Abstracts
The course of experimental T. evansi infection in four dogs was followed for 82 days and hematological, biochemical and anatomopathological findings were studied. Infected animals showed progressive decrease in red blood cell count and hemoglobin concentration, leading to anemia which persisted from the third week post-infection until the end of the study. Leucopenia and neutropenia were observed between weeks 2 and 5 of the infection. The infected dogs developed hyperproteinemia and a decrease in the albumin:globulin ratio was observed. Aspartate aminotransferase and alamine aminotransferase levels increased significantly in infected dogs in comparison to control dogs. Histological changes observed in all infected animals consisted of lymphoid hyperplasia in spleens and lymph nodes and centrilobular degeneration and periportal mononuclear cell accumulation in the liver. A massive mononuclear cell infiltration of the myocardium was seen in three dogs and a nonsuppurative meningoencephalitis was evidente in two infected animals.
Dog; Trypanosoma evansi; hematology; biochemistry; anatomopathology
O presente estudo acompanhou durante 82 dias o curso da infecção experimental com T. evansi em quatro cães, realizando a avaliação dos achados hematológicos, bioquímicos e anatomopatológicos. Os animais infectados mostraram declínio acentuado na contagem de hemácias, hematócrito e teor de hemoglobina, permanecendo anêmicos a partir da terceira semana de infecção até o final do período experimental. Leucopenia com neutropenia foram observadas entre a segunda e a quinta semanas após a infecção. Os cães inoculados desenvolveram hiperproteinemia, sendo constatada diminuição na relação albumina:globulina. As atividades séricas de alamina aminotransferase e aspartato aminotransferase aumentaram significativamente nos cães infectados em relação aos animais controle. O exame histopatológico revelou hiperplasia linfóide no baço e linfonodos e infiltrado mononuclear periportal e esteatose de padrão centrolobular no fígado de todos os cães infectados. Intenso infiltrado mononuclear foi observado no miocárdio de três cães e acúmulos de células mononucleares junto às meninges foram evidenciados em dois animais infectados.
Cão; Trypanosoma evansi; hematologia; bioquímica; anatomopatologia
Hematological, biochemical and anatomopathological aspects of the experimental infection with Trypanosoma evansi in dogs
[Aspectos hematológicos, bioquímicos e anatomopatológicos da infecção experimental por Trypanosoma evansi em cães]
L.P.C.T. Aquino, R.Z. Machado*, A.C. Alessi, A.E. Santana, M.B. Castro, L.C. Marques, E.B. Malheiros
Faculdade de Ciências Agrárias e Veterinárias da UNESP
Via de acesso Prof. Paulo Donato Castellane, s/n
14870-000 Jaboticabal, SP
Recebido para publicação em 5 de junho de 2001.
Trabalho financiado pela FAPESP (94/2413/8)
*Autor para correspondência
E-mail: zacarias@fcav.unesp.br
ABSTRACT
The course of experimental T. evansi infection in four dogs was followed for 82 days and hematological, biochemical and anatomopathological findings were studied. Infected animals showed progressive decrease in red blood cell count and hemoglobin concentration, leading to anemia which persisted from the third week post-infection until the end of the study. Leucopenia and neutropenia were observed between weeks 2 and 5 of the infection. The infected dogs developed hyperproteinemia and a decrease in the albumin:globulin ratio was observed. Aspartate aminotransferase and alamine aminotransferase levels increased significantly in infected dogs in comparison to control dogs. Histological changes observed in all infected animals consisted of lymphoid hyperplasia in spleens and lymph nodes and centrilobular degeneration and periportal mononuclear cell accumulation in the liver. A massive mononuclear cell infiltration of the myocardium was seen in three dogs and a nonsuppurative meningoencephalitis was evidente in two infected animals.
