Abstracts
The present study aimed to determine whether measles virus can induce apoptosis in murine neuroblastoma cells and the behavior of these cells under acute infection with measles virus or persistent infection with canine distemper virus upon treatment with etoposide. Measles virus induced necrosis in murine neuroblastoma cells. Canine distemper virus-persistent infection did not alter murine neuroblastoma cells behavior when treated with etoposide.
apoptosis; measles virus; canine distemper virus; murine neuroblastoma cells
O presente trabalho foi realizado tendo como objetivo determinar se o vírus de sarampo induz apoptose em células de neuroblastoma murino e avaliar o comportamento de células de neuroblastoma murino agudamente infectadas com vírus do sarampo ou persistentemente infectadas com o vírus da cinomose canina quando tratadas com etoposídeo. A infecção pelo vírus de sarampo induziu principalmente necrose em células de neuroblastoma murino. A infecção persistente pelo vírus de cinomose canina não alterou o comportamento de células de neuroblastoma murino tratadas com etoposídeo.
apoptose; vírus do sarampo; vírus da cinomose canina; célula de neuroblastoma murino
Etoposide-induced apoptosis in murine neuroblastoma (N2A) cells infected with Paramyxoviruses
[Apoptose induzida por etoposídeo em células de neuroblastoma murino (N2A) infectadas por paramixovírus]
L. MoroI; A.C. VasconcelosI; A.S. MartinsII
IDepartamento de Patologia Geral, ICB/UFMG Avenida Antônio Carlos, 6627 30270-901, Belo Horizonte, MG
IIDepartamento de Fisiologia e Biofísica, ICB/UFMG
Correspondence to Correspondence L. Moro E-mail: moro@icb.ufmg.br
ABSTRACT
The present study aimed to determine whether measles virus can induce apoptosis in murine neuroblastoma cells and the behavior of these cells under acute infection with measles virus or persistent infection with canine distemper virus upon treatment with etoposide. Measles virus induced necrosis in murine neuroblastoma cells. Canine distemper virus-persistent infection did not alter murine neuroblastoma cells behavior when treated with etoposide.
Keywords: apoptosis, measles virus, canine distemper virus, murine neuroblastoma cells
RESUMO
O presente trabalho foi realizado tendo como objetivo determinar se o vírus de sarampo induz apoptose em células de neuroblastoma murino e avaliar o comportamento de células de neuroblastoma murino agudamente infectadas com vírus do sarampo ou persistentemente infectadas com o vírus da cinomose canina quando tratadas com etoposídeo. A infecção pelo vírus de sarampo induziu principalmente necrose em células de neuroblastoma murino. A infecção persistente pelo vírus de cinomose canina não alterou o comportamento de células de neuroblastoma murino tratadas com etoposídeo.
Palavras-chave: apoptose, vírus do sarampo, vírus da cinomose canina, célula de neuroblastoma murino
INTRODUCTION
During morphogenesis, cell death is involved not only in sculpting shapes but also in optimizing functions (Golstein, 1998). The importance of apoptosis in regulating the number and type of cells in different regions of the developing central and peripheral nervous system is now well accepted (Sastry, Rao, 2000). Apoptosis is an evolutionarily conserved form of cell death that requires specialized machinery (Thornberry, Lazebnik, 1998), energy consumption, protein synthesis(Kerr, Searle, 1972) and degradation. Furthermore, severe neuronal death is common to many neurodegenerative diseases (Leist, Nicotera, 1998).
Under light microscopy, apoptotic cells are shrunken and with an acidophilic cytoplasm. Nuclei undergo into a series of changes including chromatin margination, condensation and fragmentation, followed by cytoplasmic fragmentation into apoptotic bodies (Wyllie et al., 1980; Alison, Sarraf, 1992). Apoptotic cells are rapidly sequestered by professional phagocytes or by neighboring cells before they lyse and cause inflammatory reactions (Leist, Nicotera, 1998). By transmission electronic microscopy, chromatin compaction around the nuclear membrane occurs and the nuclear membrane presents convolutions (Wyllie et al., 1980) preceding nuclear fragmentation (Alison, Sarraf, 1992). Simultaneously with these nuclear alterations, the cytoplasm condenses, microvilli disappear, blebs are formed in the cellular surface, cells separate and junctional structures are lost (Wyllie et al., 1980).
