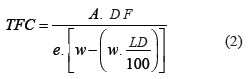Abstract
Tea from Phyllanthus niruri L., Phyllanthaceae, aerial parts is commonly used by Brazilian folk medicine for its benefits on the treatment of genitourinary disorders, for what the polyphenolic compounds are mainly responsible. The yield of such compounds may be influenced by several variables related with the plant growth. The effects of planting periods and harvesting conditions are investigated in this work, including four different seasons. The cultivation was characterized by dry mass yield of aerial parts, and the effect of pruning was analyzed. Leaves, stems and their mixtures were analyzed after drying and milling. Loss on drying and water soluble extractives were used as physical parameters for quality control. Flavonoid content and gallic acid were chosen as chemical markers for this work. The spectrophotometric trial based on the aluminum chloride complexes was applied to evaluate the total flavonoids content. Gallic acid contents were measured from the water extractive solutions by high-performance liquid chromatography. The pruning caused a positive influence on the amount of leaves and stems. The highest flavonoids and gallic acid contents were found in the leaves, which were developed over the summer and the winter, respectively, both from the second harvesting (after pruning). Chomatographic profile by HPLC was characterizes by the presence of gallic acid and two other major peaks (not identified substances), which relation was peculiar to each aerial part. In conclusion, these results suggest that even under less favorable climatic conditions, in winter, the pruning seems to cause a strong influence over the P. niruri polyphenolics production. Indeed, the total flavonoids content, as well as the HPLC profile, can be used as indicative parameters of the ratio of leaves and stem in the vegetal raw material.
Flavonoids; gallic acid; herbal drug; pruning; seasonality; Phyllanthus niruri
Chemical and technological evaluation of the Phyllanthus niruri aerial parts as a function of cultivation and harvesting conditions
Angélica G. CoutoI; Marli L. KunzlerII; Bárbara SpaniolIII; Pedro M. MagalhãesIV; George G. OrtegaV; Pedro R. PetrovickV
IPrograma de Pós-graduação em Ciências Farmacêuticas, Universidade do Vale do Itajaí, Brazil
IIFaculdade de Farmácia, Universidade Federal do Rio Grande do Sul, Brazil
IIICurso de Farmácia, Universidade Feevale, Brazil
IVDivisão de Agrotecnologia, Centro Pluridisciplinar de Pesquisas Química Biológicas e Agronômicas, Universidade de Campinas, Brazil
VPrograma de Pós-graduação em Ciências Farmacêuticas, Faculdade de Farmácia, Universidade Federal do Rio Grande do Sul, Brazil
Correspondence Correspondence: Angélica G. Couto Programa de Pós-graduação em Ciências Farmacêuticas, Universidade do Vale do Itajaí Rua Uruguai 458; 88302-202 Itajaí-SC, Brazil angelica@univali.br Tel: + 55 47 9967 7517 Fax: +55 47 3341 7744
ABSTRACT
Tea from Phyllanthus niruri L., Phyllanthaceae, aerial parts is commonly used by Brazilian folk medicine for its benefits on the treatment of genitourinary disorders, for what the polyphenolic compounds are mainly responsible. The yield of such compounds may be influenced by several variables related with the plant growth. The effects of planting periods and harvesting conditions are investigated in this work, including four different seasons. The cultivation was characterized by dry mass yield of aerial parts, and the effect of pruning was analyzed. Leaves, stems and their mixtures were analyzed after drying and milling. Loss on drying and water soluble extractives were used as physical parameters for quality control. Flavonoid content and gallic acid were chosen as chemical markers for this work. The spectrophotometric trial based on the aluminum chloride complexes was applied to evaluate the total flavonoids content. Gallic acid contents were measured from the water extractive solutions by high-performance liquid chromatography. The pruning caused a positive influence on the amount of leaves and stems. The highest flavonoids and gallic acid contents were found in the leaves, which were developed over the summer and the winter, respectively, both from the second harvesting (after pruning). Chomatographic profile by HPLC was characterizes by the presence of gallic acid and two other major peaks (not identified substances), which relation was peculiar to each aerial part. In conclusion, these results suggest that even under less favorable climatic conditions, in winter, the pruning seems to cause a strong influence over the P. niruri polyphenolics production. Indeed, the total flavonoids content, as well as the HPLC profile, can be used as indicative parameters of the ratio of leaves and stem in the vegetal raw material.
