Abstracts
PURPOSE: Regeneration and/or healing of tissues is believed to be more difficult in elderly people. The liver is one of the most complex organs in the human body, and is involved in a variety of functions. Liver regeneration is the body's protection mechanism against loss of functional liver tissue. The aim of this study is to identify the regenerative capacity of the liver in older animals and to compare it with that of young adult animals. METHODS: Thirty-four Wistar rats were used, of which 17 were 90 days old (young animals) and 17 were 460 days old (old animals). Approximately 70% of the liver was surgically removed. Examinations were carried out after 24 hours and on day 7, using 3 methods: KWON et al.'s formula to identify increase in volume; mitotic figure count in 5 fields; and the percentage of PCNA-positive nuclei in 5 fields. RESULTS: The increase in volume of the remaining liver was greater in the young animals after both 24 hours (p=0.0006) and on day 7 (p=0.0000). Histological cuts showed a greater mitotic figure count in young animals evaluated after 24 hours (p=0.0000). Upon evaluation on day 7, recovery was observed in the old animals. This recovery was similar to that of the young ones (p=0.2851). The PCNA-positive nucleus count was greater in the young animals' liver cuts after 24 hours (p=0.0310), and, while it had decreased in young animals by day 7, recovery was observed in the older animals (p=0.0298). CONCLUSION: The data confirm that age is related to delay in liver regeneration in rats.
Liver Regeneration; Aging; Hepatectomy
OBJETIVO: Acredita-se que idosos tenham maior dificuldade de regenerar e/ou cicatrizar tecidos. O fígado é um dos mais complexos órgãos do corpo humano, e está envolvido em diversas funções. A regeneração hepática representa um mecanismo de proteção orgânica contra a perda de tecido hepático funcionante. O objetivo do presente estudo é reconhecer a capacidade regenerativa do fígado de animais velhos e compará-la com a de animais adultos jovens. MÉTODOS: Foram utilizados 34 ratos Wistar, 17 com 90 dias (jovens) e 17 com 460 dias (velhos). Aproximadamente 70% do fígado foi cirurgicamente removido. As aferições foram feitas com 24 horas e com 7 dias, com 3 métodos: Fórmula de KWON et al para reconhecer ganho de volume, contagem das figuras de mitose existentes em 5 campos e percentual dos núcleos PCNA positivos em 5 campos. RESULTADOS: O remanescente hepático ganhou maior volume nos animais jovens tanto com 24 horas (p=0,0006) quanto com 7 dias (p=0,0000). Os cortes histológicos revelaram maior número de figuras de mitose nos fígados dos jovens avaliados com 24 horas (p=0,0000). Na avaliação com 7 dias houve recuperação nos velhos, que se aproximaram dos jovens (p=0,2851). A contagem dos núcleos PCNA positivos foi maior nos cortes dos fígados dos jovens, com 24 horas (p=0,0310) e enquanto diminuiu nos jovens com 7 dias, nos velhos houve recuperação (p=0,0298). CONCLUSÃO: Os dados confirmam que a idade está relacionada com o atraso da regeneração hepática, em ratos.
Regeneração Hepática; Envelhecimento; Hepatectomia
ORIGINAL ARTICLE
Effect of aging on liver regeneration in rats11 Study performed at the Surgical Techniques and Experimental Surgery Program in the School of Medicine at Federal University of Paraná (UFPR). Brazil.
Efeitos do envelhecimento na regeneração hepática em ratos
Maria de Lourdes Pessole Biondo-SimõesI; Jorge Eduardo Fouto MatiasII; Guilherme Ramina MontibellerIII; Letícia Cristina Dalledone SiqueiraIV; Eduardo da Silva NunesIV; Cristiano Antonio GrassiIV
IAssociate Professor, Department of Surgery, UFPR. Brazil
IIPhD. Associate Professor, Experimental Surgery, Department of Surgery, UFPR
IIIStudent, Scholarship National Council for Scientific and Technological Development (CNPq), Brazil
IVStudent, Scientific Initiation Program - UFPR. Brazil
Correspondence Correspondence: Maria de Lourdes Pessole Biondo-Simões Rua Ari José Valle, 1987 82030-000 CuritibaPR Brazil Phone: (55 41)223-4637 / 9991-5566 biondo@avalon.sul.com.br
ABSTRACT
PURPOSE: Regeneration and/or healing of tissues is believed to be more difficult in elderly people. The liver is one of the most complex organs in the human body, and is involved in a variety of functions. Liver regeneration is the body's protection mechanism against loss of functional liver tissue. The aim of this study is to identify the regenerative capacity of the liver in older animals and to compare it with that of young adult animals.
