ABSTRACT
Chemical profile analyses of artichoke (Cynara scolymus L., Asteraceae) edible parts (fleshy receptacle, inner bracts) as well as roots are compared with the commercially usable leaf extract using HPLC-DAD-ESI-MS via chlorogenicacid as a marker. Overall polyphenolic constituents demonstrated by means of LC/MS profiling. The nutritional values and inulin contents of different assessed parts were investigated. The present study was designed to determine the effect of artichoke: leaves, bracts, receptacles and roots alcoholic extracts against CCl4-induced acute hepatotoxicity and hyperlipidemia in rats by means of histopathological and biochemical parameters. Serum liver enzymes levels of aspartate amino transferase, alanine amino transferase, alkaline phosphatase and lipid peroxidase content (malondialdehyde MDA) were estimated. Blood glutathione, total cholesterol, triacylglycerides and high density lipid level were estimated in plasma. The ethanol extract of roots, leaves, bracts and receptacles were standardized to (0.82 ± 0.02, 1.6 ± 0.06, 2.02 ± 0.16 and 2.4 ± 0.27 mg chlorogenic acid/100 mg extract), respectively. The receptacle showed the highest content of polyphenols and exhibits the highest antioxidant activity. HPLC analysis of inulin in the receptacles of globe artichoke revealed high content of inulin (41.47 mg/g) dry extract. All artichoke parts contain comparable vitamins and minerals. Artichokes receptacles extract when taken in dose of (500 mg/kg/day) reduce the lesion caused by CCl4 alone more than groups receiving silymarin. Bracts and leaves extract exert nearly the same effect.
Keywords:
Antioxidant; Anti-hyperlipidemic; Chlorogenic acid; Globe artichoke; Hepatoprotective; Silymarin
Introduction
Developing countries generally have greater share of burden liver diseases (Elizabeth, 2008Elizabeth, M.L., (PhD thesis) 2008. Dynamics of Liver Disease in Egypt. University of Michigan, USA.). The load of liver diseases in Egypt is exceptionally high maintaining the highest prevalence of hepatitis C virus worldwide, as well as rising rates of hepato-cellular carcinoma (Strickland, 2006Strickland, G.T., 2006. Liver disease in Egypt: hepatitis C superseded schistosomiasis as a result of iatrogenic and biological factors. Hepatology 43, 915-922.). Egypt has the highest serological prevalence of hepatitis C virus in the world ranging from 6 to 28% with average approximately 15% in the general population (Saad et al., 2011Saad, Y., Zakaria, S., Ramzy, I., Raziky, E.L.M., Shaker, M., Elakel, O., Said, W., Wosre, M., Eldaly, M., Abdel Hamid, M., Esmail, G., 2011. Prevalence of occult hepatitis Cin Egyptian patients with non alcoholic fatty liver disease. Open J. Int. Med. 1, 33-37.). The above serious problem in Egypt demonstrates the urgency of finding a hepatoprotective drug from natural source in order to face this intense ongoing endemic outbreak.
Globe artichoke (Cynara scolymus L.) is a perennial plant belonging to Asteraceae. It is cultivated worldwide for its immature edible flower heads consisting of fleshy leaves (bracts) and receptacles (Jonne et al., 2007Jonne, B., Anderson, L.A., Phillipson, D., 2007. Herbal Medicines, 3rd ed. Pharmaceutical Press, London.). Globe artichoke was known by the ancient Egyptians as food and medicinal entity and known by Greeks and Romans. It was an important menu item at feasts until the fifteenth century. It is popular for its pleasant bitter taste which is mostly attributed to bioactive material called cynarin (Rottenberg and Zohary, 1996Rottenberg, A., Zohary, D., 1996. The wild ancestry of the cultivated artichoke. Genet. Resour. Crop Evol. 43, 53-58.).
The plant is recognized in herbal medicine where foliage leaves are utilized for production of commercial extracts used as hepatoprotective and choleretic in food supplements (Gebhardt, 2005Gebhardt, R., 2005. Choleretic and anticholestatic activities of flavonoids of artichoke. Acta Hortic. 681, 429-435.). These important activities have been attributed to several metabolites including polyphenols such as cynarin, caffeoylquinic, chlorogenic acid, flavonoids such as luteolin or its glycosides (Lattanzioa et al., 2009Lattanzioa, V., Kroon, P.A., Linsalatac, V., Cardinalic, A., 2009. Globe artichoke: a functional food and source of nutraceutical ingredients. J. Funct. Food 1, 131-144.; El Senousy et al., 2014El Senousy, A.S., Farag, M.A., Al-Mahdy, D.A., Wessjohann, L.A., 2014. Developmental changes in leaf phenolics composition from three artichoke cultivars (Cynara scolymus) as determined via UHPLC-MS and chemometrics. Phytochemistry 108, 67-76.). Other constituents are also reported such as sesquiterpenes (grosheimin, cyanoropicrin), saponins, fatty acids and others. Metabolite variability are reported among the different cultivars of C. scolymus (Farag et al., 2013Farag, M., El-Ahmady, S., Elian, F., Wessjohann, L., 2013. Metabolomics driven analysis of artichoke leaf and its commercial products via UHPLC-q-TOF-MS and chemometrics. Phytochemistry 95, 177-187.) as determined by analysis of principle components. Variation of constituents has been documented depending on genotype, harvest time, part used, soil, climate etc. (El Senousy et al., 2014El Senousy, A.S., Farag, M.A., Al-Mahdy, D.A., Wessjohann, L.A., 2014. Developmental changes in leaf phenolics composition from three artichoke cultivars (Cynara scolymus) as determined via UHPLC-MS and chemometrics. Phytochemistry 108, 67-76.). Globe artichoke has been the subject of study by many investigators (Gebhardt, 2005Gebhardt, R., 2005. Choleretic and anticholestatic activities of flavonoids of artichoke. Acta Hortic. 681, 429-435.; Wagenbreth and Eich, 2005Wagenbreth, D., Eich, J., 2005. Pharmaceutically relevant phenolic constituents in artichoke leaves are useful for chemical classification of accessions. Acta Hortic. 681, 469-474.; Jonne et al., 2007Jonne, B., Anderson, L.A., Phillipson, D., 2007. Herbal Medicines, 3rd ed. Pharmaceutical Press, London.). These investigations focused mainly on using the artichoke leaf extract for liver protection against chemical pollutant and viral infections. Artichoke plant is traditionally used for treating liver diseases as well as hyperlipidemia (Gebhardt and Fauseel, 1997Gebhardt, R., Fauseel, M., 1997. Antioxidant and hepatoprotective effects of artichoke extract and constituents in cultured rat hepatocytes. Toxicol. In Vitro 11, 669-672.).
The aim of this work is: (i) to evaluate and set up standard measures for C. scolymus cultivar balady growing at Nahia region at Giza; (ii) to reveal the characteristic profile of major phenolics and structurally related ingredients in the extracts of different organs, especially the edible parts (receptacle and bracts) using optimized HPLC conditions; (iii) to quantify the contents of dietary constituents; (iv) to compare the therapeutic value of this plant as a hepatoprotective drug versus the well-established silymarin obtained from milk thistle.
Experimental
Plant material
The samples of Cynara scolymus L., Asteraceae, were collected from Nahia region at Giza, Egypt, May 2014. The cultivar is known as Balady. A voucher specimen (numbered 8.5.2014) has been located at the herbarium of Pharmacognosy Department, Faculty of Pharmacy, Cairo University, Egypt. Three kilogram of air-dried powdered different parts (leaves, receptecals, bracts and roots) of C. scolymus was extracted by cold percolation (5 × 3 l) with ethanol (70%) till exhaustion. Greenish brown residues were collected after evaporation of the solvent. Solvent in each case was totally evaporated below reduced pressure and residues obtained were kept for the study.
Chemical measures
Solvent: ethanol 70% (commercial grade). Reagants: Folin-Ciocalteu reagent (Sigma-Aldrich). The DPPH (2,2-diphenyl-1-picrylhydrazyl) reagent (Sigma-Aldrich). Authentic reference materials: chlorogenic acid, benzoic acid, gallic acid, caffeic acid, cinnamic acid, ellagic acid and inulin (Sigma-Aldrich).