Keywords: Flavonoids; gallic acid; herbal drug; pruning; seasonality; Phyllanthus niruri
Introduction
The technological development research of pharmaceutical forms containing herbal raw materials has shown clear growth in these last decades (Calixto, 2000; Simões & Schenkel, 2001; Bassani et al., 2005; Calixto, 2005). Such as in many European countries, supported by World Health Organization normatives (WHO, 1999; Marques & Petrovick, 2003), the Brazilian legislation regulates the criteria of quality for these medicines (Anvisa, 2010a).
Phyllanthus niruri L., Phyllanthaceae, is an annual herb, widely distributed both in tropical and subtropical countries, and represents a promising medicinal plant to phytopharmaceutical development, considering pharmacological evidence for many popular uses, beyond the phytochemical (Calixto et al., 1998), botanical (Garcia et al., 2004) and agronomic (Magalhães, 1997) studies. In Brazil, its main application is centered in the treatment of the related genitourinary disorders for the elimination of kidney stones (Barros et al., 2006).
The quality of herbal raw materials is decisive to reach the desired specifications of intermediate and final products in the phytopharmaceutical production (List & Schmidt, 1989; Sharapin, 2000). Official pharmaceutical specifications for raw material of medicinal plants represent an important step for the establishment of minimum criteria of quality acceptance. With this goal, the Brazilian Pharmacopoea published the monograph of Phyllanthus niruri (Farmacopeia Brasileira, 2003), describing the aerial parts as the official drug.
However, it is important to take into account the biological variability as a consequence of extrinsic factors, such as environment, light, temperature, wind, soil conditions and agronomic practices, which are responsible for causing peculiar physiological responses to each species, reflecting in the variation of its chemical constitution, which could be related to its therapeutic activity (Evans, 1996). Therefore, the knowledge of how much these factors influence on the characteristics of pharmaceutical quality, is crucial for acceptance criteria adoption of herbal species as raw material for phytopharmaceuticals.
The medicinal plants cultivation should be always prioritized, since it allows the control of the agronomic conditions, assures the continuous supply and avoids the adulteration of herbal species in comparison to the extractivism (Magalhães, 1997; Ferreira, 1998). In this sense, the analysis of planting and harvesting variables, such as the climatic conditions in the growing period, as well as the plant growth techniques, represents a strategy to establish the allowed variations as acceptance limits of some quality parameters for herbal drugs (Farias, 2003).
Therefore, the present work aimed to contribute for the establishment of criteria for the acceptance of the quality of P. niruri as pharmaceutical raw material, through the study of the factors related to the chemical variability of the cultivated plants, such as the planting and harvesting period, and the influence of harvesting conditions, especially of the pruning.
Material and Methods
Plant materials
The aerial parts of Phyllanthus niruri L., Phyllanthaceae, were collected from an experimental field of the Chemical, Biological and Agricultural Pluridisciplinary Research Center (22°45′40″S and 47°9′15″W) (Paulinia-SP, Brazil). A voucher specimen (number ICN 111765) of P. niruri is deposited in the herbarium of Botanical Department of Federal University of Rio Grande do Sul, Brazil.
Four plantings were carried out in April, May and October, 2002, and in May, 2003. The plants were kept for two months in a vegetation house, for their development from the seeds, followed by their transference for the plantation in the field. For each planting, two subsequent harvests were carried out, intercalated by the period of about three months, corresponding to the complete plant development in the field (Table 1). The aerial parts were completely removed in the first harvest, following a second harvest after the development of new aerial parts. For each harvesting, the leaves and stems were analyzed, separately, as well as the mixtures of the aerial parts.
After harvesting and drying in an air oven at 40 oC, the aerial vegetal parts were sent for the present study. The samples were treated separately, initially for the selection and elimination of strange materials, additional dried in the air oven at 40 oC, if the loss on drying of herbal material was greater than 10%. Each aerial part was separately reduced in a knife mill (1.0 mm outlet sieve). The corresponding ratios of the dry mass of leaves and stems for each harvesting were used for composition of the mixtures of the aerial parts.