METHODS: Thirty-four Wistar rats were used, of which 17 were 90 days old (young animals) and 17 were 460 days old (old animals). Approximately 70% of the liver was surgically removed. Examinations were carried out after 24 hours and on day 7, using 3 methods: KWON et al.'s formula to identify increase in volume; mitotic figure count in 5 fields; and the percentage of PCNA-positive nuclei in 5 fields.
RESULTS: The increase in volume of the remaining liver was greater in the young animals after both 24 hours (p=0.0006) and on day 7 (p=0.0000). Histological cuts showed a greater mitotic figure count in young animals evaluated after 24 hours (p=0.0000). Upon evaluation on day 7, recovery was observed in the old animals. This recovery was similar to that of the young ones (p=0.2851). The PCNA-positive nucleus count was greater in the young animals' liver cuts after 24 hours (p=0.0310), and, while it had decreased in young animals by day 7, recovery was observed in the older animals (p=0.0298).
CONCLUSION: The data confirm that age is related to delay in liver regeneration in rats.
Key words: Liver Regeneration. Aging. Hepatectomy.
RESUMO
OBJETIVO: Acredita-se que idosos tenham maior dificuldade de regenerar e/ou cicatrizar tecidos. O fígado é um dos mais complexos órgãos do corpo humano, e está envolvido em diversas funções. A regeneração hepática representa um mecanismo de proteção orgânica contra a perda de tecido hepático funcionante. O objetivo do presente estudo é reconhecer a capacidade regenerativa do fígado de animais velhos e compará-la com a de animais adultos jovens.
MÉTODOS: Foram utilizados 34 ratos Wistar, 17 com 90 dias (jovens) e 17 com 460 dias (velhos). Aproximadamente 70% do fígado foi cirurgicamente removido. As aferições foram feitas com 24 horas e com 7 dias, com 3 métodos: Fórmula de KWON et al para reconhecer ganho de volume, contagem das figuras de mitose existentes em 5 campos e percentual dos núcleos PCNA positivos em 5 campos.
RESULTADOS: O remanescente hepático ganhou maior volume nos animais jovens tanto com 24 horas (p=0,0006) quanto com 7 dias (p=0,0000). Os cortes histológicos revelaram maior número de figuras de mitose nos fígados dos jovens avaliados com 24 horas (p=0,0000). Na avaliação com 7 dias houve recuperação nos velhos, que se aproximaram dos jovens (p=0,2851). A contagem dos núcleos PCNA positivos foi maior nos cortes dos fígados dos jovens, com 24 horas (p=0,0310) e enquanto diminuiu nos jovens com 7 dias, nos velhos houve recuperação (p=0,0298).
CONCLUSÃO: Os dados confirmam que a idade está relacionada com o atraso da regeneração hepática, em ratos.
Descritores: Regeneração Hepática. Envelhecimento. Hepatectomia.
Introduction
The liver is one of the most complex organs in the human body and is involved in about 5,000 functions. It has 2 different blood supplies, and is made up of 5 different types of cells and a complex extracellular structure1. It is vulnerable to a large range of metabolic, toxic, microbial, circulatory and neoplasic insults. In some cases, the disease may be a primary liver disease, for example viral hepatitis or hepatocellular carcinoma. It is more common for the involvement of the liver to be secondary. This is often a result of diseases that are more frequent in humans, such as cardiac decompensation, disseminated cancer, alcoholism and extrahepatic infections. The liver's enormous functional reserve hides the initial clinical impact of the hepatic lesion up to a certain extent. However, as the diffuse disease progresses, or if there is a strategic interruption of biliary flow, the consequences of hepatic dysfunction become life-threatening. The liver has 5 general responses to these harmful events: degeneration and intracellular accumulation, necrosis and apoptosis, inflammation, fibrosis and regeneration. Liver regeneration represents an organic protection mechanism against loss of functional liver tissue through chemical or viral lesion, traumatic loss or partial hepatectomy2-4.
The term regeneration, which is widely accepted, only means recovery of organ volume and not regeneration of the part lost. What actually takes place is global hyperplasia of the whole parenchyma5,6.