Keywords: Dog, Trypanosoma evansi, hematology, biochemistry, anatomopathology
RESUMO
O presente estudo acompanhou durante 82 dias o curso da infecção experimental com T. evansi em quatro cães, realizando a avaliação dos achados hematológicos, bioquímicos e anatomopatológicos. Os animais infectados mostraram declínio acentuado na contagem de hemácias, hematócrito e teor de hemoglobina, permanecendo anêmicos a partir da terceira semana de infecção até o final do período experimental. Leucopenia com neutropenia foram observadas entre a segunda e a quinta semanas após a infecção. Os cães inoculados desenvolveram hiperproteinemia, sendo constatada diminuição na relação albumina:globulina. As atividades séricas de alamina aminotransferase e aspartato aminotransferase aumentaram significativamente nos cães infectados em relação aos animais controle. O exame histopatológico revelou hiperplasia linfóide no baço e linfonodos e infiltrado mononuclear periportal e esteatose de padrão centrolobular no fígado de todos os cães infectados. Intenso infiltrado mononuclear foi observado no miocárdio de três cães e acúmulos de células mononucleares junto às meninges foram evidenciados em dois animais infectados.
Palavras-chave: Cão, Trypanosoma evansi, hematologia, bioquímica, anatomopatologia
INTRODUCTION
Trypanosoma evansi is the causative agent of surra, an important disease widely distributed in tropical and subtropical regions. Surra affects a great variety of domestic and also wild mammals. In Brazil, the disease is also known as mal de cadeiras and is endemic in the Pantanal of Mato Grosso and Mato Grosso do Sul states where it affects equines, capybaras, coatis and dogs (Stevens et al., 1989; Nunes & Oshiro, 1990; Nunes et al., 1993). Natural infections in dogs with manifestation of severe clinical symptons have been reported in different regions of Brazil (Moreira & Machado, 1985; Franke et al., 1994; Silva et al., 1995a). Surra in dogs is characterized by high morbity and mortality rates and anemia has been recorded as a consistent finding in naturally infected dogs (Moreira & Machado, 1985; Galhorta et al., 1986; Sandoval et al., 1994; Silva et al., 1995b) but its origin remains unclear and many hypothesis are proposed. The leucogram of infected dogs seems not to show a defined trend; leucopenia with no change in differencial count has been reported by some authors (Moreira & Machado, 1985; Silva et al., 1995b), while others registered no change in total white blood cell count (Hellebrekers & Slappendel, 1982; Sandoval et al., 1994). Some alterations in blood biochemistry, including hypoglucemia and decrease in albumin:globulin rate, were verified in naturally infected dogs (Moreira & Machado, 1985; Sandoval et al., 1994). The main histophatological lesions described in dogs infected with T. evansi consisted of mononuclear cells accumulations in the myocardium and meningoencephalitis (May, 1968; Hellebrekers & Slappendel, 1982). Despite the importance and the worldwide distribution of surra, very little is known about the pathogenesis of this trypanosomiasis. Moreover, there are few reports about the disease in dogs great part of which refers to isolated cases of natural infection, what justifies additional investigation. The present work was designed to study hematological, biochemical and anatomopathological alterations in dogs experimentally infected with T. evansi.
MATERIALS AND METHODS
A cryopreserved strain of T. evansi originally isolated from a naturally infected dog by Moreira & Machado (1985) was inoculated intravenously in a healthy 8-month-old mongrel dog. Blood with high parasitemia was used for infection of experimental animals.
Eight male and female mongrel dogs about eight months of age were used. The animals were raised in the kennel of the Department of Veterinary Pathology, FCAV-Unesp and kept in flyproof individual households. Dogs were fed a commercial ration and water was available ad libitum. Before inclusion in this study the animals were treated with anthelmintics and immunized against infeccious diseases. Four dogs were inoculated intravenously each with 2.2 x 105 trypanosomes (T1, T2, T3 e T4) and four were used as control (C1, C2, C3 e C4).
Blood samples were collected from the jugular vein of all animals once a week from day 5 until day 82 of infection. Samples for hemogram were collected in tubes containing ethylenediaminetetraacetic acid (EDTA) as anticoagulant and sodium fluoride for plasma glucose assays. Blood for serum used in other biochemical analyses was collected without anticoagulant. Blood and serum samples were also obtained from all animals before experimental infection and considered as week 0 (mean of three collections).