Dry mass yield and loss on drying
The productivity of leaves and stems from each harvesting was calculated from the dry mass and analyzed as the percentage in relation to the aerial parts and for unit of plant. The loss on drying of leaves, stems and their mixtures were obtained gravimetrically in an oven at 105 oC (Anvisa, 2010b), using samples of 500 mg.
Water soluble extractives (Bundesvereinigung, 1986a)
About 1.0 g of each sample, accurately weighed, was submitted to the extraction with 100.0 g of water, under decoction, over 10 min. After cooling, the weigh was reconstituted and filtered, discharging the first 20 mL. In weighting-filters (i.d. 27 mm x 55 mm), previously desiccated, 20.0 g of the solution were evaporated to the dryness in water bath. The solid residues were desiccated in an oven at 100±5 oC, initially for 3 h, transferred to a desiccator, where they were kept per 20 min, for the cooling, before weighing. This sequence was repeated until constant weight, however with period of 1 h in the oven dryer.
The results were expressed as the average of 3 determinations, with 4 repetitions, according the equation 1,
where, WSE=water soluble extractives (%, w/w); g=dry residue weight (g); DF=dilution factor (5); w=sample weight (g) and LD=loss on drying (%, w/w).
Total flavonoids content assay
Total flavonoids content (TFC, g%) was determined from 400 mg of each sample, according to a validated method (Soares et al., 2003) from the Medicine Code German (Bundesvereinigung, 1986b). The absorbance was read in the Hewlett-Packard 8452A spectrophometer in 420 nm, and TFC was calculated according equation 2.
where, A=absorbance at 420 nm (AUFS); DF=dilution factor (625); w=sample weight (g); LD=loss on drying (%, w/w); e=specific absorption of the AlCl3-quercetin complex. The results were expressed as an average of 3 determinations with 3 repetitions.
HPLC assay (De Souza et al., 2002)
The analysis was carried out using a Shimadzu (Kyoto, Japan) liquid chromatograph equipped with a pump (LC-10AD), a gradient controller (FCV-10AL), an auto sampler (SIL-10A) and a UV/VIS detector (SPD-10A), controlled by CLASS LC-10 software. The column was a RP-18 LiChrospher 250 x 4 mm i.d., 5 μm particle diameter (Merck, Darmstadt, Germany). A pre-column Shimadzu (10 x 4 mm i.d.) packed with Bondapak C18 125 Å (Waters, Milford, USA) was employed.
Acetonitrile (HPLC grade, Merck, Darmstadt, Germany), phosphoric acid (Merck, Darmstadt, Germany) and ultrapure water from Milli-Q system (Millipore, Bedford, USA) with resistivity above 18.0 MΩ•cm were used for the mobile phase preparation. Gallic acid (R. Ph. Eur., Merck, Darmstadt, Germany) was used as external standard.
The chromatographic separation was carried out using a mobile phase with phosphoric acid 1% (w/w) as solvent A and acetonitrile:phosphoric acid 1% (w/w) (50:50 (v/v)) as solvent B at a flow rate of 0.6 mL•min-1. The gradient program was as follows: 22-24% B (7 min), 24-40% B (10 min), 40-100% B (8 min), 100-22%B (15 min). The injection volume was 20 μL. The peaks were detected at 275 nm and were identified by comparison of the retention time with standard gallic acid.
Preparation of plant extracts
Leaves (L), stems (S), as well as leaves plus stems (L+S) were taken as starting material for extraction. The aqueous extracts (7.5% w/v) were prepared by adding boiling water to the ground plant and keeping the mixture at decoction for 15 min under reflux. After cooling, the extract was separated from the extraction residue by pressing (Hafico 5L, Germany) and then clarified by filtration under vacuum.
Each extractive solution was firstly diluted in distilled water and after in acetonitrile : water (20:80 v/v) in order to produce concentrations of 4.8 µg/mL for leaves and leaves plus stems samples and 40 µg/mL for stems samples, according previous work (Lionço et al., 2001). Each sample was injected three times. Equation 3 was employed to calculate the gallic acid concentration (GAC) in the raw materials.
where, Cam=gallic acid concentration in the sample (mg/mL), obtained from the calibration curve data, DF=dilution factor=20833 for leaves and leaves plus stems samples and 5000 for stems samples, w=sample weight (g), LD=loss on drying (%, w/w).
