Abstract
Many pharmacological effects have been ascribed to extracts of Psidium guajava L. (guava) leaves. However, in spite of its widespread use in Brazilian folk medicine and a reasonable number of scientific reports about it, we could not find any study dealing with its action on the mammalian myocardium. In the present study, by measuring isometric force, we observed that the crude extract of P. guajava (water-alcohol extract obtained by macerating dry leaves) depresses the guinea pig atrial contractility in a concentration-dependent fashion (N = 8 hearts, 15 trials). The compound with cardiac activity was concentrated by extraction in a Soxhlet apparatus using 17 M glacial acetic acid after removing the less polar fractions (hexane, chloroform, acetone, ethanol and methanol), suggesting that this compound is a highly polar substance. In the isolated guinea pig left atrium the acetic acid fraction (10-800 mg/l) of P. guajava 1) reversibly decreased myocardial force in a concentration-dependent fashion (EC50 = 0.07g/l, N = 5 hearts, 9 trials, P<0.05), 2) increased the atrial relaxation time measured at 20% of the force amplitude up to 35% (91 ± 15 to 123 ± 30 ms, N = 3 hearts, 6 trials, P<0.05), 3) abolished the positive staircase effect (Bowditch phenomenon) in a concentration-dependent fashion suggesting a decrease of the cellular inward calcium current (N = 4 hearts, 8 trials, P<0.05), and 4) its inotropic effect was abolished by cholinergic receptor blockade with 1.5 mM atropine sulfate, indicating a cholinergic involvement in the mechanism of action of the extract (N = 7 hearts, 15 trials, P<0.05). The acetic acid extract was 20 times more potent than crude extract (EC50 = 1.4 g/l). The results showed that extracts from P. guajava leaves depress myocardial inotropism.
Heart contractility; Guinea pig; Myocardial inotropism; Psidium guajava L.; Folk medicine
Braz J Med Biol Res, May 2003, Volume 36(5) 661-668
Inotropic effects of extracts of Psidium guajava L. (guava) leaves on the guinea pig atrium
E.A. Conde Garcia, V.T. Nascimento and A.B. Santiago Santos
Laboratório de Biofísica dos Tecidos Excitáveis, Departamento de Fisiologia, Centro de Ciências Biológicas e da Saúde, Universidade Federal de Sergipe, São Cristóvão, SE, Brasil
References
Correspondence and Footnotes Correspondence and Footnotes Correspondence and Footnotes
Abstract
Many pharmacological effects have been ascribed to extracts of Psidium guajava L. (guava) leaves. However, in spite of its widespread use in Brazilian folk medicine and a reasonable number of scientific reports about it, we could not find any study dealing with its action on the mammalian myocardium. In the present study, by measuring isometric force, we observed that the crude extract of P. guajava (water-alcohol extract obtained by macerating dry leaves) depresses the guinea pig atrial contractility in a concentration-dependent fashion (N = 8 hearts, 15 trials). The compound with cardiac activity was concentrated by extraction in a Soxhlet apparatus using 17 M glacial acetic acid after removing the less polar fractions (hexane, chloroform, acetone, ethanol and methanol), suggesting that this compound is a highly polar substance. In the isolated guinea pig left atrium the acetic acid fraction (10-800 mg/l) of P. guajava 1) reversibly decreased myocardial force in a concentration-dependent fashion (EC50 = 0.07g/l, N = 5 hearts, 9 trials, P<0.05), 2) increased the atrial relaxation time measured at 20% of the force amplitude up to 35% (91 ± 15 to 123 ± 30 ms, N = 3 hearts, 6 trials, P<0.05), 3) abolished the positive staircase effect (Bowditch phenomenon) in a concentration-dependent fashion suggesting a decrease of the cellular inward calcium current (N = 4 hearts, 8 trials, P<0.05), and 4) its inotropic effect was abolished by cholinergic receptor blockade with 1.5 mM atropine sulfate, indicating a cholinergic involvement in the mechanism of action of the extract (N = 7 hearts, 15 trials, P<0.05). The acetic acid extract was 20 times more potent than crude extract (EC50 = 1.4 g/l). The results showed that extracts from P. guajava leaves depress myocardial inotropism.