Red cell count (RBC), white cell count (WBC) and hemoglobin (Hb) concentration were provided by an automated blood cell counter (CELM, Barueri SP) (CC-510) connected to a hemoglobinometer1 (HB-520). The packed cell volume (PCV) was determined using the standard microhematocrit method. Mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC) were calculated according to Ferreira Neto et al. (1981).
Seric phosphatase alkaline, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, indirect bilirubin, total protein and albumin as well as plasmatic glucose were determined by enzymatic colorimetric methods using commercial available kits ( [1] LAB-TEST, Belo Horizonte MG). Serum globulin was recorded as the difference between serum total protein and albumin.
After 82 days of infection, the animals were euthanatized and submitted to necropsy. Samples from spleen, lymph nodes, heart, liver, lungs, brain, kidneys and gut were collected and placed immediately in 10% buffered formalin. After fixation for 24 hours the tissues were paraffin embedded, cut into 5mm sections and stained with hematoxilin and eosin (HE).
Data related to hemogram and biochemical assays were analysed using a simple split splot design. Within each plot, the two experimental groups were allotted at random. The times of infection were the subplots.
RESULTS
Infected animals showed a significant decrease (P<0.01) in red blood cell count (RBC), hemoglobin concentration (Hb) and packed cell volume (PCV) mean values (Fig. 1). There was a marked and progressive decrease in such values between weeks 0 and 4. Thereafter they tended to stabilize, but remained bellow normal levels from week 3 until the end of the experimental period. In the control group the mean values for these parameters were within normal range. A significant difference (P<0.01) was detected between the infected and control groups.
MCV and MCHC mean values fluctuated irregularly throughout the experimental period and no significant difference between the infected and control groups was detected. MCV values in infected animals became above normal levels on weeks 4 and 5, the same period when the lowest RBC values were registered, denoting a macrocytic normocromic anemia. Despite a slight decrease at weeks 7, 9 and 11, MCHC values in infected animals remained within normal ranges.
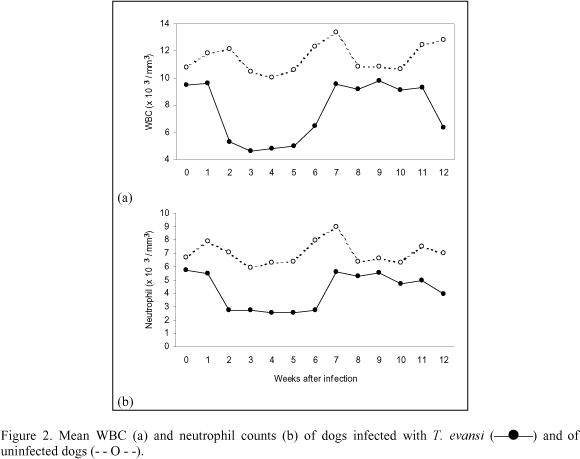
Mean absolute values for total white blood cell count (WBC) in infected group were significantly different from those of the control animals (P<0.05). Infection caused leucopenia between weeks 2 and 5 as consequence of a significant decrease (P<0.05) in neutrophils counts (Fig. 2a and 2b). Following this period, the mean values for WBC and neutrophils in infected group remained within normal ranges, but lower than in control group until the end of the study. The mean absolute lymphocyte values in infected dogs also showed a downward trend between weeks 2 and 5. Nevertheless, lymphocyte values remained within normal levels and there was no significant difference between the groups. No significant changes were observed in basophils, eosinophils and monocytes counts and no diferences were found between infected and control groups.