Gallic acid calibration curve
Gallic acid was dissolved in acetonitrile:water (20:80 v/v) to produce concentrations of 0.2, 0.4, 0.8, 1.2 and 1.6 μg•mL-1. The samples were filtered through 0.45 μm membrane (Millipore, Bedford, USA) prior to injection. Each analysis was repeated three times and the calibration curves were fitted by linear regression.
Statistical analysis
The results were evaluated by ANOVA, Tukey multi-comparison test (p=0.05) and linear correlation analysis (Snedecor & Cochran, 1989).
Results and Discussion
The productivity of dry mass, which was calculated both for the plants developed after planting (first harvesting) or after pruning (second harvesting), had demonstrated that the percentage of leaves tended to be higher than stems (Table 1), and the ratio between the leaves and stem amount belonging to the same plant varied from 1.03 to 1.67 for the first harvest and from 2.29 to 3.51 for the second one, suggesting that pruning causes a stimulant effect for the aerial parts development. This effect was especially observed for stems which amount per unit of plant suffered an increase by up to five times.
After drying and milling, the loss on drying was determined for both leaves and stems, as well as for their mixture powders. Loss on drying results approached the acceptable range for the raw vegetal material preservation and storage (Zhi-Chen, 1980), with a general average of 7.72%. These results varied from a minimal of 5.95% for stems to 9.97% for leaves. Although both leaves and stems were dried as follow by the same protocol, in general, the residual moisture of the leaves were higher than stems, suggesting the influence of peculiar anatomical and hygroscopic properties of aerial parts especially of the leaves.
Approximately, the loss on drying of the mixtures of aerial parts reflected the loss on drying of leaves and stems, so that estimated and obtained results differed only at 8.1%. This amplitude is probably due to the residual moisture oscillation that solid particles normally suffer after milling, in order to restore the equilibrium with the relative humidity of the air.
The water soluble extractives (WSE) represent one of the criteria of characterization that allows comparative evaluation among vegetable raw materials. In the present work, the WSE of leaves was about 45% higher than stems (Table 2). Among the batches of stems it was observed more variation (14.99%), in comparison to the leaves (5.20%), indicating the increased sensitivity of stems to the variables associated to the different harvestings and growing seasons.
However, none of the studied variables was preponderant for both aerial parts, signifying that neither the climatic factors nor the pruning influenced on water soluble extractives (Table 2).
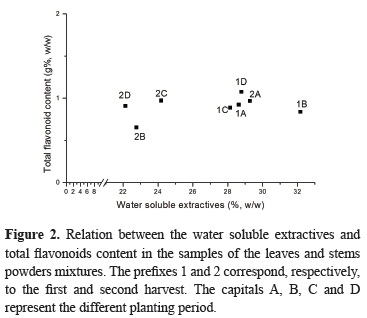
In the mixtures of aerial parts, there was only a correlation tendency between the WSE and the leaves proportion. However, the results indicated that only when the proportion of leaves is over 60%, the WSE is higher than the general average (Figure 1).
The total flavonoids content (TFC) results suggested that the aerial parts development after pruning (second harvesting) caused a positive effect on this response, independent from the season (Tables 1 and 2). This effect was highlighted in the leaves, as it was expected, since they have the highest TFC.
In the mixtures of aerial parts, there was not a linear correlation between the leaves proportion and TFC, since the most important factor was not the quantity, but the quality in terms of flavonoids content, which probably suffered more influence of the pruning effect associated with the growing season. Therefore, the analysis can be conducted by comparison of experimental (Table 2) and estimated results, taking into account the proportion of leaves and stems (Table 1) and TFC of each of them. This analysis allows observing that estimated and experimental results are in agreement, and shows that TFC determination in mixtures can be used to infer the major aerial part component, especially when the raw material is purchased as a mixture.