Several biochemical features have been identified as associated to apoptotic cell death. Among these, cleavage of genomic DNA into multiple fragments of 180-200 bp is the most typical feature of the apoptotic process (Wyllie, 1980). This characteristic ladder pattern genome fragmentation is well visualized by agarose gel electrophoresis of the isolated DNA (Wyllie et al., 1980).
Some drugs, particularly etoposide, can induce apoptosis in neuroblastoma cells (Solovyan et al., 1998; Solovyan et al., 1999). Etoposide inhibits DNA-topoisomerase II and induces apoptosis through a p53-dependent pathway (Solovyan et al., 1998). Measles virus (MV), a negative-sense enveloped RNA virus, is a member of the Morbillivirus genus in the Paramyxoviridae family (Lamb, Kolakofsky, 1996). MV has been shown to induce apoptosis in several cell types (Esolen et al., 1995; Fugier-Vivier et al., 1997; Ito et al., 1996; Ito et al., 1997; Engelkin et al., 1999). Additionally, it is known that a related paramyxovirus, canine distemper virus (CDV), causes disorganization of cytoskeletal structures with most the changes occurring in microtubules and intermediate filaments (Howard et al., 1983). Recently, it has been shown that MV infection causes disruption of the glial-fibrillary-acidic protein (GFAP) network in a line of astrocyte (Duprex et al., 2000). Cytoskeleton disruption can also induce apoptosis (Suria et al., 1999).
The aim of this study is to test whether MV infection induces apoptosis in murine neuroblastoma Neuro2a cells (N2A cells) and to determine the behavior of these cells (N2A) under acute and persistent infection with MV and CDV, respectively, in the presence of etoposide.
MATERIALS AND METHODS
Murine neuroblastoma N2A cells were purchased from American Type Culture Collection (ATCC). N2A cells were cultured in MEM (GIBCO BRL Gran Island, New York, 14072, USA) supplemented with 10% FCS, glutamine, NaHCO3 and antibiotics. N2A cells were maintained in a humidified atmosphere of 5% CO2 at 37oC. C1300B cells consisted of N2A cells persistently infected with a virulent CDV strain (R252) isolated from a dog (Oglesbee et al., 1993). Edmonston strain of measles virus (ED-MV) was purchased from ATCC (ATCC P.O. BOX 1549, Manassas, VA 20108 USA).
At first, 106 N2A cells/culture flask were seededin 10% FCS media. After attachment (24h of incubation) cells were rinsed with serum-free media. Subsequently, cell cultures were divided in three groups: one was supplied with 10% FCS media; another group was supplied with serum-free media and the last one was supplemented with serum-free media containing 15m M etoposide (Calbiochem, cat# 341205 - La Jolla, California 92039-2087, USA) for 24 and 48h. After the incubation period, N2A cells were harvested. Attached and unattached cells were processed for electron microscopy and DNA electrophoresis
For determining whether acute ED-MV infection could induce apoptosis in N2A cells 2 x 106 N2A cells each culture flask were seeded and divided into two groups: control cells were supplied with 10% FCS media; treated cells were supplied with the same media and infected with Ed-MV (multiplicity of infection=1). Cells were harvested at 1, 2, 3 and 4 days post infection for DNA extraction and agarose gel electrophoresis.
For determiningthe effect of acute infection in apoptotic pathway, it was carried out an acute ED-MV infection plus etoposide treatment in N2A cells. Two x 106 N2A cells/culture flask were seeded after 24h cells were rinsed with serum-free media and culture cells were divided into four different groups: (1) cells supplied with 10% FCS media; (2) cells supplied with serum-free media; (3) cells supplemented with serum-free media containing 15m M etoposide and (4) cells supplied with serum free-media containing 15m M etoposide and simultaneously infected with Ed-MV (multiply of infection=1). At 48h post infection, cells were harvested with trypsin.