HPLC standardization of artichoke extracts
Artichoke particularly the edible organs namely receptacle and bracts were reported to contain chlorogenic acid. Hence, it deemed necessary to shed light on its concentration indifferent plant extracts. The artichoke extracts of leaves, bracts, receptacles and roots were standardized according to their chlorogenic acid content (European Pharmacopoeia, 2006European Pharmacopoeia, 2006. Council of Europe, France, 4th ed.). An Agilent 1200 series HPLC was used, equipped with a quaternary pump and an auto sampler. Samples were dissolved in methanol, filtered through PTFE 0.45 µm syringe filter (Macherey-Nagel, Germany) and injected into Zorbax C8 (5 µm, 4.6 × 250 mm) column. The mobile phase was methanol (solvent A) and 0.3% phosphoric acid in water (solvent B). Gradient elution was carried out at a flow rate of 1 ml/min. Measurements were made with an injection volume of 20 µl and UV detection at 330 nm.
Preparation of standard for HPLC
Standard stock solution of chlorogenic acid was prepared by dissolving 10 mg chlorogenic acid in 50 ml methanol. Transfer 5 ml of this solution to a volumetric flask, add 5 ml of methanol and dilute to 20 ml with water. Then concentrations of 0.2, 0.4, 0.6, 0.8 and 1 mg/ml were prepared.
Preparation of test solution
To 50 mg extract (leaves, bracts, receptacles and roots) 5 ml methanol added then filtered, 5 ml of filtrate was taken in volumetric flask and 5 ml methanol was added then completed to 20 ml with water. These concentrations were separately subjected to HPLC analysis according to condition mentioned before then calibration curve were established. Another sample of Super Artichoke capsules® (Western Medical) which is a commercially available drug, consisting of cynarin at a concentration of 320 mg, was analyzed under the same conditions for comparison with four artichoke extracts.
LC-DAD-ESI-MS separation technique
Separation of the sample was carried by a bounded silica C18 column (4.6 × 150 mm 3 µm). The sample filtrate by Teflon 0.45 membrane filter, then 10 µm of sample dissolved in 1 ml of MeOH to carry out analysis (Fritsche et al., 2002Fritsche, J., Beindorff, C.M., Dachtler, M., Zhang, H., Lammers, J.G., 2002. Isolation, characterization, and determination of minor artichoke leaf extract compounds. Eur. Food Res. Technol. 215, 149-157.). Chemical compounds were analyzed via reversed phase HPLC using a binary gradient consisting of solvent A: isopropanol/acetonitrile/methanol/0.3% aqueous formic acid 18:30:12:40 (v/v) and solvent B: 0.3% aqueous formic acid. A linear gradient from 8% (A) (0 min) to 48%(A) (35 min) at a flow rate of 1 ml/min was applied, at temperature 25 ºC. The column is interfaced with electrospray (Bruker daltonic Esquire-LC mass spectrometer (Bremen, Germany), and Dionex Ultimate 300 (Germany) equipped with a quaternary pump with an on line degasser, a thermostatted column compartment, a photodiode array detector (DAD), an auto sampler, and HyStar software. Mass spectra were acquired in positive ion mode at a voltage of 70 V. Nitrogen was used as drying gas at a flow rate of 10 l/min and 300 ºC. Nebulizer pressure was set to 60 psi. The capillary voltage was optimized to 3500 V. For all spectra manual baseline subtraction was performed. This analysis was kindly performed at institute for Environmental Studies and Research.
Spectrophotometric determination of total polyphenols
Determination of total phenolics according to Singleton and Rossi (1965)Singleton, V.L., Rossi, A.J., 1965. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Viticult. 16, 144-158..
Preparation of standard solution
The standard stock solution of gallic acid was prepared with different concentrations (0.025, 0.05, 0.1, 0.2, 0.3 and 0.4 mg/ml) in ethanol.
Preparation of test samples
Extract (0.1 g) was dissolved in 100 ml distilled water, 0.8 ml was taken to which 0.4 ml Folin-Ciocalteu and 4 ml distilled water were added, then diluted to 10 ml with sodium carbonate (290 g/l). The solution was thoroughly mixed and incubated for 30 min, then total phenols were spectrophotometrically estimated at 760 nm. The concentration of total phenolics was expressed as gallic acid equivalent (GAE) per 1 g of fresh sample. All experiments were carried out in triplicates.
HPLC analysis and identification of phenolic compounds
The phenolic compound analysis was carried out using an Agilent Technologies 1100 series liquid chromatograph (RP-HPLC) coupled with a UV-Visible multi wavelength detector. The separation was carried out on a 250 × 4.6 mm, 4 µm Hypersil ODS C18 reversed phase column at ambient temperature. The mobile phase consisted of acetonitrile (solvent A) and water with 0.2% sulfuric acid (solvent B). The flow rate was kept at 0.5 ml/min. The gradient programme was as follows: 15% A/85% B 0–12 min, 40% A/60% B 12–14 min, 60% A/40% B 14–18 min, 80% A/20% B 18–20 min, 90% A/10% B 20–24 min, and 100% A 24–28 min (Bourgou et al., 2008Bourgou, S., Ksouri, R., Bellila, A., Skandarani, I., Falleh, H., Marzouk, B., 2008. Phenolic composition and biological activities of Tunisian Nigella sativa L. shoots and roots. C. R. Biol. 331, 48-55.). The injection volume was 20 µl, and peaks were monitored at 280 nm. Samples were filtered through a 0.45 µm membrane filter before injection. The identification of each compound was based on a combination of retention times as well as spectral matching to those of authentic samples and relative area percentages computed by integration. All analyses were compared with injected standards of each phenolic acid separately and calculated according to following equation:
Nutritional values of artichoke
Moisture content, total ash, protein, fibers, carbohydrates content, minerals as (sodium, calcium, iron) and vitamins (A, C, and E) were tested using 100 g fresh receptacles according to (AOAC, 2005AOAC, 2005. Official Method of Analysis of AOAC International, revision 1, 2006, Gaitherburg, Maryland, USA.)
Determination of inulin content
Inulin content was determined by HPLC technique in the receptacle only because it is the edible portion. One gram of fresh receptacle was accurately weighed and extracted with 90 ml hot water in a shaking water-bath at 85 ºC for 25 min, then the beaker was cooled to 60 ºC. At this temperature 100 µl of inulinase was added to the beaker and incubated in a shaking water-bath at 60 ºC for 30 min for total digestion of inulin or inulin oligomers. Then, the beaker was cooled to room temperature and transferred to 100 ml volumetric flask and completed to 100 ml with water. Then after the processes of centrifugation (10,000 × g, 20 min) and filtration the solution was injected into the HPLC Agilent 1200 series equipped with a quaternary pump and auto sampler, Zorbax column 5 µm, 4.6 × 250 mm with a flow rate 1 ml/min and detected at wave length 330 nm (Ronkart et al., 2007Ronkart, S.N., Blecker, C.S., Fourmanoir, H., Foungnies, C., Deroanne, C., Van Herek, J.C., Paquot, M., 2007. Isolation and identification of inulooligosaccharides resulting from inulin hydrolysis. Anal. Chim. Acta 604, 81-87.). Results were compared to standard inulin according to the following equation:
Materials for biological study
The methanol extract of artichoke (leaves, receptacles, bracts and roots) was investigated at a dose level of 500 and 900 mg/kg/day. Animals: 120 male albino rats weighing between 80 and 100 g were purchased from Research Institute of Ophthalmology, Cairo, Egypt. They were kept under standard conditions with temperature at 23 ± 2 ºC and a 12/12 h light/dark cycle and allowed free access to food and water throughout the experiment. To facilitate measures of food intake, rats were housed conventionally in individual stainless steel hanging wire-mesh cages, and fed with standard pellets, commercial standard chow (18% protein; Global 2018, Harlan Teklad, Madison, WI) and water ad libitum. This study was conducted in accordance with the standard guidelines used in handling of the experimental animals and approved by the Institutional Animal Care and Use Committee (IACUC) (No. 9-031), College of Pharmacy, Cairo University. Carbontetrachloride reagent 99.9% (Sigma-Aldrich), silymarin from market as Legalon®), kits for hepatoprotective and hypocholesterolemic activities (BioDiagnosteic Company) and formal saline (formalin 100 g, sodium chloride 8.5 g, water 900 ml).