According the Tukey multiple comparison test analysis (Table 2), the highest TFC was obtained in the leaves that grew during the summer and after pruning (second harvest) (2C) (Tables 1 and 2). In general, all the samples collected in the second harvest showed the greatest TFC. Among the other ones, the unique leaves sample (1C) which approached to the greatest TFC, originated from the same planting which gave the greater TFC (2C). In the stems an opposite behavior was observed, as it means, from the first to the second harvest it was observed a reduction in the TFC in these samples. Besides, no statistical correlation was verified between TFC and WSE for the mixtures of aerial parts (Figure 2). These results probably mean that the variation on the qualitative and quantitative chemical composition of leaves, where the WSE are more expressive, is not only related to the flavonoids content. The involved climatic factors in the period of the plant development, the cultivation technique, as well the time of harvesting and age of the plant (Table 1), probably influenced on the herb chemical composition.
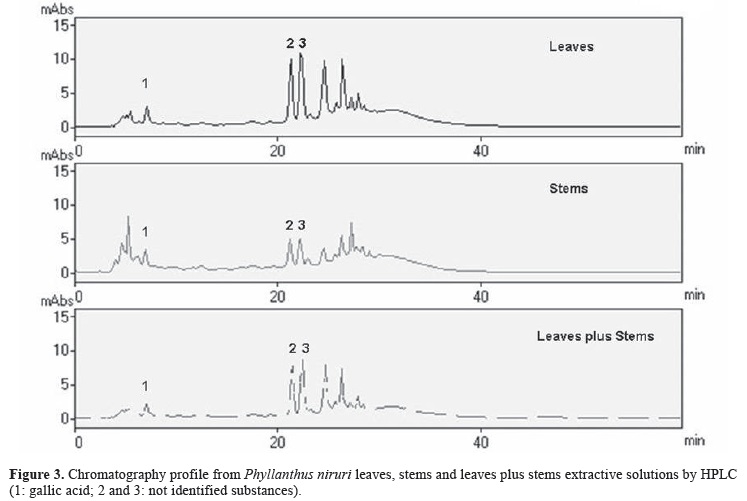
In the present work, the HPLC profile of the aqueous solutions of leaves and stems of P. niruri was reproduced, in accordance with previous work (Lionço et al., 2001), that identified quantitative and qualitative differences in the chromatogram of them (De Souza et al., 2002). In all samples gallic acid (peak 1), and other peaks (peak 2 and 3) were obtained in the chromatogram region between 20 and 30 min, which can be named as phenolic region (Figure 3).
Gallic acid concentration (GAC) was quantified in the same samples to evaluate the influence of the cultivation variables on this chemical marker (Table 2). The analysis of the variation of the gallic acid concentration also showed the positive influence of pruning in the majority of the plantings. The highest concentrations occurred in leaves that had grown over the winter (2A). However, in another planting (D), the second highest concentration happened in the leaves that had grown predominantly over the spring (2D). The interventions of pruning and, with lesser extent, the winter season, probably were decisive on this response.
Differently from the TFC in the mixtures (Table 2), there was not the same correspondence behavior between the estimated values and the experimental ones with relation to the gallic acid quantification. This fact might be related to heterogeneity of the samples, when they occur as a mixture of leaves and stems, which have different size distribution and density. This kind of problem increases the need of sample leaves and stems separately in order to make up a sample of the mixture. In the present work, this problem was evidenced only by gallic acid quantification, probably due to the most sensitivity of analytical technique employed.
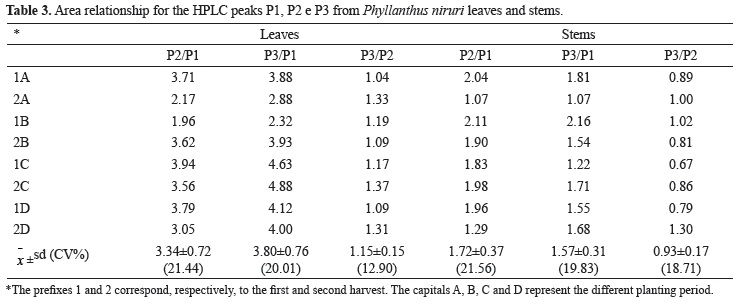
Besides the gallic acid quantification, the chromatographic profile of leaves and stem was also analyzed as a qualitative analysis, through the relation among the peak areas of peak 2 and 3 with peak 1 (gallic acid) (Table 3).