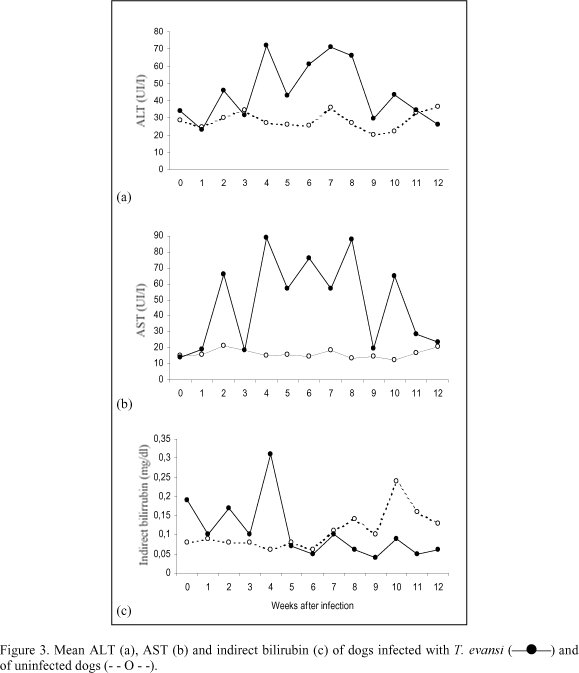
AST and ALT activity fluctuated irregularly in infected dogs and the mean values remained higher than those in control animals during most of the studied period. A significant difference was found in AST (P<0.01) and ALT (P<0.05) activity between control and infected groups. AST values were above normal levels on weeks 4, 6 and 8 after infection (Fig. 3b). Despite remaining within normal levels, ALT mean values fluctuated in infected animals and were higher than the values found in the control animals for most of the experimental period (Fig. 3a). There was no significant difference in alkaline phosphatase activity in control and infected dogs. In both groups alkaline phosphatase mean values remained within normal ranges.
Bilirubin concentrations remained within normal levels in both infected and control groups and no significant difference was found between them. However an increase in total bilirubin mean values due to a rise in indirect bilirubin was observed on week 4 in infected animals (Fig. 3c). The mean glucose levels fluctuated considerably in both infected and control groups, but remained within normal values, displaying no particular trends.
Concentrations of total protein in control animals mantained at a nearly constant value during the infection, but in the infected dogs, total protein values rose significantly (P<0.05) throughout the experiment and was found above normal ranges from week 10 to 12 (Fig. 4a). A significant difference in total protein levels was found between control and infected groups (P<0.01).
A significant increase in globulin (P<0.01) and a significant decrease in albumin concentration (P<0.01) were observed in infected dogs. Infected animals showed hyperglobulinemia at weeks 4, 10, 11 and 12 after infection. Globulin and albumin concentrations in infected group were significantly different (P<0.01) from controls (Fig. 4b and 4c).
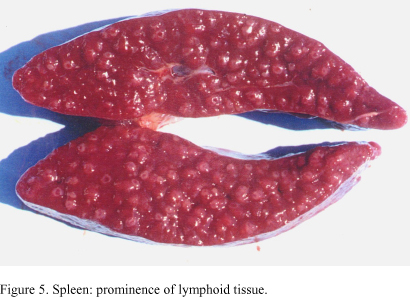
In all infected dogs, necropsy showed nonspecific changes including emaciation, pale mucous membranes, enlarged and edematous lymph nodes and markedly enlarged spleens which on section displayed prominence of white pulp (Fig. 5). Lesions found in the heart of three infected dogs consisted of pale areas in both right ventricles and auricles, the latter showing hemorragic areas (Fig. 6). One of the animals presented serous fluid accumulation in pericardic sac.
Predominance of mononuclear cell infiltration of the myocardium affecting mainly right ventricle and auricle and presence of degenerated cardiac fibers were observed in three infected dogs. In some regions the infiltrates were more pronounced and a dense focal infiltrate in a area of fibers destruction could be observed (Fig. 7). The spleens showed lymphoid hyperplasia of various degrees, scattered hemorrhagic areas and erythrophagocytosis. There was marked enlargement of lymphoid follicles with presence of mitotic cells in the lymph nodes. Proliferation of hystiocitic cells, plasmocytosis in medullar cords and a marked sinusal dilation with presence of edema were evident. There was centrilobular fatty degeneration of various degrees and periportal mononuclear cells accumulation in the liver. Lesions observed in the brain of two animals consisted of mononuclear cell accumulation in white and grey matter areas of cerebral cortex and also in cerebellae penducle. Some lesions displayed the aspect of glial nodule (Fig. 8) and moderated perivascular mononuclear cuffing was observed, usually nearby the infiltration areas. In addition, moderate to severe mononuclear cell accumulation was observed in the meninges of the cerebral cortex, especially in the sulcus and around meningeal vessels.