In order to test if persistent infection could interfere with the apoptotic pathway, N2A cells persistently infected with CDV (C1300B) were treated with etoposide. First of all, 2x106 C1300B (N2A persistently infected) cells were seeded. After 24h, cells were rinsed once with serum-free media, and divided into two different groups: (1) control supplied with serum-free media; (2) supplied with serum-free media containing 15m M etoposide. After 48h, cells were harvested with trypsin.
DNA extraction was performed as follows: supernatant media was collected and kept on ice bath. Cells were harvested with trypsin. Unattached and attached cells were centrifuged (1000 rpm/5min at 4oC) and rinsed twice in PBS. Cells were resuspended in TTE (10mM TRIS, 1mM EDTA, 0.2% TRITON X 100) with added proteinase k (final concentration of 100m g/ml). Cells lysate was incubated at 56oC/2.5h 3h and RNAse to a final concentration of 20m g/ml was added. Cells lysate was incubated at 37oC/1h. DNA was extracted with two steps of 25 phenol: 24 chloroform: 1 isoamyl alcohol and precipitated with 1/20v sodium acetate 3M and 2v cold 96o ethanol. DNA was measured in a GeneQuantpro (Pharmacia Biotech (Biochrom) Ltd. 22 Cambridge Science Park, Milton Road, Cambridge, Cambridgeshire, CB4 4FJ UK) and 1.5% agarose gel electrophoresis was performed adding 1.5 - 3.0 m g of DNA/well.
For transmission electron microscopy cells were harvested with trypsin 24h after treatment with etoposide. Attached and unattached cells were used. Cells and media were centrifuged at 1000rpm/5min at 4oC, washed twice in PBS, fixed in 3% glutaraldehyde, post fixed in 1.33% osmium tetroxide, dehydrated and embedded in eponate 12 (Ted Pella). Ultrathin sections were obtained in a LKB Ultrotome and examined with a Philips 300 Electron Microscope.
RESULTS
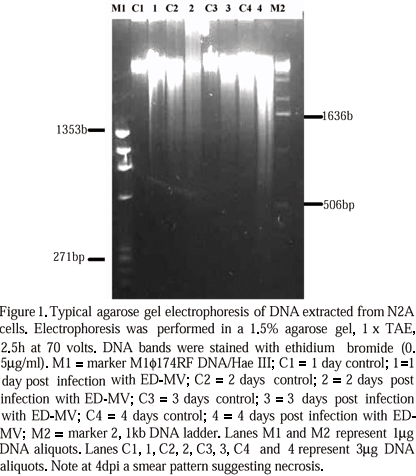
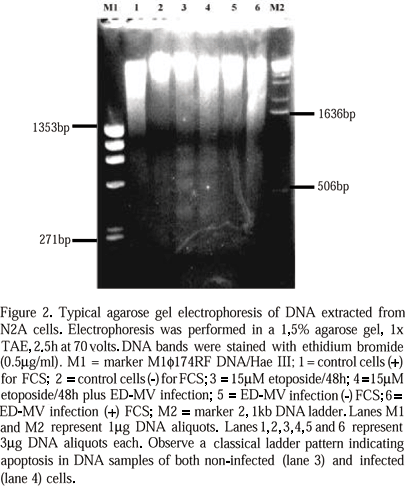
After 20h treatment with 15m M etoposide there was an increase in the number of unattached cells in all experiments. Under inverted microscope, these cells were rounded and had condensed or fragmented nuclei, suggesting apoptosis. Some cells were detaching and fragmenting into apoptotic bodies. Agarose gel electrophoresis from DNA extracted from N2A cells acutely infected with ED-MV showed a smear pattern (Fig. 1). DNA extracted from N2A cells, after 48h etoposide treatment, with or without ED-MV infection, showed the same ladder pattern characteristic of apoptosis (Fig. 2). By transmission electron microscopy, all cultures treated with etoposide showed shrunken cells with indented nuclei, chromatin displaced to the periphery of nuclear membrane, sometimes in crescent shape. Some nuclei were fragmented (Fig. 3A) and some cells were fragmented into apoptotic bodies.