In the leaves, the major substance is referring to the peak 3, and in the stems, the same one mentions to the peak 2. Moreover, the gallic acid concentration and other substances (P2 and P3) are much higher in leaves, in agreement to the results from another vegetal batch (Lionço et al., 2001).
This information can be useful in the characterization of the mixtures of the aerial parts, recognized vegetal raw material according to the Brazilian Pharmacopea (Farmacopeia Brasileira, 2003), in order to indicate which vegetal part is present in higher ratio.
The different relations between area peaks (P2/GA; P2 and P3/GA), in leaves and stem had been reflected in their mixtures (Table 4). Although qualitative analysis must be used only to identified compounds, as gallic acid, it was observed a peculiar relation P3/P2 (Table 4) for stems chromatographic profile, which knowledge can assist in the identification of harmful alterations on the homogeneity of the botanical and chemical compositions of the vegetal raw material and in the optimization of the extraction process.
With the aim to identify the most favorable conditions in terms of the studied parameters, an analysis on the behavior of all joint was carried out centered on the characteristics of leaves, since the same ones represent the more abundant vegetal part, where WSE, TFC and the GA is much more expressive in relation to stems.
It is considered that the WSE are a sufficiently important parameter in this evaluation, however only of qualitative nature, therefore WSE presented poor discriminative power among the batches, since the observed differences had not been of same magnitude in relation to the variation of TFC and GAC.
Among all the samples, the water soluble extractives were much similar, independent of the season, pruning or plant age. Taking into account the inexistent correlation between the TFC and the GAC, it seems that the factors that interfere on the TFC are not the same ones that act on the gallic acid concentration. The samples 2A, 2C e 2D expressed the greatest TFC and GA. These results indicate that pruning caused a positive influence, independently of the plant age or its growing season.
The pruning was the common factor among the samples whose had the same age (A x C and B x D) or the predominant growing season (B x D), even when only the season was different (A x C).
The water soluble extractives from stems were more sensible to the studied factors related to the planting and harvesting variations. The presence of stem causes a diminutive effect on the mixtures, especially when they represent more than 40% of the total content. The presence of leaves, overall above of 60%, in the vegetal raw material, destined to the water extraction, is determinative in the attainment of extractive solutions with bigger dry residue, and, consequently, of products with higher yield mass when resulted from a drying process.
Beyond the flavonoids and gallic acid accumulation in leaves, the results suggest that even in less favorable climatic conditions, as in the winter, the pruning seems to cause a strong influence on the production of these chemical markers.
In the majority of the analyzed batches, the growth of the plant from the pruning, caused positive effect on flavonoids and gallic acid, being more prominent the effect on the total flavonoids content.
The highest total flavonoids content was proceeding from second harvest of leaves whose had grown during the summer, while the gallic acid concentration was bigger in the leaves whose development gave predominantly during the winter. Indeed, the TFC, as well as the HPLC profile, can be used as indicative parameters of the ratio of leaves and stem in the vegetal raw material.
Acknowledgements
The authors thank the Brazilian Agencies CAPES and CNPq for financial support and scholarship grants (three first authors).
Received 30 Jun 2012
Accepted 29 Sep 2012
- Anvisa 2010a. Agência Nacional de Vigilância Sanitária, Ministério da Saúde. Resolução de Diretoria Colegiada nº 14 de 31 de março de 2010. Dispõe sobre o registro de medicamentos fitoterápicos. DOU, 5 April 2010.
- Anvisa 2010b. Agência nacional de Vigilância Sanitária, Ministério da Saúde. Farmacopeia Brasileira http://www.anvisa.gov.br/hotsite/cd_farmacopeia/index.htm, accessed Jun 2012.
- Barros ME, Lima R, Mercuri LP, Matos JR, Schor N, Boim MA 2006. Effect of extract of Phyllanthus niruri on crystal deposition in experimental urolithiasis. Urol Res 34: 351-357.