Key words: Heart contractility, Guinea pig, Myocardial inotropism, Psidium guajava L., Folk medicine
Introduction
Guava (Psidium guajava L.), a plant of the Myrtaceae family, is native to Tropical America. It is found widely in hot climate countries and grows up to 7 m high. Its fruits, which are rich in vitamin C, are consumed fresh or industrialized as jam, juice, etc. South American folk medicine uses tea from its leaves, leaf buds, and flowers to treat intestinal colic and diarrhea (1-3). In guinea pig ileum the hexane, methanol, and aqueous extracts of leaves decrease the peristaltic waves, probably due to quercetin, a glycoside (flavonoid) found in the leaves (1). Quercetin antagonizes the inward calcium membrane current leading to a decrease of smooth muscle contractile force (2,3). It also reduces acetylcholine release in neuromuscular junctions due to its interaction with calcium channels of presynaptic membranes (4).
Many other effects of extracts of Psidium leaves have been reported, such as antinociceptive (5,6), CNS depressor (7), antimutagenic (8,9), antihyperglycemic (10) - but such effect was controversial (11) -, antidiarrheic (12), antibiotic (12,13), antiamebic and antispasmodic (14) effects, anticough (15), and narcotic-like activity (16).
Phytochemical analysis of Psidium leaves has revealed the presence of tannins, phenols, triterpenes, essential oils, saponins (17), lectins (18), carotenoids (19), ascorbic acid (20), and fatty acids (21). Its essential oil contains cineol, D-limonene, caryophyllene, eugenol, alpha pinene, and myrcene, but no alkaloids or anthocyanides. The volatile acids (E)-cinnamic acid and (Z)-3-hexenoic acid are the major constituents of the volatile principles of P. guajava (22).
During the screening of several plants we observed that a crude extract of P. guajava depressed myocardial contractility. The objective of the present study was to characterize its inotropic effects on the guinea pig atrial myocardium.
Material and Methods
Extract preparation
Leaves were collected on the campus of the Federal University of Sergipe (Aracaju, SE, Brazil), from agrotoxic-free trees during the winter season (June-July, 2000). A voucher specimen of P. guajava was compared with that deposited in the Herbarium of the Federal University of Sergipe (code ASE 03304; collectors' number: 4122; voucher No. 04531). A crude water-alcohol extract was obtained by macerating dry leaves in 6:4 ethanol:water (v/v) at 27ºC for 10 days and by concentrating the extract in a rotary evaporator (BUCHII RE 111, Buchi Laboratoriums-Technik AG, Flawil, Switzerland). The hexane, chloroform, acetone, ethanol, methanol, and acetic acid extracts were prepared with a Soxhlet extractor using analytical PA grade solvents. The extracts were then stored at 27 ± 2ºC in a dry atmosphere without protection from light for 1 to 6 months until use. The Na+ and K+ contents of each extract were analyzed by flame photometry using an ENGRO 456/E analyzer (Analyser Comércio e Indústria Ltda., São Paulo, SP, Brazil).
Experimental procedure
The experiments were carried out on guinea pig left atria isolated from animals of both sexes (300-500 g) killed by a blow to the head. The heart was promptly excised and the left atrium was transferred to an organ bath where it was mounted and immersed in modified Tyrode solution (137.0 mM NaCl, 5.0 mM KCl, 0.5 mM MgCl2, 12.0 mM NaHCO3, 1.8 mM CaCl2, 6.0 mM glucose, and 1.8 mM NaH2PO4). All salts were of analytical grade, manufactured by Merck S.A. Indústrias Químicas, Rio de Janeiro, RJ, Brazil. The bath was oxygenated and buffered with a carbogen mixture (95% O2 + 5% CO2, purchased from Aga S.A., São Paulo, SP, Brazil or White Martins Gases Industriais S.A., São Paulo, SP, Brazil). The bath temperature was maintained at 27 ± 0.1ºC by means of a thermostatically controlled water jacket. The atria were stretched to 10 mN as a control diastolic tension. Suprathreshold current pulses of 2 Hz, 100 V, and 0.5 ms provided by a Digitimer 4030 and a Digitimer 3072 apparatus (Digitimer Limited, Welwyn Garden City, Hertfordshire, England) were delivered by a pair of Ag/AgCl electrodes placed inside the organ bath to pace the preparations. The electrodes were arranged along the atrium in order to provide simultaneous stimulation of the whole preparation. The isometric force (HP FTA10-1 Sunborn, HP 8805B, Chicago, IL, USA) was recorded with a thermal paper polygraph (HP8805B, HP7754A, HP7754B) and stored in a computer (A/D converter DI-400, WINDAQ Pro Acquisition, DATAQ Instruments, Akron, OH, USA; 512 sample/s) for off-line processing. The preparations were allowed to stabilize for 1-2 h before measurements were made. They were considered well adapted when the force amplitude and the resting tension were stabilized.