Antioxidant activity
The antioxidant activity was assayed using a DPPH assay (Burda and Oleszek, 2001Burda, S., Oleszek, W., 2001. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 49, 2774-2779.). DPPH solution was prepared with an HPLC grade methanol at a concentration of 0.004%. Each standard extract (0.05 ml) was added to 5 ml of DPPH solution (0.025 g/l). Blank samples were run using 100 µl water in place of the plant extract. After a 30 min incubation period in the dark at room temperature, the absorbance was measured against a blank at 517 nm spectrophotometrically using Unicam UV Series vision 32 software operating manual. Gallic acid was used as positive control at a concentration of 0.02, 0.04, 0.06, 0.08 and 0.1 mg/ml. Inhibition of free radical in percent (I%) was calculated according to this formula:
where A0 is the absorbance of the control reaction (containing all reagents except the extract), and Ai is the absorbance of the extract. Measurements were carried out in triplicates.
Hepatoprotective, hypocholesterolimic and hypolipidemic activities
Liver damage in rats was induced by intraperitoneal injection of carbon tetrachloride (CCl4) at a dose of 0.4 ml/kg body weight (diluted in olive oil 1:4, v/v) twice weekly for one month (Canturk et al., 1999Canturk, N.Z., Ozbilim, G., Yenisey, C., 1999. Experimental cirrhosis of liver and cytoprotective effects of alpha tocopherol. East Afr. Med. J. 76, 223-227.). Liver protection activity of four organs of artichoke extracts at a dose of 500, 900 mg/kg/day were compared with silymarin at a dose of 500 mg/kg/day. Rats were divided into twenty groups, each contains six rats. At the end of the experimental period, the animals were sacrificed by decapitation and blood samples were collected into plain centrifuge tubes (to separate the serum by centrifugation) and kept in EDTA tubes to keep the blood. Determination of serum liver enzymes levels by the methods described as by (Reitman and Frankel, 1957Reitman, S., Frankel, S., 1957. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 28, 56-63.; Belfield and Goldberg, 1971Belfield, A., Goldberg, D.M., 1971. Normal ranges and diagnostic value of serum 5′ nucleotidase and alkaline phosphatase activities in infancy. J. Clin. Chim. Acta 46, 842-846.; Uchiyama and Mihara, 1978Uchiyama, M., Mihara, M., 1978. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 86, 271-278.) were carried out. Blood glutathione (GSH) level was also determined (Beutler et al., 1963Beutler, E., Olga, D., Barbara, M., 1963. Improved method for determination of blood glutathione. J. Lab. Clin. Med. 61, 882-888.). Total cholesterol (Natio and David, 1984Natio, H.K., David, J.A., 1984. Laboratory consideration: determination of cholesterol, triglyceride, phospholipid, and other lipids in blood and tissues. Lab. Res. Methods Biol. Med. 10, 1-76.), triacylglycerides (Buccolo and David, 1973Buccolo, G., David, H., 1973. Quantitative determination of serum triacylglycerides by the use of enzymes. J. Clin. Chem. 19, 476-482.) and high density lipids were estimated (Burstein et al., 1970Burstein, M., Scholnick, H.R., Monfin, R., 1970. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J. Lipid Res. 11, 585-595.). The results of biochemical analysis were expressed as mean ± standard error (SE).
Histopathological examination
After scarification, the liver tissues were rapidly isolated from each rat and possess the following steps for histopathological examination (Drury and Wallington, 1967Drury, R., Wallington, E., 1967. Carleton's Histological Technique, 4th ed. Oxford University Press, London.).
Fixation
The livers were fixed in formal saline 10% for one week. After fixation, livers were cut into slices then washed under running water gently for 3 h.
Dehydration
The formalin fixed livers were dehydrated in ascending grades of ethyl alcohol. A commonly used range of dehydration solution is 70% for 24–48 h, then 90% alcohol for 24 h, then three changes of absolute alcohol each for 1 h.
Clearing
The tissue was then cleared in 1% celloidine in methyl benzoate for 24 h. This was followed by treatment with two changes of benzene, half an hour each.
Impregnation
It was carried out in soft paraffin (M.P.50–55 ºC) for 2 h in an oven at 55 ºC. This is changed 2–3 times then tissue was transferred to hard paraffin (55–60 ºC) also for 2–3 h.
Sectioning and staining
Sectioning was at 8 µm thickness using the microtome. Staining was done with hematoxylin and eosin. Histology of the liver was studied immediately after sacrificing the animals (Drury and Wallington, 1967Drury, R., Wallington, E., 1967. Carleton's Histological Technique, 4th ed. Oxford University Press, London.).
Statistical analysis
The results of biochemical analysis were expressed as mean ± standard error (SE). Statistical analysis was conducted using one way analysis of variance (ANOVA). The level of statistical significance was taken at p < 0.05.
Results
HPLC standardization of artichoke extracts
Chlorogenic acid content in different extracts is found to be (0.82 ± 0.02, 1.6 ± 0.06, 2.02 ± 0.16 and 2.4 ± 0.27) in alcoholic extracts of roots, leaves, bracts and receptacles, respectively. HPLC chromatograms of the finger prints of the four extracts showing the peak of chlorogenic acid at Rt 7.6–7.9. The calibration curve showed good linearity for chlorogenic acid with a correlation co-efficient (R2) of 0.992. Another sample of Super Artichoke capsules® (Western Medical), which is a commercially available drug, consisting of cynarin at a concentration of 320 mg, was analyzed under the same conditions for comparison with four artichoke extracts.
The results of chlorogenic acid content in different extracts are summarized in Table 1, and are shown in Fig. 1A–E. From the presented results it can be concluded that C. scolymus receptacles extract contain the highest amount of chlorogenic acid followed by bracts extract, then by leaves and roots extracts. Chlorogenic acid present in each of the four extracts was greater than that in the imported extract marketed as Super Artichoke® originated from the leaves which were grown in Germany. Hence, the edible portions of globe artichoke (receptacle and bracts) are rich in chlorogenic acid and cynarin more than the leaves or roots. These results encourage the use of receptacles or bracts as source for the medicinally active constituents namely, cynarin and chlorogenic acid. The extract of C. scolymus bracts was previously reported containing 8.82 g% caffeoylquinic acid, calculated as chlorogenic acid (El Senousy et al., 2012El Senousy, A.S., Seida, A.A., El Tanboul, N.D., Islam, W.T., Eid, H.H., (PhD thesis) 2012. Agro-food industry waste processing and conversion to useful products. Faculty of Pharmacy, Cairo University.).