This is known to be an event that promotes highly ordered and organized tissue growth until the liver reaches its original weight, with a small variation between 5 and 10%4,7. The first successful experimental model for studying liver regeneration was introduced by Higgins and Anderson in 19318. This model involved surgical removal of the left lateral and median lobes in rat livers, making up approximately 67 to 70% of the total liver mass of the animals1,4,9,10.
Massive tissue loss induces temporary liver insufficiency until a significant part of the organ is restored. This has encouraged further research for factors capable of influencing hepatocellular proliferation and the regenerative process3,11.
Hepatocytes are highly differentiated cells of an epithelial nature, which rarely divide. At any given point in time in an animal's or a human's life only one out of 20,000 hepatocytes is actually dividing, and this division can occur once or twice, at most, in each cell 4,12.
All liver cells (hepatocytes and endothelial, Kupffer, Ito, and ductal cells) proliferate in order to replace lost liver tissue. Nevertheless, hepatocytes are the first to proliferate and most studies give prominence to these cells as they make up almost 90% of the liver's mass and 60% of the total number of cells4,13.
Perhaps even more extraordinary than the hepatocytes' ability to proliferate is the fact that these cells simultaneously carry out essential functions for maintaining organic homeostasis, such as regulation of the level of glycemia, synthesis of plasmatic proteins and coagulation factors, bile secretion, the urea cycle and biodegradation of toxic compounds4,11,13.
Orderly repair (regeneration and fibroplasia) and healing of tissues in normal individuals can be affected by a number of factors that jeopardize the quality and end result of the process. These influences include both local and systemic host factors. Ageing is one of these factors. Age is directly related to the healing and repair process. There is a decrease in the number of cells in the elderly, which compromises macrophage function, reduces inflammatory response, limits mitogenic response, reduces collagen quality and delays epithelization.
Four groups of age-related changes can be outlined: general tissue, cardiovascular and nervous and endocrine system changes, as well as changes related to other organs important in body homeostasis. Our aim in this study is to understand the influence of ageing on liver regeneration in partially hepatotectomized rats.
Methods
Thirty-four male Wistar rats (Rattus norvegicus albinus, Rodentia mammalia) were used, divided into two groups. There were 17 young 90-day-old rats in one group and 17 old rats with an average age of 560 days in the other group. The animals were kept in an animal colony on a light/dark cycle, at room temperature and humidity, and unlimited access to water and commercial feed.
After being anaesthetized by intramuscular injection of 0.2 ml/100g of a 1 ml ketamine (50 mg) and 1 ml of xylazine (20 mg) solution, the animals were weighed and both groups were submitted to manual depilation of the abdominal ventral wall and antisepsis with polyvinyl pyrrolidone-iodine. A median laparotomy and a partial hepatectomy were carried out, and the left lateral and median lobes were resected. This hepatectomy is equivalent to sectioning approximately 67% of the organ's volume. After checking for hemostasis, laparorraphy was carried out. The resected segment was weighed and the value recorded in the protocol.
During the post operation period, the rats were kept under the above-mentioned conditions. The animals were anesthetized and weighed once more and relaparotomy was carried out after 24 hours and again on day 7. The remaining liver was completely sectioned in 9 rats after 24 hours and in 8 rats on day 7 in both groups. The animals were submitted to euthanasia with a lethal intraperitoneal dose of thiopental. The resected parts were weighed and the results recorded in the protocol. After this process, the resected livers were fixed in 10% buffered formalin and sent for histological examination. Slides with 4-micrometer-thick cuts were prepared, stained with hematoxylin-eosin for mitotic figure assessement. Immunohistochemistry with anti-PCNA was used to evaluate the PCNA-positive nuclei.
Regeneration was assessed by 3 methods: KWON et al.'s formula (1990); the average number of mitotic figures in 5 fields; and the average of PCNA-positive nuclei in 5 fields.
Kwon et al.'s formula 14 gives the regeneration rate based on weight.
% = D/E .100 where E = R/0.7
Where: D = liver weight per 100 g body weight on the day of sacrifice,
E = represents the estimated weight per 100g body weight before the hepatectomy, calculated by using the weight of the resected liver (R).
The existing mitotic figure count was carried out in 5 fields.