Data processing
Stored data were processed automatically with Force2000 (a software developed in our laboratory) to determine the values for the following variables: a) force amplitude, b) contraction time, c) relaxation time (both times were measured at 20, 50, and 80% of force amplitude), and d) force first derivatives related to the contraction and relaxation phases. These results were obtained by processing 50 successive contractions from each experimental procedure. The Student t-test for independent samples was used to determine differences between means at the 5% level of significance (Statistic for Windows). Data are reported as means ± SD. To determine the extract concentration required to produce 50% of the maximum extract effect (EC50) the experimental data were fitted by the Hill-Langmuir curve (23-25) by adjusting the EC50 and the Hill constant for the best fitting to the experimental data.
Drugs and membrane receptors
To study the involvement of membrane receptors in the mechanism of action of extracts of P. guajava on the guinea pig myocardium, the following drugs were used: 1.5 mM atropine sulfate, 0.7 mM propranolol, 0.3 µM acetylcholine chloride, 0.1 µM epinephrine bitartrate, and 120 mM naloxone (Narcan). Drugs were purchased from Sigma-Aldrich (St. Louis, MO, USA) and naloxone from Rhodia Farma (São Paulo, SP, Brazil).
Inward calcium current
The experimental protocol proposed by Nayler and Merrillees (26) was employed to study the effect of extracts of P. guajava on the inward calcium current. The experiments were carried out on animals injected ip with 5 mg/kg reserpine (Gross Laboratory, Rio de Janeiro, RJ, Brazil) 24 h before the experiment. The protocol consisted of measuring atrial force at different stimulation rates: 1) low frequency (12 bpm, control rate), 2) high frequency (60 bpm, 40 s, test rate), 3) no stimulation (silent period: 30 s), and 4) low frequency (12 bpm). This was done using normal Tyrode (control solution) or extract of P. guajava in the bath (test solution).
Results
Figure 1 shows the negative inotropic effect of the crude extract of Psidium. The reduction of atrial force was concentration-dependent and at 3 g/l the extract abolished contractions. In spite of its magnitude, this effect promptly disappeared when the extract was removed from the bath (washout). Similar results were obtained in 15 trials carried out on 8 atria. The Na+ and K+ contents in the crude extract (1 g/l), determined by flame photometry, were 0 and 0.3 mM, respectively. These results indicate that the effect of P. guajava could not be ascribed to these ions.
The experiments with the ethanol and acetic acid extracts showed that these preparations can depress the atrial inotropic mechanism. Figure 2 shows the Hill-Langmuir curves used to determine the EC50 of these extracts. The EC50 for the crude extract was 1.4 g/l (Hill constant = 2), and the EC50 for the ethanol and acetic acid fractions was 4.5 (Hill constant = 3) and 0.07 g/l (Hill constant = 1.2), respectively.
The acetic acid fraction of P. guajava (the most potent extract), used at 0.3 g/l, increased the relaxation time by 35% (Figure 3) when measured at 20% of the force amplitude (91 ± 15 to 123 ± 30 ms, N = 4). The maximum force derivatives for the contraction and relaxation phases decreased in a concentration-dependent fashion, following the same pattern as observed for the force amplitude.
The involvement of the ß-adrenergic, cholinergic, or opioid membrane receptors in the mechanism of action of the acetic acid fraction of P. guajava was also studied. The results showed that neither the opioid nor the ß-adrenergic receptors participate in such mechanism because neither naloxone (120 µM) nor propranolol (0.7 µM) impaired the effect of the acetic acid fraction (0.3 g/l) on the reduction of atrial force. On the other hand, 1.5 mM atropine sulfate was able to block the effect of P. guajava (Figure 4). This result was observed in three different preparations.