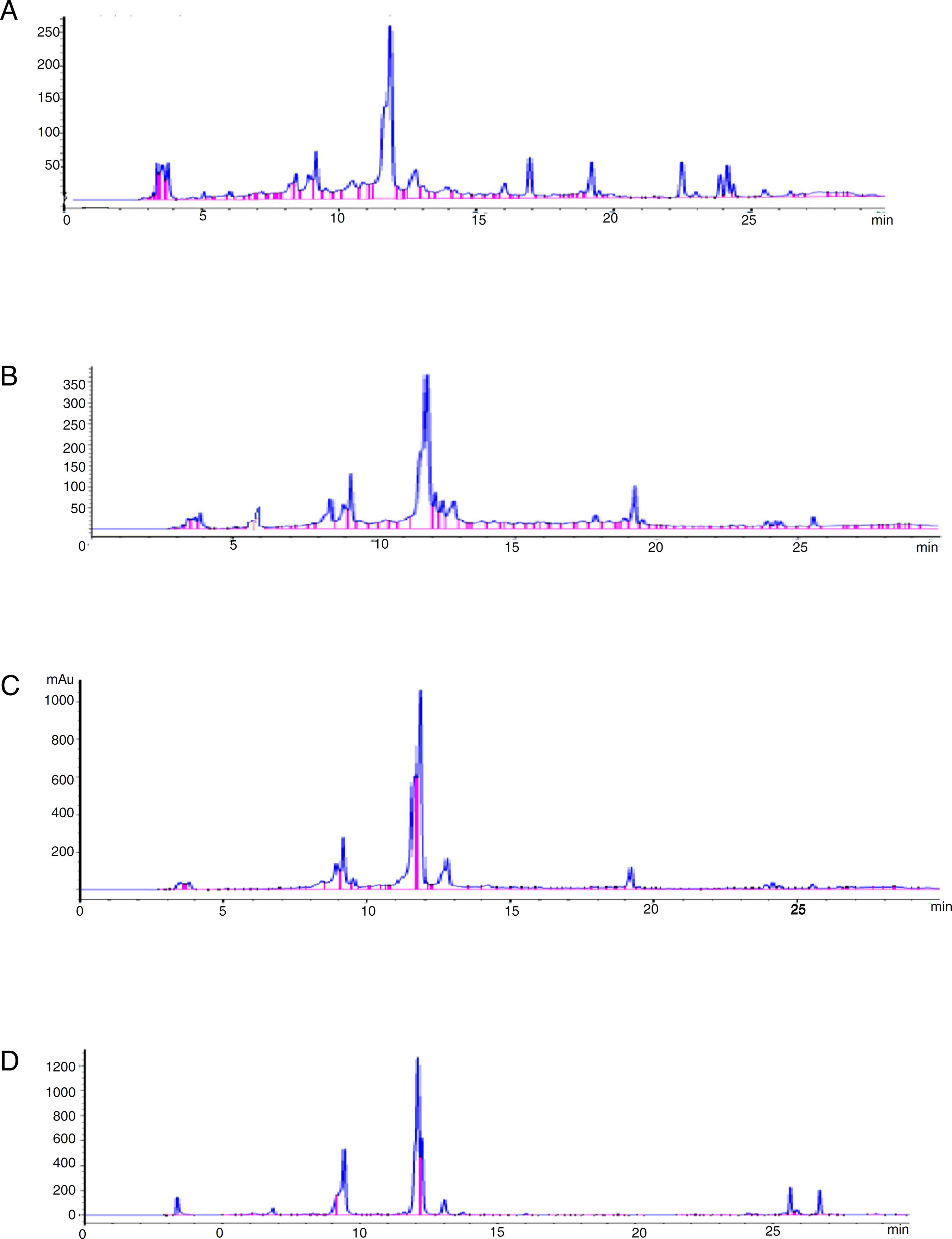
Standardization of alcoholic extracts of (roots, bracts, receptacles and leaves) of Cynara scolymus using chlorogenic acid.
(A–E) HPLC chromatogram of alcoholic extract of globe artichoke roots, leaves, bracts, receptacles and leaf alcoholic extract marketable as super artichoke capsules respectively.
LC-DAD-ESI-MS separation technique
A total of 24 chromatographic peaks belonging to various metabolite classes were derived from examined samples including phenolic acids, flavonoids, sesquiterpenes, fatty acids and saponins (Table 2) (Fig. 2A–D).
Components detected in artichoke extracts (roots, bracts, receptacles and leaves) using HPLC-DAD-ESI-MS.
(A–D) LC-DAD-ESI-MS base peak chromatogram of the artichoke methanol extract of roots, leaves bract and receptacles respectively.
Phenolic acid derivatives
The hydroxycinnamic derivatives detected in this work belong to mono- and di-caffeoylquinic acid compounds. This finding is agreed with the previously found in the literature (Jun et al., 2007Jun, N.J., Jang, K.C., Kim, S.C., Moon, D.Y., Seong, K.C., Kang, K.H., 2007. Radical scavenging activity and content of cynarin (1,3-dicaffeoylquinic acid) in artichoke (Cynara scolymus L.). J. Appl. Biol. Chem. 50, 244-248.; Pinelli et al., 2007Pinelli, P., Agostini, F., Comino, C., Lanteri, S., Portis, E., Romani, A., 2007. Simultaneous quantification of caffeoyl esters and flavonoids in wild and cultivated cardoon leaves. J. Food Chem. 105, 1695-1701.). The peaks number 4 and 6 gave the [M+H]+ ions at m/z 193.1, 353.07 with molecular formula C7H12O6 and C16H15O9 assigned as quinic acid and chlorogenoquinone. Peaks number 9 and 20 gave the [M+H]+ ions at m/z 355.3 in accordance with the molecular formula C16H17O9 and C16H18O9. These compounds have been assigned as chlorogenic acid and neochlorogenic acids (Sánchez et al., 2003Sánchez, F., Jáuregui, O., Raventós, R., Bastida, J., Viladomat, F., Codina, C., 2003. Identification of phenolic compounds in artichoke waste by high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 1008, 57-72.; Moglia et al., 2008Moglia, A., Lanteri, S., Comino, C., Acquadro, A., Vos, R., Beekwilder, J., 2008. Stress-induced biosynthesis of dicaffeoylquinicacids in globe artichoke. J. Agri. Food Chem. 56, 8641-8649.). Peaks number 10 and 11 with a precursor ions at [M+H]+ m/z 171.12 and 517.1188, and identical molecular formula C7H6O5 and C25H23O12, were identified as gallic acid and dicaffeoylquinic acid (cynarin) (Sánchez et al., 2003Sánchez, F., Jáuregui, O., Raventós, R., Bastida, J., Viladomat, F., Codina, C., 2003. Identification of phenolic compounds in artichoke waste by high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 1008, 57-72.; Moglia et al., 2008Moglia, A., Lanteri, S., Comino, C., Acquadro, A., Vos, R., Beekwilder, J., 2008. Stress-induced biosynthesis of dicaffeoylquinicacids in globe artichoke. J. Agri. Food Chem. 56, 8641-8649.).
Flavonoids
Peaks number 5, 7 and 14 showed precursor ions at [M+H]+ m/z 287.04, 449.0 and 595.15 with molecular formula C15H9O6, C21H21O11 and C27H29O15, was proposed to be luteolin, luteolin-O-glycoside and luteolin-7-O-rutinoside (Schutz et al., 2006Schutz, K., Persike, M., Carle, R., Schieber, A., 2006. Characterization and quantification of anthocyanin in selected Cynara scolymus L. cultivars by HPLC. Anal. Bioanal. Chem. 384, 1511-1517.). Peak number 12 showed precursor ion at [M+H]+ m/z 271.24 with molecular formula C15H10O5 was proposed to be apigenin (Schutz et al., 2006Schutz, K., Persike, M., Carle, R., Schieber, A., 2006. Characterization and quantification of anthocyanin in selected Cynara scolymus L. cultivars by HPLC. Anal. Bioanal. Chem. 384, 1511-1517.).
Sesquiterpene glycosides
Two sesquiterpene glycosides were readily identified by high resolution mass analysis at peaks number 21 and 22) with [M+H]+ m/z 429.1958 and 427.1799, respectively, assigned as cynarascoloside A/B and cynarascoloside C (Farag et al., 2013Farag, M., El-Ahmady, S., Elian, F., Wessjohann, L., 2013. Metabolomics driven analysis of artichoke leaf and its commercial products via UHPLC-q-TOF-MS and chemometrics. Phytochemistry 95, 177-187.).
Sesquiterpene lactones
Cynaropicrin showed an [M+H]+ peak at m/z 347.2 corresponding to the molecular formula C19H22O6. Grossheimin showed an [M+H]+peak at m/z 263.3 corresponding to the molecular formula C15H18O4 (Fritsche et al., 2002Fritsche, J., Beindorff, C.M., Dachtler, M., Zhang, H., Lammers, J.G., 2002. Isolation, characterization, and determination of minor artichoke leaf extract compounds. Eur. Food Res. Technol. 215, 149-157.).
Triterpene saponins
Cynarasaponin E, J, C, A/H and F/I were also identified (Farag et al., 2013Farag, M., El-Ahmady, S., Elian, F., Wessjohann, L., 2013. Metabolomics driven analysis of artichoke leaf and its commercial products via UHPLC-q-TOF-MS and chemometrics. Phytochemistry 95, 177-187.).