No significant changes were detected in the other organs examined. No trypanosome were found in blood vessels or in the intersticial tissue in any organ.
DISCUSSION
Anemia was a consistent finding as reported previously in different hosts infected with T. evansi (Jaktar & Purohit, 1971; Oshiro et al., 1989; Monzon et al., 1991) including dogs (Hellebrekers & Slappendel, 1982; Moreira & Machado, 1985; Silva et al., 1995b). Despite being considered a significant pathological feature of the disease, the origin of this anemia is not completely elucidated. Proposed mechanisms include hemolysis as a result of erythrophagocytosis, hemodilution and depression of erythropoiesis. Increase in serum unconjugated bilirubin levels in infected animals occurred at week four after inoculation, coinciding with the lowest RBC values observed, suggesting the occurrence of an hemolytic crisis in this period of the infection. Hyperbilirubinemia has been reported in naturally infected dog as consequence of an increase in unconjugated bilirubin (Sandoval et al., 1994) and conjugated bilirubin (Hellebrekers & Slappendel, 1982). Increase in serum bilirubin levels was not a consistent finding during the infection and did not exceed normal limits suggesting that extravascular destruction of red cells is a more likely explanation for anemia. Infected dogs showed leucopenia with neutropenia from week 2 until week 5 of the infection. Leucopenia (Moreira & Machado, 1985) and normal leucograms (Hellebrekers & Slappendel, 1982; Sandoval et al., 1994) have been reported in naturally infected dogs. The marked rise in AST levels, compared to the rather modest increase in ALT observed in infected animals, indicate that little of the former is derived from the liver. It is likely that the AST originated from heart muscle since myocarditis was found in three infected dogs. Increases in ALT and AST activity have been also observed in a T. evansi naturally infected dog (Sandoval et al., 1994). A significant increase in seric protein levels, as consequence of globulin rise, and a parallel decrease in albumin concentrations were observed in infected dogs. The decrease in albumin:globulin ratio has been frequently reported in T. evansi infection in various studied hosts (Jaktar et al., 1973; Boid et al., 1980; Moreira & Machado, 1985; Galhorta et al., 1986; Monzon et al., 1991). It is suggestive that fall in albumin levels was secondary to hyperglobulinemia as a compensatory mechanism for the maintenance of normal blood viscosity increased by high globulin levels. Hypoalbuminemia as consequence of liver damage may be rejected since serum albumin levels fall only after extensive and chronic liver malfunction, what was not a feature in this experimental infection, as evidenced by liver function tests and anatomopathological findings. There is evidence to suggest that increase in immunoglobulin levels was responsible for hyperglobulinemia observed in infected dogs. Study of electrophoretic patterns of serum proteins in T. evansi infected camels (Jaktar et al., 1973; Boid et al., 1980), equines (Raza et al., 1981) and cattle (Verma & Gautman, 1977)showed a marked increase in g-globulin fraction.
Gross examination of all infected dogs revealed lymphadenopathy and splenomegaly which, despite being always reported in T. evansi infection, are not pathognomonic for the disease. Histological lesions in spleen and lymph nodes consisted of marked lymphoid hyperplasia and infiltrate of macrophages and histiocytes in the paracortical zone. According to Woodruff (1973) a progressive enlargement of spleen may occur when small amounts of antigen are released successively over a prolonged period, leading to a slight and continuous hemolysis. If red cells are coated with immune complexes or sensitized in some way, they are likely to be removed, at least in some measure, by the splenic reticuloendothelial tissue. Centrilobular fatty degeneration and periportal accumulation were observed in all infected dogs. It is possible that degenerative lesions resulted from anemia that developed in all infected animals. Experimental studies have suggested that centrilobular hepatic lesions result in less hyperbilirubinemia than when cells of the outer zone are damaged (Cornelius, 1989). This fact is consistent with the almost unaltered bilirubin levels found in infected animals.