The PCNA-positive nucleus count in 5 fields was carried out, using the immunohistochemical method with PCNA (proliferating cell nuclear antigen) antibodies and the strepto-avidin-biotin-peroxidase technique for class PC (10) anti-PCNA monoclonal antibodies.
Results
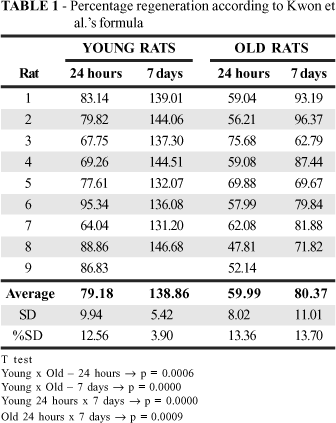
Regeneration evaluation according to Kwon et al.'s formula showed that the volume of the organ at 24 hours was larger in the young animals (p=0.0006). This was still the case on day 7 (p=0.0000). It can be seen that on day 7 the liver volume of the old animals had reached the same liver volume as the young animals at 24 hours (Table 1 and Figure 1).
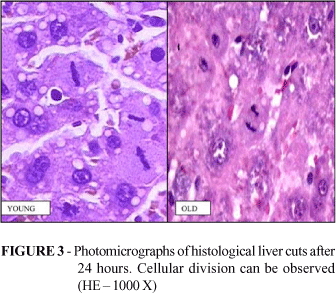
The mitotic figure count in five fields showed that the count was twice as high (p=0.0000) in the group of young animals when evaluated after 24 hours. There is not, however, a difference in evaluation on day 7 (Table 2 and Figure 2). Figure 3 shows cells dividing in both groups.
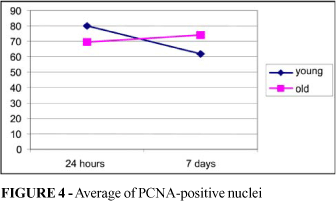
The PCNA-positive nucleus count after 24 hours was higher in the histological cuts from the young animals (p=0.0310) but higher in the old animals on day 7 (p=0.0298). While it had decreased in young animals on day 7 (p=0.0000), it remained stable in the old animals group (p=0.4681) (Table 3 and Figure 4). Figure 5 shows various pictures of the PCNA-positive nuclei in both groups.
Discussion
An additional factor when carrying out surgery on elderly patients is the concern shared by both physician and family regarding possible complications. A number of authors have shown impaired healing in elderly patients15-20. Studies have shown delayed reepithelization in the elderly21-22 and reduced proliferative response of keratinocytes with advancing age23-24.
Opinions diverge with regard to collagen synthesis. Chyun and Griminger25 and Tan et al.26 showed a reduction in collagen I synthesis in rats and humans respectively, whilst Ashcroft et al.27 stated that there is a reduction in collagen I and collagen II synthesis in mice. Holt et al.22, however, reported a build-up of hydroxyproline and DNA similar to that in patients who were not elderly. Kletsas et al.28 stated that age is not related to decline in response of human fibroblasts to various growth factors.
It can be observed that there is concern about skin healing and collagen synthesis. Few people have studied liver regeneration relating it to age. With increased life expectancy, the number of elderly patients and common diseases in hospitals can be expected to increase. Metastatic lesions that are secondary to tumors will result in a greater number of liver resections. This means that efforts will have to focus on the processes involved in the regeneration of the organ.
According to Baratta et al.12, at least 2 waves of cellular proliferation can be detected. One starts the S phase around 18 hours after the hepatectomy and finishes at 26 hours, and the other starts the S phase around 26 hours and finishes at 34 hours. The first phase can be detected at the periphery of the lobule, and the second can be seen in both the central and peripheral areas.
There are a number of methods for quantifying liver regeneration. These include: liver mass, mitotic cell count, identification of DNA synthesis (measurement of 3H-thymidine incorporation, bromodeoxydeuridine incorporation and flow cytometry), and histochemical methods based on the use of antibodies of endogenous tissue molecules, such as proliferating cell nuclear antigen (PCNA), alpha DNA polymerase, Ki-67, anti-PAA, anti-ribonucleotide reductase, C5F10 or p53 transformation-related protein. The most commonly used is PCNA. It is a DNA polymerase delta auxiliary protein, essential for DNA replication in eukaryotic cells. Its expression is dependent on the cellular cycle. It is detected initially in the final phase of G1, and its maximum detection is in the S phase. This is also the phase in which the incorporation of the radioactive isotopes takes place (marked thymidine). It is also possible to quantify liver regeneration by NOR (nucleolus organizer region) protein detection, by identifying tissue enzymes and proteins associated with liver regeneration (thymidine-kinase, ornithine decarboxylase and putrescine), and by blood tests (thymidine-kinase, fibronectin and alpha-fetoprotein)1.