Amino and fatty acids
Hydroxy-octadecatrienoic acid, dihydroxy-octadecatrienoic acid, hydroxy-oxo-octadecatrienoic acid and tyrosyl-L-leucin were previously reported (Farag et al., 2013Farag, M., El-Ahmady, S., Elian, F., Wessjohann, L., 2013. Metabolomics driven analysis of artichoke leaf and its commercial products via UHPLC-q-TOF-MS and chemometrics. Phytochemistry 95, 177-187.). Peak number 8 was identified as dihydroxy benzoic acid with molecular formula C7H6O4 as it showed a precursor ion at [M+H]+, m/z155.02.
This analysis revealed that polyphenols are present in all parts of globe artichoke but more strenuous in receptacles and bracts. Flavonoids are also present in all parts and more concentrated in bracts. Sesquiterpene lactones are detected in all parts but mainly in the leaves. Amino acids are found in all parts but more concentrated in roots.
Spectrophotometric determination of total polyphenols
Exploration of the amount of polyphenols and their relative distribution in the receptacles, bracts, leaves and roots were performed using Folin-Ciocalteu colorimetric method by means of gallic acid as positive control. Results are tabulated in Table 3. Polyphenols estimation in different parts of artichoke (cultivar balady) has evidenced higher proportion of total polyphenols in the edible receptacles followed by bracts. The medicinally used foliage leaves contain lower proportions of polyphenols in addition roots evidenced the lowest amount.
Results of total phenolic contents of leaves, bracts, receptacle and roots of artichoke (cultivar balady) growing in Egypt.
HPLC analysis and identification of phenolic compounds
The results are in Table 4 and presented in Fig. 3A–D. The presented comparative A study between different parts of Egyptian artichoke revealed variation in the polyphenol content, especially the potent anti-oxidant chlorogenic acid in the receptacles (66,015 µg/100 g), and bracts (30,445 µg/100 g), compared with the medicinally used leaves (13,539 µg/100 g), and unused roots (8522 µg/100 g). Hence, the receptacles contain the highest proportion of chlorogenic acid in all edible parts, followed by bracts and leaves. Caffeic acid was found as a major constituent in the leaves. The total content of all phenolic acids are intense in the receptacles followed by bracts, leaves and roots, respectively.
Phenolic acid content identified by HPLC in different alcoholic extracts of Cynara scolymus growing in Egypt.
(A–D) HPLC chromatogram of phenolic acids determined in artichoke roots, leaves, bracts and receptacles extract respectively.
Nutritional values
Artichoke was found to be rich in fibers, minerals and vitamins as shown in Table 5. Artichoke (medium sized 15 cm length and diameter 8 cm) has evidenced several nutritional health benefits. Every 100 g fresh plant material (receptacles, bracts, leaves and roots) provide the body by 24, 13, 12 and 5.3%RDA (Recommended Dietary Allowances), respectively. Artichoke is low in fats and carbohydrates and evidenced zero content of cholesterol. The edible portions of artichoke are super source of folic acid (Table 5). Every 100 g fresh (receptacles, bracts, leaves and roots) provide the body by folic acid 17, 14.7, 13.7 and 3.3% RDA, respectively. Fresh artichoke contains moderate amounts of the anti-oxidant vitamins C 9.1, 7.2, 6.6 and 5.3% RDA by receptacles, bracts, leaves and roots, respectively. It shows vitamin E as 2.5, 2, 2 and 1.9% RDA by receptacles, bracts, leaves and roots, respectively. Artichoke is one of the very good vegetable sources for vitamin K. Artichoke provides it as 18.5, 15.5, 14.5 and 0.12% RDA by receptacles, bracts, leaves and roots, respectively.
Results of nutritional facts about fresh Cynara scolymus (receptacle, leaf, bract and root).
Artichoke is rich in B-complex group of vitamins such as, pyridoxine (vitamin B6) which is detected as 5.5, 5, 5 and 3.5% RDA by receptacles, bracts, leaves and roots, respectively. Thiamin (vitamin B1) is presented as 5, 3.5, 2.8 and 1.4% RDA by receptacles, bracts, leaves and roots, respectively. Furthermore, riboflavin (vitamin B2) exhibited as 3.6, 2.6, 2.1 and 0.6% RDA by receptacles, bracts, leaves and roots, respectively. Artichoke is rich source of minerals such as copper, calcium, potassium, iron, manganese and phosphorus. Potassium presented as 9.2, 0.6, 0.56 and 0.5% RDA by receptacles, bracts, leaves and roots, respectively. Copper existing as 1.3, 1.2, 1.2 and 1% RDA by receptacles, bracts, leaves and roots, respectively. While iron was represented as 6.3, 5.7, 5.3 and 4% RDA by receptacles, bracts, leaves and roots, respectively. Trace metals such as zinc was detected as 3.3, 2.5, 2.8 and 0.8% RDA from receptacles, bracts, leaves and roots, respectively, where selenium found as 8.7, 8.5, 8.4 and 0.2% RDA by receptacles, bracts, leaves and roots, respectively.
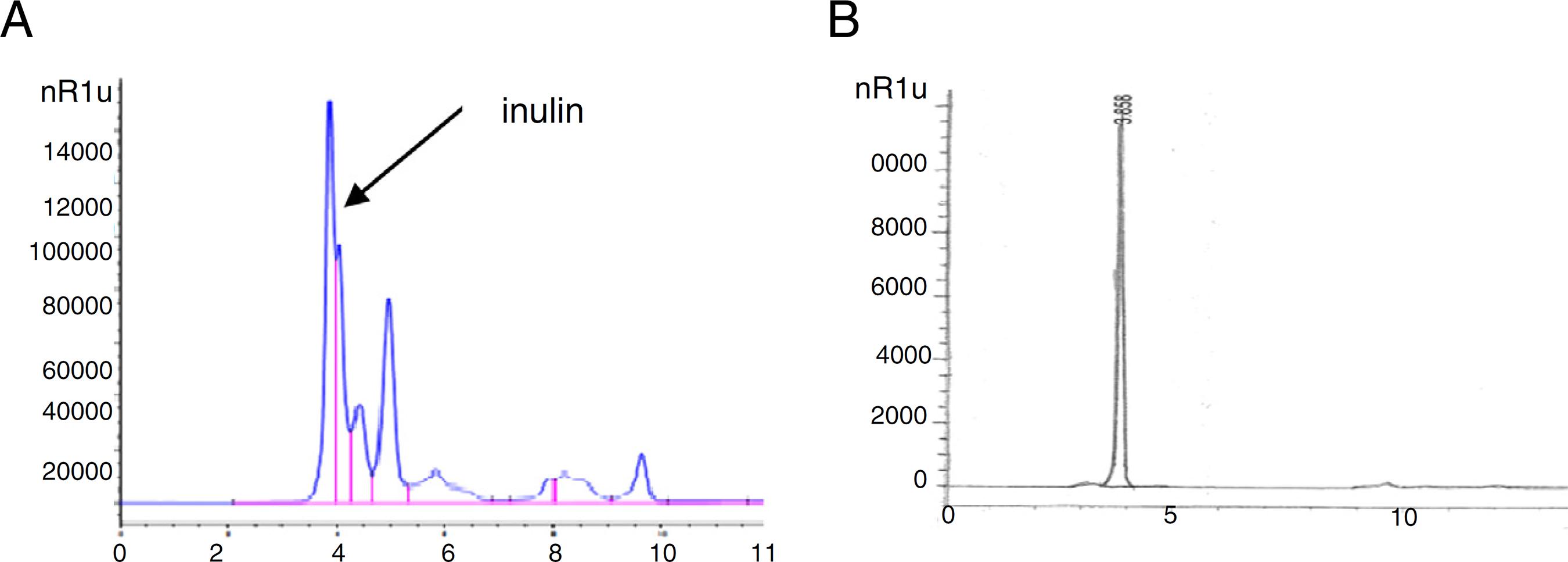
HPLC determination of inulin content
HPLC chromatogram of inulin analysis in the receptacles of globe artichoke (Fig. 4A and B) revealed high content of inulin (41.47 mg/g) dry extract. Inulin decreases the body's ability to make certain kinds of fats.
(A) HPLC chromatogram of inulin analysis in the receptacles of globe artichoke (cultivar balady). (B) HPLC chromatogram of standard inulin.
