132(1):72-9. PMID: 16798305.
-
18Lee CY, Sit WH, Fan ST, Man K, Jor IW, Wong LL, Wan ML, Tan-Un KC, Wan JM. The cell cycle effects of docosahexaenoic acid on human metastatic hepatocellular carcinoma proliferation. Int J Oncol. 2010 Apr;36(4):991-8. PMID:20198345.
-
19Ziegler TR, Ogden LG, Singleton KD, Luo M, Fernandez-Estivariz C, Griffith DP, Galloway JR, Wischmeyer PE. Parenteral glutamine increases serum heat shock protein 70 in critically ill patients. Intensive Care Med. 2005 Aug;31(8):1079. PMID: 15973519.
-
20Moloney TC, Hoban DB, Barry FP, Howard L, Dowd E. Kinetics of thermally induced heat shock protein 27 and 70 expression by bone marrow-derived mesenchymal stem cells. Protein Sci. 2012 Jun;21(6):904-9. doi: 10.1002/pro.2077.
» https://doi.org/10.1002/pro.2077 -
21Zuliani G, Galvani M, Leitersdorf E, Volpato S, Cavalieri M, Fellin R. The role of polyunsaturated fatty acids (PUFA) in the treatment of dyslipidemias. Curr Pharm Des. 2009;15(36):4087-93. PMID: 20041810.
-
22Denke MA. Dietary prescriptions to control dyslipidemias. Circulation. 2002 Jan 15;105(2):132-5. PMID: 11790687.
-
23Gupta A, Butts B, Kwei KA, Dvorakova K, Stratton SP, Briehl MM, Bowden GT. Attenuation of catalase activity in the malignant phenotype plays a functional role in an in vitro model for tumor progression. Cancer Lett. 2001 Nov 28;173(2):115-25. PMID: 11597785.
-
Financial source: National Council for Scientific and Technological Development (CNPq)
-
1
Research performed at "Waldemar Alcantara" General Hospital and Molecular Biology and Genomics Laboratory (RENORBIO), Federal University of Rio Grande do Norte (URFN), Brazil. Part of Master degree thesis, Postgraduate Program in Surgery, Federal University of Ceara (UFC). Tutor: Paulo Roberto Leitao de Vasconcelos.
Publication Dates
-
Publication in this collection
Aug 2014
History
-
Received
26 Mar 2014 -
Reviewed
27 May 2014 -
Accepted
24 June 2014