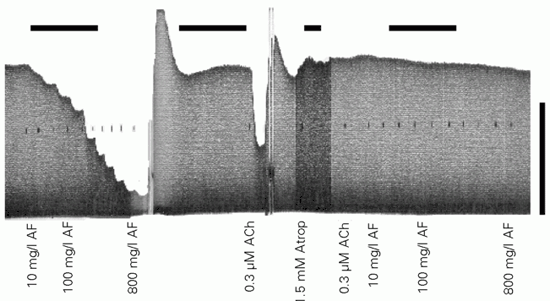
The cholinergic receptor block prevented the acetic acid fraction of P. guajava from reducing the atrial force (Figure 5). Concentration-effect tests were performed by cumulatively increasing the acetic acid fraction in the organ bath (10, 20, 35, 50, 100, 150, 200, 300, 500, and 800 mg/l) before and after adding 1.5 mM atropine sulfate to the bath. Before the cholinergic blockade, both the extract (800 mg/l) and acetylcholine (0.3 µM) reduced the atrial force by 90 and 50%, respectively. These effects, however, disappeared when the cholinergic receptor was blocked by atropine sulfate (N = 7, 15 trials).
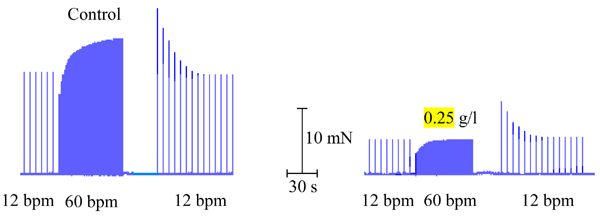
Calcium inward current was studied by the Nayler and Merrillees (26) protocol, which is based on the Bowditch phenomenon. The results showed that, in the control solution, the force overshoot at the end of the positive staircase that was induced by increasing the stimulation rate disappeared when the acetic acid fraction of P. guajava (0.25 g/l) was added to the organ bath (Figure 6). Similar results were observed in 4 hearts (8 trials).
Negative inotropic effect promoted by a crude extract of Psidium guajava L. on an artificially paced guinea pig left atrium. The pace conditions were 2 Hz, 100 V, 0.5 ms, 27 ± 0.1ºC. Similar results were observed in 10 other hearts. Forces were statistically different from that measured for the control before addition of the extract (P<0.05, Student t-test).
Hill-Langmuir plots constructed to estimate the potency of acetic acid (squares, EC50 = 0.07 g/l, Hill constant = 1.2), crude (diamonds, EC50 = 1.4 g/l, Hill constant = 2), and ethanol extracts (triangles, EC50 = 4.5 g/l, Hill constant = 3) of Psidium guajava L. The experiments were carried out on paced guinea pig left atria (2 Hz, 100 V, 0.5 ms, 27 ± 0.1ºC, N = 6 hearts, 13 trials, resting tension: 10 mN).
Effect of acetic acid extract of Psidium guajava on the guinea pig atrial contraction (tc80, tc50, tc20) and relaxation (tr80, tr50, tr20) times. Times were measured at different levels of the force amplitude (80, 50, and 20%). The extract did not significantly change the contraction times, but increased the relaxation times when used at 0.3 g/l. This effect was more evident at the lowest force level (20%, open circles). The effect of P. guajava on the force amplitude (diamonds) is illustrated as reference (N = 3, 6 trials, 27 ± 0.1ºC, 2 Hz).
Neither opioid (upper panel) nor adrenergic (lower panel) receptor blockers inhibited the effect of the acetic acid extract of Psidium guajava on guinea pig atrium myocardial contractility. AF, acetic acid fraction; Nx, naloxone; Adr, adrenaline; Prop, propranolol (N = 3, 6 trials, 27 ± 0.1ºC, 2 Hz).