Antioxidant activity
The DPPH radical-scavenging capacity has been used to determine the free radical scavenging activity of different parts of globe artichoke extracts. Results were presented in Fig. 5, which indicates that all samples tested have noticeable effect on DPPH radical. The demonstrated high anti-oxidant activity in the edible receptacles and bracts are attributed to higher contents of phenolic constituents since a direct proportional association was observed between total phenolic content and activity.
Evaluation of hepatoprotective, hypocholesterolimic and hypolipidemic activities
At a dose of 500 mg/kg/day, the receptacle extract is the most effective extract as hepatoprotective and hypolipidemic agent, comparable to silymarin at the same dose. The results were tabulated in Table 6. Bracts and leaves extract showed similar activity but root extract evidenced the least hepatoprotective and hypolipidemic activities. At a dose of 900 mg/kg/day, receptacle extract elevate the liver enzymes, total cholesterol and triacylglycerides, then to lesser extent, bracts, leaves, and roots extract. The results were represented in Table 7.
Protective effect of Cynara scolymus leaves, receptacles, bracts and roots against CCl4 induced damage of hepatic cells.
Effect of aqueous extracts of artichoke leaves on serum levels of total cholesterol, triacylglycerides and high density lipoprotein in CCl4-intoxicated rats.
Acute intoxication of rat's liver by CCl4 induced significant increases in serum levels of liver enzymes AST, ALT, ALP, and MDA also induced significant decrease in blood GSH when compared to the control group (Table 6). Also, causes significant increase in serum levels of total cholesterol (TC) and triacylglycerides (TG) (Table 7). Artichoke alcoholic extracts of (leaves, bracts, receptacles and roots) significantly at p < 0.05 reduced cholesterol, low density lipoprotein cholesterol (LDL-C), but no change was observed with high density lipoprotein cholesterol (HDL-C).
At a dose of 500 mg/kg/day
The receptacle extract showed most significant reduction in serum AST, ALA, ALP, MDA, total cholesterol and triacylglycerides followed by silymarin, bracts, leaves then roots extracts. GSH was increased in the same sequences. No change in serum HDL, when compared with normal control group.
At a dose of 900 mg/kg/day
The receptacle extract showed the most significant increase in serum AST, followed by bracts, leaves then roots extracts by +28.7, +18.7, +16.8 and +9.3%, respectively. ALT level increased in the same pattern +47.9, +27.6, +26.8 and +15.2%, respectively. ALP level increased in similar mold by +24.7, +14.9, +13.8 and +7.9%, respectively. MDA level was increased in matching model by +71.4, +50, +42.8 and +21.4%, respectively. GSH was decreased with the same sequences by −25, −15.1, −13.42 and −7%, respectively. However, total cholesterol increased in alike outline by +24.8, +12.1, +14.6 and +7.9%, respectively, and serum triacylglycerides increased in same pattern by +24.5, +15.4, +14.2 and +7.4%, respectively, but no change in serum HDL, when compared with normal control group.
At a dose of 500 mg/kg/day+CCl4
The receptacle extract showed the most significant reduction in serum AST, followed by silymarin, bracts, leaves then roots extracts by −18.5, −14.2, −9.3, −8.5 and −5%, respectively. The same for ALT decreased in the similar pattern by −24.1, −18.2, −11.1, −10.4 and −5.9%, respectively. ALP decreased in the alike model by −18.8, −14.8, −9.2, −8.8 and −4.8%, respectively; MDA by −31.2, −23.7, −16.2, −15.6 and 7.5%, respectively. GSH was increased with the same sequences by +57.2, +43.2, +23.6, 21.8 and +7.2%, respectively. Total cholesterol as well as serum triacylglycerides decreased by receptacle extract followed by silymarin, bracts, leaves then roots extracts by −17.5, −13.5, −9.6, −9.1 and −5.5%, and by −20, −15.4, −10.6,− 10 and −4.8%, respectively, but no change in serum HDL, when compared with group receiving CCl4 only.
At a dose of 900 mg/kg/day+CCl4
The receptacle extract showed the most significant increase in serum AST, followed by bracts, leaves then roots extracts by +14.3, +10.3, +9.2 and +4.2%, respectively. ALT level was increased in the same pattern by +20.4, +13.3, +12.8 and 6.3%, respectively. Furthermore, ALP increased in alike example by +16.1, +10.2, +9.7 and 4.5%, respectively; MDA level increased in similar pattern by +25, +16.2, +15 and +7.5%, respectively. GSH level was decreased with the same sequences by −43.2, −28.6, 26.3, and −14.1%, respectively. Total cholesterol and serum triacylglycerides levels increased as a result of receptacle extract followed by bracts, leaves then roots extracts with +39.3, +35.1, +34.8 and +29.8% and +14.3, +9.9, +9.3 and +3.8%, respectively, but no change in serum HDL, when compared with group receiving CCl4 only.
Interpretation of the above results revealed that at a dose of 500 mg/kg/day, the receptacle extract is the most effectual in both hepatoprotective and hypolipidemic activities, comparable to sylimarin at the same dose. Bracts and leaves extract showed similar activity but root extract evidenced least hepatoprotective and hypolipidemic activities.
At a dose of 900 mg/kg/day, receptacle extract elevate the liver enzymes, total cholesterol and triacylglycerides, then to lesser extent, bracts, leaves, and roots extract. The high dose of artichoke extracts is not recommended, it may be due to side effect of polyphenols (Mennen et al., 2005Mennen, L., Walker, R., Pelissero, C., Scalbert, A., 2005. Risks and safety of polyphenol consumption. Am. J. Clin. Nutr. 81, 326S-329S.).
Histopathological finding
Histology of the liver was studied immediately after sacrificing the animals. Analysis of the histopathological sections were presented (Fig. 6A–O). The histopathological examination of liver sections of normal control (Fig. 6-A) rats showed normal histological structure of hepatic lobule with normal hepatocytes and hepatic sinusoids. Injection of CCl4 in rats induced severe fatty degeneration with leukocyte inflammatory cells infiltration, associated with coagulative necrosis of hepatocytes and inflammatory cells surrounding the dilated ventral vein. Liver sections of rats given orally silymarin as a standard drug and artichoke receptacles, bracts, leaves and roots extract at dose of (500 mg/kg/day) revealed almost normal histological architecture of hepatic lobule. The dose (900 mg/kg/day), revealed mild fatty degeneration and necrosis were most seen in group receiving artichoke receptacles extract, then to lesser extent groups receiving bracts, leaves and roots extract, respectively. Artichoke receptacles extract when taken with dose of (500 mg/kg/day) with CCl4, reduce the lesion caused by CCl4 alone more than groups receiving silymarin, then groups receiving bracts and leaves extract with nearly same effect and finally roots extract. Artichoke receptacles extract when taken with dose of (900 mg/kg/day) with CCl4, showed increase in the lesion caused by CCl4 then groups receiving silymarin, followed by groups receiving bracts and leaves extract with nearly same effect and finally roots extract.
(A) Liver cells from rats treated with artichoke extracts at 500 mg/kg/day showing normal structure of liver tissue. (B) Cells treated with 900 mg/kg/day leaf extract showing severe sinusoidal dilatation. (C) Cells treated with receptacle extracts at 900 mg/kg/day showing sinusoidal dilatation. (D) Cells treated with bract extracts at 900 mg/kg/day showing sinusoidal dilatation and extravasation of blood elements. (E) Cells treated with root extracts at 900 mg/kg/day showing intracellular edema of hepatocytes. (F) Cells treated with leaf extracts at 500 mg/kg/day + CCL4 showing slight sinusoidal dilatations. (G) Cells treated with receptable extracts at 500 mg/kg/day + CCL4 showing sinusoidal dilatations. (H) Cells treated with bract extracts at 500 mg/kg/day + CCL4 showing severe sinusoidal dilatations and hemorrhage. (I) Cells treated with root extracts at 500 mg/kg/day + CCL4 showing sinusoidal dilatations and loss of cellular details. (J) Cells treated with leaf extratcts at 900 mg/kg/day + CCL4 showing severe loss of cellular architecture and narrowing of blood sinusoids. (K) Liver cells from rats treated with receptable extracts at 900 mg/kg/day + CCL4 showing severe loss of cellular architecture and narrowing of blood sinusoids. (L) Cells treated with bract extracts at 900 mg/kg/day + CCL4 showing dilatation of blood sinusoids. (M) Cells treated with root extracts at 900 mg/kg/day + CCL4 showing sinusoidal dilatation and increased phagocytic activity. (N) Cells treated with silymarin at 500 g/kg/day + CCL4 showing slight sinusoidal dilatation and increased phagocytic activity. (O) Cells treated with CCL4 showing loss of cellular architecture of hepatocytes and displayed hyper-chromatism of their nuclei.