It can be seen that regeneration, evaluated in this experiment by organ weight and Kwon et al.'s formula14, was lower in the old animals. The volume increase in the remaining liver was greater in the young animals both after 24 hours (p=0.0006) and on day 7 (p=0.0000). On day 7, the remaining liver in the old animals had half the volume of the young animals'. The histological cuts revealed a larger number of mitotic figures in the livers of the young animals evaluated after 24 hours (p=0.0000). Evaluation on day 7 confirmed that there was not a significant difference between young and old animals, and that while the number of mitotic figures in the young animals' liver cuts showed a slight decrease over time, in the old animals there was an increase. This suggests that cellular replication is delayed in the livers of old animals. This is supported by the PCNA-positive nuclei analysis, as the number of positive nuclei is greater in the histological cuts in young animals (p=0.0310). There was reduction in the livers of the young animals, while there was a slight increase in the old animals'. It therefore seems clear that in old animals the liver needs more time to recover its volume.
In young adult rats, a 2/3 partial hepatectomy (resection of the median and left lobes) causes compensatory hyperplasia. Following the pre-replication phase, when RNA and proteins are synthetized, DNA synthesis starts around 18 hours after the hepatectomy and reaches a maximum at 24 hours. At this time, 3H-thymidine incorporation in DNA increases substantially and 30% of the hepatocyte nuclei are found to contain 3H-thymidine. The mitoses are more active between 24 and 48 hours, with 3 to 4% of the hepatocytes dividing. In 1-year-old rats, the time for 3H-thymidine incorporation extends beyond 24 hours after the hepatectomy, and the number of hepatocytes marked with 3H-thymidine is lower. These data enabled Ogawa et al.29 to state that age reduces liver regeneration in rats.
Beyer et al9 studied liver regeneration, and compared 6-week-old and 1-year-old animals. The young animals regenerated 97% of the mass after 1 week, compared to 67% in the old animals. They observed that 93% of the DNA content was recovered in the young animals and only 65% in the old animals. Transcription of thymidine-kinase, an enzyme that catalyzes the addition of phosphate to thymidine and is involved in DNA synthesis, decreased with age. Although Beyer et al did not detect a significant difference in terms of RNA content, in our study this content also decreased.
Conclusion
The present data confirm that age is related to liver regeneration delay in rats.
Received: January 08, 2006
Review: February 10, 2006
Accepted: March 16, 2006
Conflict of interest: none
Financial source: none
How to cite this article: Biondo-Simões MLP, Matias JEF, Montibeller GR, Siqueira LCD, Nunes ES, Grassi CA. Effect of aging on liver regeneration in rats. Acta Cir Bras. [serial on the Internet] 2006 July-Aug;21(4). Available from URL: http://www.scielo.br/acb
- 1. Assy N, Minuk GY. Liver regeneration: methods for monitoring and their applications. J Hepatol. 1997; 26 (4):945-52.
- 2. Kalil AN, Sperb D, Lichtenfels E. Efeito da pilocarpina na regeneração hepática pós hepatectomia parcial em ratos. Acta Cir Bras. 1998; 13(4):222-6.
- 3. Ramalho FS. A regeneração hepática e os inibidores da enzima conversora da angiotensina. Acta Cir Bras. 2000; 15 (Supl 2):14-7.
- 4. Ramalho FS, Ramalho LNZ, Zucoloto S, Castro e Silva Jr O. Regeneração hepática: algumas definições num universo de incertezas. Acta Cir Bras. 1993; 8(4):177-89.
- 5. Cressman DE, Diamond RH, Taub R. Rapid activatin of the start 3 transcription complex in the liver regeneration. Hepatology. 1995; 21(5):1443-9.
- 6. Westwick JK, Weitzel C, Leffert HL, Brenner DA. Activation of jun kinase is a early event in hepatic regeneration. J Clin Invest. 1995; 95(2):803-10.
- 7. LaBrecque D. Liver regeneration: a picture emerges from the puzzle. Am J Gastroenterol. 1994; 89(Suppl 8):S86-96.