Macroscopical lesions observed in the heart of three infected dogs could not be reported in the available bibliography. The histological lesions observed in myocardium of these animals close resembled those described in trypanosomiasis caused by T. cruzi, although without the presence of the parasite. Similar lesion has been reported in a naturally infected dog (Hellebrekers & Slappendel, 1982) and in horses experimentally infected with T. evansi (Marques, 1996). Non suppurative meningoencephalitis, similar to that found in two of the infected dogs, has been reported in naturally and experimentally infected horses (Seiler et al., 1981; Ikede et al., 1983; Marques, 1996)and less frequently in naturally infected dogs (May, 1968; Hellebrekers & Slappendel, 1982). Microglial nodules, similar to those observed in two infected dogs, are very commonly a feature of viral encephalitis, occuring in both gray and white matter, but are not specific for viral infections. Microglial response to tissue injury may be a proliferative reaction, although their ability to do so seems limited and many of the cells in proliferative foci are probably derived from imigrant histiocytes (Jubb & Huxtable, 1993).
The aetiology of tissue lesions in animals infected with T. evansi is unknown. However, it is hypothesized that the deposition of immune complex in organs and body fluids is important and leads to the activation of Hageman factor and a cascade of enzymatic reactions resulting ultimately in the release of important pharmacologically active substances involved in the more chronic phases of the inflammatory response (Seed & Hall, 1985). It is clearly that a final explanation of the mechanisms of pathogenesis in trypanosomiasis will be complex and obviously much more detailed investigation is need.
ACKNOWLEDGEMENTS
We are gratefull to Eugênio de Campos Filho, Maria Inês Y. de Campos, Francisca de Assis Ardison for their technical assistance and Ronaldo Del Vechio for the care of the animals.
- BOID, R.; LUCKINS, A.G.; GRAY, P.F. et al. Serum immunoglobulins levels and eletrophoretic patterns of serum proteins in camels infected with Trypanosoma evansi Vet. Parasitol, v.6, p.333-345, 1980.
- CORNELIUS, C.E. Liver function. In: KANEKO, J.J. Clinical biochemistry of domestic animals San Diego: Academic, 1989. p.364-397.
- FERREIRA NETO, J.M.; VIANA, E.S.; MAGALHĂES, L.M. Patologia clínica veterinária Belo Horizonte: Rabelo, 1981. 279p.
- FRANKE, C.R.; GREINER, M.; MEHLITZ, D. Investigation on naturally ocurring T. evansi infections in horses, cattle, dogs and capybaras (Hydrochaeris hydrochaeris) in Pantanal de Poconé (Mato Grosso, Brazil). Acta Trop., v.58, p.159-169, 1994
- GALHORTA, A.P.; SINGH, M.P.; GAUTMAN, O.P. Biochemical changes and therapeutic trials in experimental trypanosomiasis in dogs. Indian J. Parasitol., v.10, p.253-7, 1986.
- HELLEBREKERS, L.J.; SLAPPENDEL, R.J. Tripanosomiasis in a dog imported in the Netherlands. Vet. Q, v.4, p.182-6, 1982.
- IKEDE, B.O.; FATIMAH, W.; SHARIFUDDIN, W. et al. Clinical and pathological features of natural Trypanosoma evansi infections in ponies in West Malaysia. Trop. Vet, v.1, p.151-157, 1983.
- JAKTAR, P.R.; PUROHIT, M.S. Pathogenesis of anemia in Trypanosoma evansi infection. I Hemathology. Indian Vet. J., v.48, p.239-244, 1971.
- JAKTAR, P.R.; GHOSAL, A.K.; SINGH, M. Pathogenesis of anemia in Trypanosoma evansi infection. III Studies on serum proteins. Indian Vet. J, v.50, p. 634-636, 1973.
- JUBB, K.V.F.; HUXTABLE, C.R. The nervous system. In: JUBB, K.V.F.; KENNEDY, P.C.; PALMER, N. Pathology of domestic animals San Diego: Academic, 1993. p.267-439.
- MARQUES, L.C. Infecçăo experimental em eqüinos com Trypanosoma evansi Steel, 1885 (Sarcomastigophora: Trypanosomatidae) 1996. 134f. Tese (Livre Docęncia) Faculdade de Cięncias Agrárias e Veterinárias, Universidade Estadual Paulista, Jaboticabal.