Discussion
Cynara scolymus is cultivated worldwide for its immature flower heads consisting of receptacles and fleshy bracts where it is served in many food recipes for nutrition value. Our outcomes are in harmony with the result of Lutz et al. (2011)Lutz, M., Henriquez, C., Escobar, M., 2011. Chemical composition and antioxidant properties of mature and baby artichokes (Cynara scolymus L.) raw and cooked. J. Food Comp. Anal. 24, 49-54.; they were found that the moisture content of artichoke heads was 76.6%. Basay and Tokusoglu (2013)Basay, S., Tokusoglu, O., 2013. The proximate composition and quality characteristics in artichoke (Cynara scolymus L.) varieties developed with clonal selection. J. Food Agric. Environ. 11, 584-587. reported that the moisture content of artichoke leaves was in they range of 79.86–83.03%. The highest amount of crude protein was for the bract and the root recorded 6.60 and 3.77 correspondingly. Our finding are in agreement with results of Hosseinzadeh et al. (2013)Hosseinzadeh, M., Shekari, F., Janmohammadi, M., Sabaghnia, N., 2013. Effect of sowing date and fliar application of salicylic acid on forage yields and quality of globe artichoke (Cynara scolymus L.). Annales 8, 50-59. who found that the protein content of artichoke leaves (d.w) was in the range of 8.05–12.35%. However, our results was in disagreement with Lutz et al., 2011Lutz, M., Henriquez, C., Escobar, M., 2011. Chemical composition and antioxidant properties of mature and baby artichokes (Cynara scolymus L.) raw and cooked. J. Food Comp. Anal. 24, 49-54. who found that the protein content of artichoke heads was only 15.96% which is nearly three time as our finding. The ash content for the different organs was non-significant. However, the ash content of the receptacles was nearly as the leaves higher than the other parts. Our results are in conflict with the results of Lutz et al. (2011)Lutz, M., Henriquez, C., Escobar, M., 2011. Chemical composition and antioxidant properties of mature and baby artichokes (Cynara scolymus L.) raw and cooked. J. Food Comp. Anal. 24, 49-54. as percentage of total carbohydrates (d.w) of the receptacles nearly as that of the leaves. Where, the root is the richest organ in carbohydrates.
Cynara scolymus receptacles contain the highest amount of chlorogenic acid followed by bracts, leaves and roots extracts. Chlorogenic acid present in each of the four extracts was greater than that in the imported extract marketed as Super Artichoke® originated from the leaves which were grown in Germany. Our results are in accordance with the results of Wang et al. (2003)Wang, M.F., Simon, J.E., Aviles, I.F., He, K., Zheng, Q.Y., Tadmor, Y., 2003. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.). J. Agric. Food Chem. 51, 601-608. who reported that the total phenolic content of leaves of green globe was in the range of 8.76–9.56%(as chlorogenic acid). Meanwhile, they found that the total phenolic content of leaves of violet variety was 6.806%, which is slightly higher than our results. Hence, the edible portions of globe artichoke (receptacle and bracts) are rich in cynarin more than the leaves or roots. Shen et al. (2010)Shen, Q., Dai, Z., Yanbin, L., 2010. Rapid determination of caffeoylquinic acid derivatives in Cynara scolymus L. byultrafast liquid chromatography/tandem mass spectrometry based on a fused core C18 column. J. Sep. Sci. 33, 3152-3158. reported that the three compounds (5-O-caffeoylquinic acid (chlorogenic acid), 1,3-di-O-caffeoylquinic acid (cynarin) and 1,5-di-O-caffeoylquinic acid) are the major active compounds in artichoke, they are considered as to be responsible for hepatoprotective action. The caffeoylquinic acid with potential health benefits as natural antioxidant. The extract of C. scolymus bracts was previously reported to contain 8.82 g% caffeoylquinic acid, calculated as chlorogenic acid (El Senousy et al., 2012El Senousy, A.S., Seida, A.A., El Tanboul, N.D., Islam, W.T., Eid, H.H., (PhD thesis) 2012. Agro-food industry waste processing and conversion to useful products. Faculty of Pharmacy, Cairo University.). These results encourage the use of receptacles or bracts as source for the medicinally active constituents namely, cynarin and chlorogenic acid.
Metabolite profiling of artichoke heads using HPLC-DAD-ESI-MS is comparable to the foliage leaves in composition. Among the polyphenol group, caffeoylquinic, chlorogenic acids are present in addition to flavonoids. Luteolin and luteolin glycosides and apigenin are important constituents detected in cultivar Baladi and other cultivars abroad (Farag et al., 2014Farag, M., El-Ahmady, S., Elian, F., Wessjohann, L., 2013. Metabolomics driven analysis of artichoke leaf and its commercial products via UHPLC-q-TOF-MS and chemometrics. Phytochemistry 95, 177-187.).
Variation between artichoke samples are not only among polyphenol content but include saponins (cynarosaponin C, A, H, F) and fatty acids (hydroxyl- and dihydroxy-octadecatrienoic acids) which exhibit variable accumulation (Farag et al., 2014Farag, M., El-Ahmady, S., Elian, F., Wessjohann, L., 2013. Metabolomics driven analysis of artichoke leaf and its commercial products via UHPLC-q-TOF-MS and chemometrics. Phytochemistry 95, 177-187.). Our outcome are in agreement with previously published data by (Lutz et al., 2011Lutz, M., Henriquez, C., Escobar, M., 2011. Chemical composition and antioxidant properties of mature and baby artichokes (Cynara scolymus L.) raw and cooked. J. Food Comp. Anal. 24, 49-54.) in which the antioxidant activity of (mature and baby) cooked and raw receptacles was determined on plants grown in Chile and it was 28.46%in mature raw, 34.56% in mature coked, 22.97% in baby raw and 69.91% in baby cooked.
The percentage antioxidant activity of different extracts derived from the head and leaves were as good as the chemical results. Meanwhile, extracts of the receptacle significantly reduced liver enzymes (AST, ALA, ALP, and MDA) as well as total cholesterol and triacylglycerides (LDL) more than other extracts. Heidarian and Soofiniya (2011)Heidarian, E., Soofiniya, Y., 2011. Hypolipidemic and hypocglycemic effects of aerial part of Cynara scolymus in streptozotocin-induced diabetic rats. J. Med. Plants Res. 5, 2717-2723. reported that, the elevated serum triacylglyceride and total cholesterol levels significantly reduced by the oral administration of artichoke (1000 and 2000 mg/kg) in dose dependent manner. This liver protection was supported by the histopathological study performed in rat livers which revealed reduced tissue lesions and almost normal histological architecture when treated with dose 500 mg/kg/day.
Conclusion
A relative study between globe artichoke standard extracts (root, bract, receptacle and leaf) was revealed that the edible parts (receptacle and bracts) contain the highest amount polyphenols especially the potent anti- oxidant, chlorogenic acid. Where, the medicinally used leaves exhibited lower proportion of it followed by roots. These studies advocate using receptacle extract since it exerts most liver protection and cholesterol lowering activity comparable to silymarin. As the histopathological study performed in rat livers also proved our finding and revealed that artichoke extract at dose level 500 mg/kg/day reduced liver tissue lesions when damaged by CCl4 and showed almost normal histological architecture of hepatic lobule. Bract and leaf extract approximately exert the same action, as well as more than root extract. However, higher dose of artichoke (900 mg/kg/day) is unadvised since signs of fatty degenerations and necrosis of liver cells are noticed. So, our recommendation is the use of the edible parts of artichoke as commercial leaf preparation designed for liver protection.
-
Ethical disclosures
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Acknowledgement
The authors are thankful for Mrs. Therese Labib, consultant of plant taxonomy at Ministry of Agriculture, and Dr. Mohamed El-Gibaly, ex-curator of National Research Center Herbarium, Dokki, Giza, for identification of the plant.