Cholinergic receptor involvement in the atrial effect of Psidium guajava extracts. The acetic acid fraction (AF, 10, 20, 35, 50, 100, 150, 200, 300, 500, and 800 mg/l) of P. guajava was tested before and after cholinergic blockade with atropine sulfate (Atrop, 1.5 mM, 20 min). The efficacy of the cholinergic blockade was confirmed by adding acetylcholine (ACh, 0.3 µM) to the organ bath before and after atropine sulfate. In the presence of cholinergic receptor block, the acetic acid fraction of P. guajava (same concentrations as used previously) did not reduce the atrial inotropism. Similar results were observed in 7 paced hearts (2 Hz, 100 V, 0.5 ms, 27 ± 0.1ºC; horizontal bars: 10 min; vertical bar: 5 mN).
Nayler and Merrillees (26) protocol used to study the cellular inward calcium current in the guinea pig atrium. Control solution (left panel): the positive staircase (Bowditch phenomenon is seen at 60 bpm) increased the atrial force over its initial value, but in the presence of the acetic acid extract of Psidium guajava (0.25 g/l), in spite of the small positive staircase (60 bpm), the force overshoot did not occur and the final force only reached the initial control level. Similar results were obtained with 4 other preparations (8 trials, 27 ± 0.1ºC).
Discussion
The present study is the first report of the effects of extracts of P. guajava on the mammalian myocardium.
In the guinea pig atria, the crude extract from P. guajava leaves interfered with the contraction mechanism by depressing the myocardial force in a concentration-dependent manner (EC50 = 1.4 g/l). Concentrations higher than 2.5 g/l could even abolish the myocardial contractility. Furthermore, its acetic fraction 1) increased the relaxation time measured at 20 and 50% of the force curve by 30 and 15%, respectively, but 2) did not change the contraction time.
In the effort to isolate the active compound of the crude extract, the following water-soluble leaf extracts were obtained: ethanol, methanol, and acetic acid. Among them, the acetic acid extract was the most potent (EC50 = 0.07 g/l), suggesting that the active principle must be a highly polar compound. Unfortunately, there is no evidence so far for the chemical composition of such a substance. Our results showed that the acetic acid, crude and ethanol extracts exert their myocardial effect with a positive cooperation (Hill constant equal to 1.2, 2 and 3, respectively). According to Hill's theoretical formulation, the Hill constant represents the number of active molecules that are needed to interact with each binding site to promote the effect (24,27).
The negative inotropic effect of the extract of P. guajava was abolished by atropine sulfate. This result, which was also observed on isolated rat tracheal muscle (13), suggests either that the active substance acts as a cholinergic agonist or that it could release acetylcholine from parasympathetic myocardial endings.
Neither the opioid nor the ß-adrenergic membrane receptors seem to be involved in the mechanism of action of the acetic acid extract of P. guajava because neither naloxone nor propranolol blocked its effects. On the other hand, the disappearance of the Bowditch-positive staircase promoted by extracts of P. guajava indicates inhibition of the calcium inward current. Quercetin, which so far is the most important bioactive substance present in P. guajava leaves, does not seem to be directly involved in depression of myocardial contractility because it increased the contraction force in rat isolated atria and also prevented the myocardium from reducing its force due to the acetylcholine effect (28). On the other hand, quercetin antagonized the rate of spontaneous cytoplasmic calcium oscillation (29), a phenomenon related to the calcium-induced calcium release mechanism. In guinea pig ileum, quercetin showed a morphine-like inhibition of acetylcholine release (12).
In our experiments the acetic acid extract of P. guajava abolished the force overshoot (force increase over the force control value) that could be recorded in Tyrode solution when the stimulus rate was increased (Bowditch phenomenon). Such effect points to the inhibition by extracts of P. guajava of the calcium inward current perhaps by blocking the L-type calcium membrane channels. This hypothesis is similar to that proposed for other excitable tissues (2-4). Furthermore, the increased myocardial lusitropy observed in some atria when the extract of P. guajava was added (results not shown) suggests that the intracellular calcium level could be reduced either by a decrease in the calcium inward current and/or by an activation of the calcium pumping system. This effect could be due to the tannins present in the extract (30,31). However, this mechanism does not seem to be operating because the extract promoted only an increase in the relaxation time, indicating that the calcium-pumping mechanism was, in fact, slowed down by the Psidium extract. On the other hand, since atropine blocks the effect of P. guajava on myocardium, more experiments are needed to determine if the calcium inward current is being reduced indirectly as a consequence of action potential shortening due to activation of cholinergic receptors by the extract of P. guajava.