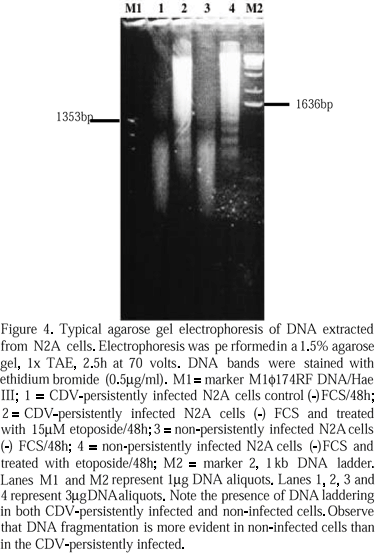
Control (C1300H) and CDV-persistently infected cells (C1300B) showed the same morphological features of apoptosis as described above when treated with etoposide. Also, control and treated cells presented the same DNA ladder pattern (Fig. 4) of apoptosis. The presence of virus nucleocapsids was confirmed in CDV-persistently infected cells through immunohistochemistry and by electron microscopy (Fig. 3B).
DISCUSSION
CDV and MV induce alterations in components of the cytoskeleton (Howard et al., 1983; Duprex et al., 2000). In vitro, MV infection can induce apoptosis in human monocyte cell lines (Esolen et al., 1995; Ito et al., 1998), Vero cells (Esolen et al., 1995), lymphocytes (Ito et al., 1997) and dendritic cells (Fugier-Vivier et al., 1997). According to some authors (Mcquaid et al., 1997), MV can also induce in vivo apoptosis in neurons. Moreover, CDV causes apoptosis in vitro (Gu, Lu, 2000) and in vivo (Moro, 2001). Taken together, these data support the possibility that MV infection might induce apoptosis in N2A cells. However, our data have shown that MV infection in N2A cells did not induce apoptosis. This conclusion is based upon the absence of DNA ladder pattern typical of apoptosis in agarose gel electrophoresis of DNA from MV infected cells. As controls, N2A cells were serum starved, a known apoptotic stimulus (Mastrangelo et al., 2000). However, this treatment induced necrosis (smear pattern and electron microscopy characteristics), not apoptosis. Gschwind and Huber (1997) also cited that serum withdrawal for two days does not induce apoptosis in N2A cells. Previous studies demonstrated that insuline-like growth factor II, acting through type 1 insulin-like growth factor receptor (IGF1R), can function as either autocrine or paracrine growth factor for neuroblastoma tumors in vivo (Sullivan et al., 1995). Consequently, overexpression of IGF1R in neuroblastoma cells might render them resistant to apoptosis in response to serum deprivation and other stimuli (Singleton et al., 1996) and might explain their behavior when infected with ED-MV.
If left undisturbed, the death program would predominantly yield an apoptotic-like morphology. When elements of the apoptotic program are disturbed or inhibited then the type of death can change (Leist, Nicotera, 1998). Present data suggest that the ED-MV infection cause unbalance in important elements of the apoptotic pathway. Therefore, the decrease in Bcl-2, Bim, Bak and small Hsps level (Craig Downs personal communication, 2000) and the possible resistance of N2A cells to programmed cell death might together contribute to a predominance of necrosis in ED-MV infection in this cell strain. Thus, if ED-MV infected N2A cells cannot complete the apoptotic process, the intracellular unbalance is so great that necrosis occurs instead.