- 8. Higgins GM, Anderson RM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931; 12:186-202.
- 9. Beyer HS, Sherman R, Zieve L. Aging is associated with reduced liver regeneration and diminished thymidine kinase mRNA content and enzyme activity in the rat. J Lab Clin Med. 1991; 117 (2):101-8.
- 10. Jesus RP, Waitzberg DL, Campos FG. Regeneração hepática: papel dos fatores de crescimento e nutrientes. Rev Assoc Med Bras. 2000; 46(3):242-54.
- 11. Michalopoulos G. Liver regenaration: molecular mechanisms of growth control. Faber J. 1990; 4(2):176-87.
- 12. Barrata B, Rizzoli R, Galliani I, Vitale M, Rizzi E, Matteucci A, Galanzi A, Zamai L, Mazzotti G. Early events of liver regeneration in rats: a multiparametric analysis. Histochem Cell Biol. 1996;105(1):61-9.
- 13. Michalopoulos G, DeFrances MC. Liver regeneration. Science.1997; 276(5309):60-6.
- 14. Kwon AH, Uetsuji S, Yamamura M, Hioki k, Yamamoto M. Effect of administration of fibronectin or aprotinin on liver regeneration after experimental hepatectomy. Ann Surg. 1990; 211(3):295-300.
- 15. Halasz NA. Dehiscence of laparotomy wounds. Am J Surg. 1968; 116(2):210-14.
- 16. Mendoza CB, Postlethwait RW, Johnson WD. Incidence of wound disruption following operation. Arch Surg. 1970; 101(3):396-8.
- 17. Schrock TR, Deveney CW, Dunphy JE. Factors contributing to leakage of colonic anastomoses. Ann Surg. 1973; 177(5):513-8.
- 18. Irvin TT, Goligher JC. Aetiology of disruption of intestinal anastomoses. Br J Surg. 1973; 60(6):461-4.
- 19. Riou JP, Cohen JR, Johnson H Jr. Factors influencing wound dehiscence. Am J Surg. 1992; 163(3):324-30.
- 20. Carlson MA. Acute wound failure. Surg Clin North Am. 1997; 77(3):607-36.
- 21. Gilchrest BA. In vitro assessment of keratinocyte aging. J Invest Dermatol. 1983; 81(Suppl 1):S184-9.
- 22. Holt DR, Kirk SJ, Regan MC, Hurson M, Lindblad WJ, Barbul A. Effect of age on wound healing in healthy human beings. Surgery. 1992; 112(2):293-8.
- 23. Rheinwold JG, Green H.Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975; 6(3):331-43.
- 24. Morris GF, Matheus MB. Regulation of proliferating cell nuclear antigen during the cell cycle. J Biol Chem. 1989; 264(23):13856-64.
- 25. Chyun JH, Griminger P. Improvement of nitrogen by arginine and glycine supplementation and its relation to collagen synthesis in traumatized mature and aged rats. J Nutr. 1984; 114(9):1697-704.
- 26. Tan EM, Rouda S, Hoffren J, Chen YQ, Uitto J, Li K. Extracellular matrix gene expression by human keratinocytes and fibroblasts from donors of varying ages. Trans Assoc Am Physicians. 1993; 106:168-78.
- 27. Ashcroft GS, Horan MA, Ferguson MWJ. Aging is associated with reduced deposition of specific extracellular matrix components, an up regulation of angiogenesis, and an altered inflammatory response in a murine incisional wound healing model. J Invest Dermatol. 1997; 108(4):430-7.
- 28. Kletsas D, Pratsinis H, Zervolea I, Handris P, Sevaslidou E, Ottaviani E, Stathakos D. Fibroblast responses to exogenous and autocrine growth factors relevant to tissue repair: the effect of aging. Ann N Y Acad Sci. 2000; 908:155-66.
- 29. Ogawa K, Mukai H, Mori M. Effect of aging on proliferative activity of normal and carcinogen-altered hepatocytes in rat liver after a two-thirds partial hepatectomy. Jpn J Cancer Res. 1985; 76(8):779-84.
Publication Dates
-
Publication in this collection
20 Sept 2006 -
Date of issue
Aug 2006
History
-
Accepted
16 Mar 2006 -
Received
08 Jan 2006 -
Reviewed
10 Feb 2006