It is clear that myocardial depressant substances are potentially useful clinically because they can be used as heart antiarrhythmic agents. Additional studies are needed on the chemical characterization of the active principle present in the acetic fraction of P. guajava, which depresses the inotropism of the mammalian heart muscle.
Acknowledgments
The authors thank the Gross Laboratory, Rio de Janeiro, RJ, Brazil, for kindly providing the reserpine used in some experiments.
Address for correspondence: E.A. Conde Garcia, Laboratório de Biofísica dos Tecidos Excitáveis, Departamento de Fisiologia, CCBS, UFSE, Av. Marechal Rondon, s/n, 49100-000 São Cristóvão, SE, Brasil. Fax: +55-79-212-6660 ou +55-79-246-3377. E-mail: egarcia@ufs.br
Research supported by CNPq and Universidade Federal de Sergipe. Received January 31, 2002. Accepted January 15, 2003.
- 1. Lozoya X, Becerril G & Martinez M (1990). Modelo de perfusión intraluminal del ileón del cobayo in vitro en el estudio de las propriedades antidiarréicas de la guayaba (Psidium guajava). Archivos de Investigacion Medica, 21: 155-162.
- 2. Morales MA, Trotoriello J, Meckes M, Paz D & Lozoya X (1994). Calcium-antagonist effect of quercetin and its relation with the spasmolytic properties of Psidium guajava L. Archives of Medical Research, 25: 17-21.
- 3. Lozoya X, Meckes M, Abou-Zaid M, Tortoriello J, Nozzolillo J & Arnason JT (1994). Quercetin glycosides in Psidium guajava L. leaves and determination of spasmolytic principle. Archives of Medical Research, 25: 11-15.
- 4. Re L, Barocci S, Capitani C, Vivani C, Ricci M, Rinaldi L, Paolucci A, Scarpantonio A, Léon-Fernandez OS & Morales MA (1999). Effects of some natural extracts on the acetylcholine release at the mouse neuromuscular junction. Pharmacological Research, 39: 239-245.
- 5. Santos FA, Rao VSN & Silveira ER (1998). Investigations on the antinociceptive effect of Psidium guajava leaf essential oil and its major constituents. Phytotherapy Research, 12: 24-27.
- 6. Shaheen HM, Ali BH, Alqarawi AA & Bashir AK (2000). Effect of Psidium guajava leaves on some aspects of the central nervous system in mice. Phytotherapy Research, 14: 107-111.
- 7. Meckes M, Calzada F, Tortoriello J, Gonzalez JL & Martinez M (1996). Terpenoids isolated from Psidium guajava hexane extract with depressant activity on central nervous system. Phytotherapy Research, 10: 600-603.
- 8. Matsuo T, Hanamure N, Shimoi K, Nakamura Y & Tomita I (1994). Identification of (+)- galoocatechin as a bio-antimutagenic compound in Psidium guajava leaves. Phytochemistry, 36: 1027-1029.
- 9. Grover IS & Bala S (1993). Studies on antimutagenic effects of guava (Psidium guajava) in Salmonella typhimurium Mutation Research, 300: 1-3.
- 10. Obatomi DK, Bikomo EO & Temple VJ (1994). Antidiabetic properties of the African mistletoe in streptozotocin-induced diabetic rats. Journal of Ethnopharmacology, 43: 13-17.
- 11. Roman-Ramos R, Flores-Saenz JL & Alarcon-Aguilar FJ (1995). Anti-hyperglycemic effect of some edible plants. Journal of Ethnopharmacology, 48: 25-32.
- 12. Lutterodt GD (1989). Inhibition of gastrointestinal release of acetylcholine by quercetin as a possible mode of action of Psidium guajava leaf extracts in the treatment of acute diarrhoeal disease. Journal of Ethnopharmacology, 25: 235-247.
- 13. Le Grand A (1989). Les phytothérapies anti-infectieuses de la forêt-savane, Senegal (Afrique occidentale) III: Un resumé des substances phytochimiques et l'activité anti-microbienne de 43 species. Journal of Ethnopharmacology, 25: 315-338.