Another explanation is that the action of ED-MV in apoptotic pathway might inhibit programmed cell death in N2A cells. If MV disturbs apoptotic pathway it could partially explain the persistence of MV. Lesions produced by MV and CDV in the nervous system are related to the ability of these viruses to persist in central nervous system (Kimoto, 1986; Schneider-Schaulies, Ter Meulen, 1999). It is known that some viruses that cause persistent infection have developed mechanisms to prevent or delay induction of apoptosis or infect cells resistant to virus-induced cell death (Magurano et al., 2000). One explanation of MV persistence is that MV might have the ability of inhibit the apoptosis pathway in neural cells. However acutely ED-MV infected and CDV-persistently infected N2A cells treated with 15 m M etoposide/48h did not show inhibition of apoptosis indicating that, in our model system, disturbances of apoptosis cannot explain in vitro viral persistence in cells of neural origin.
CONCLUSION
ED-MV infection does not stimulate apoptosis in N2A cells. Moreover, acute ED-MV infection does not alter the behavior of N2A cells treated with etoposide, nor does persistent infection with CDV in vitro.
ACKNOWLEDGMENTS
We thank: CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil) for the grant; Dr. Michael Oglesbee for his contribution with cell cultivation; Dr. Steven Krakowka for his suggestions and Anne Evelyn Handley for her assistance in the transmission electron microscopy, Candy Glendening and Jason Zhang for their assistance.
Recebido para publicação em 19 de março de 2002
- ALISON, M.R.; SARRAF, C.E. Apoptosis: a gene-directed program of cell death. J.R. Coll. Phys., v.26, p.25-35, 1992.
- DUPREX, W.P.; MCQUAID, S.; RIMA, B.K. Measles virus-induced disruption of glial-fibrillary-acidic protein cytoskeleton in an astrocytoma cell line (U-251). J. Virol., v.74, p.3874-3880, 2000.
- ENGELKIN, O.; FEDOROK, L.M.; LILISCHIKS, R. et al. Measles virus-induced immunosuppression in vitro is associated with deregulation of G1 cell cycle control proteins. J. Gen. Virol., v.80, p.1599-1608, 1999.
- ESOLEN, L.M.; PARK, S.W.; HARDWICK, J.M. et al. Apoptosis as a cause of death in measles virus-infected cells. J. Virol., v.69, p.3955-3958, 1995.
- FUGIER-VIVIER, I.; SERVET-DELPRAT, C.; RIVAILLER, P. et al. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J. Exp. Med., v.186, p.813-823, 1997.
- GOLSTEIN, P. Cell death in us and others. Science, v.281, p.1283, 1998.
- GSCHWIND, M.; HUBER, G. Detection of apoptotic or necrotic death in neuronal cells by morphological, biochemical and molecular analysis. Neuromethods, v.29, p.13-31, 1997.
- GU, A.Z.; LU, C.P., Canine distemper virus causes apoptosis of Vero cells. J. Vet. Med. B, v.47, p.183-190, 2000.
- HOWARD, J.M.; ECKERT, B.S.; BOURGUIGNON, L.Y.W. Comparison of cytoskeletal organization in canine distemper virus-infected and uninfected cells. J. Gen. Virol., v.64, p.2379-2385, 1983.
- ITO, M.; WATANABE, M.; IHARA, T. et al. Measles virus induces apoptotic cell death in lymphocytes activated with phorbol 12-myristate 13-acetate (PMA) plus calcium ionophore. Clin. Exp. Immunol., v.108, p.266-271, 1997.
- ITO, M.; YAMAMOTO, T.; WATANABE, M., et al. Detection of measles virus-induced apoptosis of human monocytic cell line (THP-1) by DNA fragmentation ELISA. FEMS Immunol. Med. Microbiol., v.15, p.115-122, 1996.
- KERR, J.F.R.; SEARLE, J. A suggested explanation for the paradoxically slow growth rate of basal cell carcinomas that contain numerous mitotic figures. J. Pathol., v.107, p.41-44, 1972.
- KIMOTO, T. In vitro and in vivo properties of the virus causing natural canine distemper encephalitis. J. Gen. Virol., v.67, p.487-503, 1986.