- MAY, C. Canine Meningo-encephalitis due to Trypanosoma evansi Infection (A report of two cases). Vet. Rec., v.83, p.663-665, 1968.
- MONZON, C.M.; VILLAVICENCIO, V.I.; ROUX, J.P. et al. Estudios hematológicos en cobaios y equinos infectados con el Trypanosoma evansi (Steel,1885). Vet. Argent., v.8, p.668-676, 1991.
- MOREIRA, R.D.; MACHADO, R.Z. Identificaçăo e isolamento do Trypanosoma equinum em um căo do município de Camapuă-MS. In: ENCONTRO DE PESQUISAS VETERINÁRIAS, 10, Jaboticabal, 1985. Resumo..., Jaboticabal: UNESP/Faculdade de Cięncias Agrárias, 1985. p.66.
- NUNES, V.L.B.; OSHIRO, E.T. Trypanosoma evansi in the coati from the Pantanal region of Mato Grosso do Sul State, Brazil. Trans. R. Soc. Trop. Med. Hyg, v.84, p.692, 1990.
- NUNES, V.L.B.; OSHIRO, E.T.; DORVAL, M.E.C. et al. Investigaçăo epidemiológica sobre Trypanosoma (trypanozoon) evansi no pantanal sul-mato-grossense. Estudo de reservatórios. Rev. Bras. Parasitol., v.2, p.41-44, 1993.
- OSHIRO, E.T.; RODRIGUES, M.; NUNUES, V.L.B. et al. Trypanosoma (Trypanozoon evansi) (Steel,1885) Balbiani, 1888, infecçăo experimental em equinos com amostra isolada de capivara, Hydrochaeris hydrochaeris (Linnaeus, 1766) (Rodentia: hydrochaeridae). Semina, v.10, p.51-55, 1989.
- RAZA, M.A.; REHMAN, Z.; CHAUDHRY, A.H. et al. Serum proteins changes in horses infected with surra. Pak. Vet. J, v. 1, p.78-79, 1981.
- SANDOVAL, G.L.; COPPO, N.B.; NEGRETTE, M.S. et al. Alteraçőes bioquímicas e histopatológicas de um căo e ratos infectados com Trypanosoma evansi Hora Vet, v.81, p.53-55, 1994.
- SEED, J.R.; HALL, J.E. Pathophisiology of African Trypanosomiasis. In: TIZARD, I. Immunology and pathogenesis of trypanosomiasis Boca Raton: CRC, 1985. p.1-11.
- SEILER, R.J.; OMAR, S.; JACKSON, A.R.B. Meningoencephalitis in naturally occurring Trypanosoma evansi infection (Surra) of horses. Vet. Pathol., v.18, p.120-122, 1981.
- SILVA, R.A.M.S.; BARROS, A.T.M.; HERRERA, H.M. Trypanosomosis outbreaks due to Trypanosoma evansi in the Pantanal, Brazil. A preliminary approach on risk factors. Rév. Élev. Méd. Vét. Pays Trop, v.48, p.315-319, 1995a.
- SILVA, R.A.M.S.; HERRERA, H.M.; DOMINGOS, L.B.S. et al. Pathogenesis of Trypanosoma evansi infection in dogs and horses: hematological and clinical aspects. Cięnc. Rural, v.25, p.223-238, 1995b.
- STEVENS, J.R.; NUNES, V.L.B.; LANHAM, S.M. et al. Isoenzyme characterization of Trypanosoma evansi isolated from capybaras and dogs in Brazil. Acta Trop., v.46, p.213-222, 1989.
- VERMA, B.B.; GAUTMAN, O.P. Serological diagnosis of experimental bovine surra (Trypanosoma evansi infection) - A comparison of passive hemagglutination, gel diffusion and indirect fluorescent antibody tests. Indian Vet. J., v.54, p.809-813, 1977.
- WOODRUFF, A.W. Mechanisms involved in anemia associated with infection and splenomegaly in the tropics. Trans. R. Soc. Trop. Med. Hyg, v.67, p.313-325, 1973.
Publication Dates
-
Publication in this collection
22 July 2002 -
Date of issue
Feb 2002
History
-
Received
05 June 2001