- 14. Tona L, Kambu K, Ngimbi N et al. (2000). Antiamebic and spasmolytic activities of extracts from some antidiarrhoeal traditional preparations used in Kinshasa, Congo. Phytomedicine, 7: 31-38.
- 15. Jaiarj P, Khoohaswan P, Wongkrajang Y, Peungvicha P, Suriyawong P, Saraya ML & Ruangsomboon O (1999). Anticough and antimicrobial activities of Psidium guajava Linn. leaf extract. Journal of Ethnopharmacology, 67: 203-212.
- 16. Lutterodt GD & Maleque A (1988). Effects on mice locomotor activity of a narcotic-like principle from Psidium guajava leaves. Journal of Ethnopharmacology, 24: 219-231.
- 17. Cuellar AC, Lara RA & Zayas JP (1984). Psidium guajava L. tamizaje fitoquímico y estudio del aceite esencial. Revista Cubana de Farmacia, 18: 92-99.
- 18. Coutiño-Rodríguez R, Hernández-Cruz P & Giles-Ríos H (2001). Lectins in fruits having gastrointestinal activity: their participation in the hemagglutinating property of Escherichia coli O157:H7. Archives of Medical Research, 32: 251-257.
- 19. Mercadante AZ, Steck A & Pfander H (1999). Carotenoids from guava (Psidium guajava L.): isolation and structure elucidation. Journal of Agricultural and Food Chemistry, 47: 145-151.
- 20. Nogueira JN, Soybihe Sobrinho J, Vencosvsky R & Fonseca H (1978). Effect of storage on the levels of ascorbic acid and beta-carotene in freeze dried red guava (Psidium guajava L.). Archivos Latinoamericanos de Nutrición, 28: 363-377.
- 21. Opute FI (1978). The component fatty acids of Psidium guajava seed fats. Journal of Scientific and Food Agriculture, 29: 737-738.
- 22. Idstein H, Bauer C & Schreier P (1985). Volatile acids in tropical fruits: cherimoya (Annona cherimolia, Mill.), guava (Psidium guajava, L.), mango (Mangifera indica, L., var. Alphonso), papaya (Carica papaya, L.). Zeitschrift für Lebensmittel-Untersuchung und -Forschung, 180: 394-397.
- 23. Voet V, Voet JG & Pratt CW (2000). Fundamentos de Bioquímica ARTMED Editora, Porto Alegre, RS, Brazil.
- 24. Volkenshtein MV (1983). Biophysics Mir Publishers, Moscow, Russia.
- 25. Rang HP & Dale MM (1991). Farmacologia Editora Guanabara Koogan, Rio de Janeiro, RJ, Brazil.
- 26. Nayler WG & Merrillees NCR (1971). Cellular exchange of calcium. In: Harris P & Opie L (Editors), Calcium and the Heart Academic Press, New York, NY, USA.
- 27. Mahler HR & Cordes EH (1971). Biological Chemistry 2nd edn. Harper & Row Publishers, New York, NY, USA.
- 28. Apisariyakul A, Chaichana N & Takemura H (1999). Dual effects of quercetin on contraction in cardiac and skeletal muscle preparations. Research Communications in Molecular Pathology and Pharmacology, 105: 129-138.
- 29. Wang Y, Wang HY, Yuan ZK, Zhao XN, Wang JX & Zhang ZX (1999). Quercetin decreased heart rate and cardiomyocyte Ca2+ oscillation frequency in rats and prevented cardiac hypertrophy in mice. Zhongguo Yaoli Xuebao (Acta Pharmacologica Sinica), 20: 426-430.
- 30. Coll KE, Johnson RG & McKenna E (1999). Relationship between phospholamban and nucleotide activation of cardiac sarcoplasmic reticulum Ca2+ adenosinetriphosphatase. Biochemistry, 38: 2444-2451.
- 31. Chiesi M & Schwaller R (1994). Reversal of phospholamban-induced inhibition of cardiac sarcoplasmic reticulum Ca(2+)-ATPase by tannin. Biochemical and Biophysical Research Communications, 202: 1668-1673.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
22 Apr 2003 -
Date of issue
May 2003
History
-
Accepted
15 Jan 2003 -
Received
31 Jan 2002