References
- AOAC, 2005. Official Method of Analysis of AOAC International, revision 1, 2006, Gaitherburg, Maryland, USA.
- Basay, S., Tokusoglu, O., 2013. The proximate composition and quality characteristics in artichoke (Cynara scolymus L.) varieties developed with clonal selection. J. Food Agric. Environ. 11, 584-587.
- Belfield, A., Goldberg, D.M., 1971. Normal ranges and diagnostic value of serum 5′ nucleotidase and alkaline phosphatase activities in infancy. J. Clin. Chim. Acta 46, 842-846.
- Beutler, E., Olga, D., Barbara, M., 1963. Improved method for determination of blood glutathione. J. Lab. Clin. Med. 61, 882-888.
- Bourgou, S., Ksouri, R., Bellila, A., Skandarani, I., Falleh, H., Marzouk, B., 2008. Phenolic composition and biological activities of Tunisian Nigella sativa L. shoots and roots. C. R. Biol. 331, 48-55.
- Buccolo, G., David, H., 1973. Quantitative determination of serum triacylglycerides by the use of enzymes. J. Clin. Chem. 19, 476-482.
- Burda, S., Oleszek, W., 2001. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 49, 2774-2779.
- Burstein, M., Scholnick, H.R., Monfin, R., 1970. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J. Lipid Res. 11, 585-595.
- Canturk, N.Z., Ozbilim, G., Yenisey, C., 1999. Experimental cirrhosis of liver and cytoprotective effects of alpha tocopherol. East Afr. Med. J. 76, 223-227.
- Drury, R., Wallington, E., 1967. Carleton's Histological Technique, 4th ed. Oxford University Press, London.
- El Senousy, A.S., Seida, A.A., El Tanboul, N.D., Islam, W.T., Eid, H.H., (PhD thesis) 2012. Agro-food industry waste processing and conversion to useful products. Faculty of Pharmacy, Cairo University.
- El Senousy, A.S., Farag, M.A., Al-Mahdy, D.A., Wessjohann, L.A., 2014. Developmental changes in leaf phenolics composition from three artichoke cultivars (Cynara scolymus) as determined via UHPLC-MS and chemometrics. Phytochemistry 108, 67-76.
- Elizabeth, M.L., (PhD thesis) 2008. Dynamics of Liver Disease in Egypt. University of Michigan, USA.
- European Pharmacopoeia, 2006. Council of Europe, France, 4th ed.
- Farag, M., El-Ahmady, S., Elian, F., Wessjohann, L., 2013. Metabolomics driven analysis of artichoke leaf and its commercial products via UHPLC-q-TOF-MS and chemometrics. Phytochemistry 95, 177-187.
- Fritsche, J., Beindorff, C.M., Dachtler, M., Zhang, H., Lammers, J.G., 2002. Isolation, characterization, and determination of minor artichoke leaf extract compounds. Eur. Food Res. Technol. 215, 149-157.
- Gebhardt, R., 2005. Choleretic and anticholestatic activities of flavonoids of artichoke. Acta Hortic. 681, 429-435.
- Gebhardt, R., Fauseel, M., 1997. Antioxidant and hepatoprotective effects of artichoke extract and constituents in cultured rat hepatocytes. Toxicol. In Vitro 11, 669-672.
- Heidarian, E., Soofiniya, Y., 2011. Hypolipidemic and hypocglycemic effects of aerial part of Cynara scolymus in streptozotocin-induced diabetic rats. J. Med. Plants Res. 5, 2717-2723.
- Hosseinzadeh, M., Shekari, F., Janmohammadi, M., Sabaghnia, N., 2013. Effect of sowing date and fliar application of salicylic acid on forage yields and quality of globe artichoke (Cynara scolymus L.). Annales 8, 50-59.
- Jonne, B., Anderson, L.A., Phillipson, D., 2007. Herbal Medicines, 3rd ed. Pharmaceutical Press, London.
- Jun, N.J., Jang, K.C., Kim, S.C., Moon, D.Y., Seong, K.C., Kang, K.H., 2007. Radical scavenging activity and content of cynarin (1,3-dicaffeoylquinic acid) in artichoke (Cynara scolymus L.). J. Appl. Biol. Chem. 50, 244-248.
- Lattanzioa, V., Kroon, P.A., Linsalatac, V., Cardinalic, A., 2009. Globe artichoke: a functional food and source of nutraceutical ingredients. J. Funct. Food 1, 131-144.
- Lutz, M., Henriquez, C., Escobar, M., 2011. Chemical composition and antioxidant properties of mature and baby artichokes (Cynara scolymus L.) raw and cooked. J. Food Comp. Anal. 24, 49-54.
- Mennen, L., Walker, R., Pelissero, C., Scalbert, A., 2005. Risks and safety of polyphenol consumption. Am. J. Clin. Nutr. 81, 326S-329S.
- Moglia, A., Lanteri, S., Comino, C., Acquadro, A., Vos, R., Beekwilder, J., 2008. Stress-induced biosynthesis of dicaffeoylquinicacids in globe artichoke. J. Agri. Food Chem. 56, 8641-8649.
- Natio, H.K., David, J.A., 1984. Laboratory consideration: determination of cholesterol, triglyceride, phospholipid, and other lipids in blood and tissues. Lab. Res. Methods Biol. Med. 10, 1-76.
- Pinelli, P., Agostini, F., Comino, C., Lanteri, S., Portis, E., Romani, A., 2007. Simultaneous quantification of caffeoyl esters and flavonoids in wild and cultivated cardoon leaves. J. Food Chem. 105, 1695-1701.
- Reitman, S., Frankel, S., 1957. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 28, 56-63.
- Ronkart, S.N., Blecker, C.S., Fourmanoir, H., Foungnies, C., Deroanne, C., Van Herek, J.C., Paquot, M., 2007. Isolation and identification of inulooligosaccharides resulting from inulin hydrolysis. Anal. Chim. Acta 604, 81-87.
- Rottenberg, A., Zohary, D., 1996. The wild ancestry of the cultivated artichoke. Genet. Resour. Crop Evol. 43, 53-58.
- Saad, Y., Zakaria, S., Ramzy, I., Raziky, E.L.M., Shaker, M., Elakel, O., Said, W., Wosre, M., Eldaly, M., Abdel Hamid, M., Esmail, G., 2011. Prevalence of occult hepatitis Cin Egyptian patients with non alcoholic fatty liver disease. Open J. Int. Med. 1, 33-37.
- Sánchez, F., Jáuregui, O., Raventós, R., Bastida, J., Viladomat, F., Codina, C., 2003. Identification of phenolic compounds in artichoke waste by high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 1008, 57-72.
- Schutz, K., Persike, M., Carle, R., Schieber, A., 2006. Characterization and quantification of anthocyanin in selected Cynara scolymus L. cultivars by HPLC. Anal. Bioanal. Chem. 384, 1511-1517.
- Shen, Q., Dai, Z., Yanbin, L., 2010. Rapid determination of caffeoylquinic acid derivatives in Cynara scolymus L. byultrafast liquid chromatography/tandem mass spectrometry based on a fused core C18 column. J. Sep. Sci. 33, 3152-3158.
- Singleton, V.L., Rossi, A.J., 1965. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Viticult. 16, 144-158.
- Strickland, G.T., 2006. Liver disease in Egypt: hepatitis C superseded schistosomiasis as a result of iatrogenic and biological factors. Hepatology 43, 915-922.
- Uchiyama, M., Mihara, M., 1978. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 86, 271-278.
- Wagenbreth, D., Eich, J., 2005. Pharmaceutically relevant phenolic constituents in artichoke leaves are useful for chemical classification of accessions. Acta Hortic. 681, 469-474.
- Wang, M.F., Simon, J.E., Aviles, I.F., He, K., Zheng, Q.Y., Tadmor, Y., 2003. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.). J. Agric. Food Chem. 51, 601-608.
Publication Dates
-
Publication in this collection
Mar-Apr 2018
History
-
Received
14 Sept 2017 -
Accepted
5 Jan 2018 -
Published
15 Feb 2018