- LAMB, A.B.; KOLAKOFSKY, D. Paramyxoviridae: the viruses and their replication. In: FIELDS, B.N.; KNIPE, D.M.; HOWLEY, P.M. (Eds.). Fundamental virology Philadelphia: Lippincott-Raven, 1996. p.577- 604.
- LEIST, M.; NICOTERA, P. Apoptosis, excitotoxicity, and neuropathology. Exp. Cell Res, v.239, p.183-201, 1998.
- MAGURANO, F.; MARCH, A.; NICOLETTI, L. Apoptosis induced by toscana virus (Bunyaveviridae, phlebovirus) in cell lines. In: INTERNATIONAL CONFERENCE ON NEGATIVE STRAND VIRUSES, 11, 2000, Quebec. Proceedings...Quebec, 2000, p.100.
- MASTRANGELO, A.L.; HARDWICK, J.M.; ZOU, S. et al. Part II: Over expression of Bcl-2 family members enhances survival of mammalian cells in response to various culture insults. Biotechnol. Bioeng., v.67, p.555-564, 2000.
- MCQUAID, S.; MCMAHON, J.; HERRON, B. et al. Apoptosis in measles virus infected human central nervous system tissues. Neuropathol. Appl. Neurobiol., v.23, p.218-224, 1997.
- MORO, L. Apoptose na patogenia da cinomose canina. 2001. 213f. Tese (Doutorado). Faculdade de Medicina, Universidade Federal de Minas Gerais, Belo Horizonte, MG.
- OGLESBEE, M.J.; KENNEY, H.; KENNEY, T. et al. Enhanced production of morbillivirus gene-specific RNAs following induction of the cellular stress response in stable persistent infection. Virology, v.192, p.556-567, 1993.
- SASTRY, P.S.; RAO, K.S. Apoptosis in the nervous system. J. Neurochem., v.74, p.1-20, 2000.
- SCHNEIDER-SCHAULIES, S.; TER MEULEN, V. Pathogenic aspects of measles virus infection. Arch. Virol., v.15, p.139-158, 1999.
- SINGLETON, J.R.; RANDOLPH, A.E.; FELDMAN, E.L. Insulin-like growth factor I receptor prevents apoptosis and enhances neuroblastoma tumorigenesis. Cancer Res., v.56, p.4522-4529, 1996.
- SOLOVYAN, V.; BEZVENYUK, Z.; HUOTARI, V. et al. Distinct mechanisms underlay DNA disintegration during apoptosis induced by genotoxic agents in neuroblastoma cells. Neurochem. Intern., v.34, p.465-472, 1999.
- SOLOVYAN, V.; BEZVENYUK, Z.; HUOTARI, V. et al. Distinct mode of apoptosis induced by genotoxic agent etoposide and serum withdrawal in neuroblastoma cells. Molec. Brain Res., v.62, p.43-55, 1998.
- SULLIVAN, K.A.; CASTLE, V.P.; HANASH, S.M. et al. Insuline-like growth factor II in the pathogenesis of human neuroblastoma. Am. J. Pathol., v.147, p.1790-1798, 1995.
- SURIA, H.; CHAU, L.A.; NEGROU, E. et al. Cytoskeletal disruption induces T cell apoptosis by a caspase-3 mediated mechanism. Life Sci., v.65, p.2697-2707, 1999.
- THORNBERRY, N.A.; LAZEBNIK, Y. Caspases: enemies within. Science, v.281, p.1312-1316, 1998.
- WYLLIE, A.H. Glucocorticoid- induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature, v.284, p.555-556, 1980.
- WYLLIE, A.H.; KERR, J.F.R.; CURRIE, A.R. Cell death: the significance of apoptosis. Int. Rev. Cytol., v.68, p.251-305, 1980.
Publication Dates
-
Publication in this collection
23 Apr 2003 -
Date of issue
Feb 2003
History
-
Received
19 Mar 2002