- Bassani VL, González OG, Petrovick PR 2005. Desenvolvimento tecnológico de produtos fitoterápicos. Fitos 1: 14-17.
- Bundesvereinigung Deutscher Apothekerverbände (Hrsg.) 1986a. Deutscher Arzneimittel-Codex. Frankfurt: Govi; Stuttgart: Deutscher Apotheker, 1986. v. 1: Codex-Probe 4, 9.
- Bundesvereinigung Deutscher Apothekerverbände (Hrsg.) 1986b. Deutscher Arzneimittel-Codex. Govi; Stuttgart: Deutscher Apotheker, 1986. v. 2: Holunderblüten, p. 1-3.
- Calixto JB 2000. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Braz J Med Biol Res 33: 179-189.
- Calixto JB 2005. Twenty-five years of research on medicinal plants in Latin America: a personal view. J Ethnopharmacol 100: 131-134.
- Calixto JB, Santos ARS, Filho VC, Yunes RA 1998. A review of the plants of the genus Phyllanthus: their chemistry, pharmacology, and therapeutic potential. Med Res Rev 18: 225-258.
- De Souza TP, Holzschuh MH, Lionço MI, Ortega GG, Petrovick PR 2002. Validation of a LC method for the analysis of phenolic compounds from aqueous extract of Phyllanthus niruri aerial parts. J Pharm Biomed Anal 30: 351-356.
- Evans WC 1996. Trease and Evan's Pharmacognosy London: W. B. Saunders.
- Farias MR 2003. Avaliação da qualidade de matérias-primas vegetais. In Simões, CMO, Schenkel EP, Gosmann G, Mello CP, Mentz LA, Petrovick PR (Eds.) Farmacognosia: da planta ao medicamento. Porto Alegre: UFRGS, Rio Florianópolis: UFSC, p. 263-288.
- Farmacopéia Brasileira 2003. Atheneu: São Paulo, Brasil. Part 2, Fasc. 5.
- Ferreira SH 1998. Medicamentos a partir de plantas medicinais no Brasil, Rio de Janeiro: Academia Brasileira de Ciências.
- Garcia CM, Zanetti GD, Zago AM, Bittencourt CF, Heinzmann BM 2004. Estudo morfo-anatômico de Phyllanthus niruri L. e Phylllanthus tenellus Roxb. Acta Farm Bonaer 23: 67-70.
- Lionço MI, De Souza TP, Petrovick PR 2001. Avaliação comparativa de polifenóis presentes nas partes morfológicas de Phyllanthus niruri. Cad Farmacia 17: 117-120.
- List PH, Schmidt PC 1989. Phytopharmaceutical Technology, London: Heyden.
- Magalhães PM 1997. O caminho medicinal das plantas, Campinas: Unicamp, p. 98-100.
- Marques LC, Petrovick PR 2003. Evolução da normatização de fitoterápicos no Brasil ao longo dos tempos. In Simões CMO, Schenkel EP, Gosmann G, Mello, JCP, Mentz LA, Petrovick PR (Eds.) Farmacognosia: da planta ao medicamento. Porto Alegre: UFRGS, Florianópolis: UFSC, p. 327-369.
- Sharapin N 2000. Fundamentos de tecnologia de produtos fitoterápicos. Santafé de Bogotá: Quebecor-Impreandes.
- Simões CMO, Schenkel EP 2001. A pesquisa e a produção de medicamentos a partir de plantas medicinais. Nexus Ciência & Tecnologia Florianópolis 1: 24-27.
- Snedecor GW, Cochran WG 1989. Statistical Methods, Ames: University Press.
- Soares LAL, Bassani VL, Ortega GG, Petrovick PR 2003. Total flavonoids determination for the quality control of aqueous extractives from Phyllanthus niruri. Lat Am J Pharm 22: 203-207.
- World Health Organization 1999. WHO Monographs on Selected Medicinal Plants. WHO: Geneva, v. 1.
- Zhi-Chen L 1980. General Control Methods for Vegetable Drugs, WHO: Geneva.
Publication Dates
-
Publication in this collection
01 Feb 2013 -
Date of issue
Feb 2013
History
-
Received
30 June 2012 -
Accepted
29 Sept 2012