ABSTRACT
Cyperus iria (CYPIR) is one of the main weeds in irrigated rice crops. The intense chemical control with acetolactate synthase (ALS) inhibiting herbicides favors the selection of cross-resistance. This study aimed to evaluate the crossresistance of CYPIR to ALS-inhibiting herbicides in irrigated rice in Rio Grande do Sul. Two experiments arranged in a factorial scheme, in a completely randomized design, with four replications were conducted. Experiment I consisted of resistant (CYPIR-R) and susceptible (CYPIR-S) biotypes and ALS-inhibiting herbicide doses: imazethapyr (106 g a.i. ha-1); pyrazosulfuron-ethyl (20 g a.i. ha-1); penoxsulam (36 g a.i. ha-1) and, as an alternative herbicide, bentazone (960 g a.i. ha-1); corresponding to 0; 1/16; 1/8; 1/4; 1/2; 1; 2; 4; 8 and 16x (x = maximum registered dose). Experiment II followed the same procedures, with doses of 0; 1/64; 1/32; 1/16; 1/8; 1/4; 1/2; 1 and 2x for CYPIR-S and 0; 1/2; 1; 2; 4; 8; 16; 32; 64 and 128x for CYPIR-R; including bispyribac-sodium (50 g a.i. ha-1). The variables evaluated were: visual control at 7, 14, 21 and 28 days after the treatments (DAT) and shoot dry matter (SDM) at 28 DAT. The results, fitted by nonlinear regression, show varied and high levels of cross-resistance of CYPIR-R to ALS-inhibiting herbicides from the group of imidazolinones, sulfonylureas, triazolopyrimidines and pyrimidinyl(thio)benzoates. Bentazone can be used as an alternative herbicide, however, not exclusively in the control of this biotype.
Keywords:
Oryza sativa; Cyperus iria; dose-response curve; resistant biotype
RESUMO
Cyperus iria (CYPIR) é uma das principais espécies daninhas em lavouras de arroz irrigado. O intenso controle químico com herbicidas de diferentes grupos dos inibidores da enzima acetolactato sintase (ALS) favorece a seleção de resistência cruzada. O objetivo deste trabalho foi avaliar a resistência cruzada de CYPIR aos herbicidas inibidores da ALS, em arroz irrigado, no Rio Grande do Sul. Foram conduzidos dois experimentos arranjados em esquema fatorial, em delineamento inteiramente casualizado com quatro repetições. O experimento I constou de biótipos resistente (CYPIR-R) e suscetível (CYPIR-S) e doses de herbicidas inibidores da ALS: imazethapyr (106 g i.a. ha-1), pyrazosulfuron-ethyl (20 g i.a. ha 1), penoxsulam (36 g i.a. ha-1) e, como herbicida alternativo, bentazone (960 g i.a. ha-1), correspondendo a 0, 1/16, 1/8, 1/4, 1/2, 1, 2, 4, 8 e 16x (x = dose máxima registrada). O experimento II seguiu os mesmos procedimentos, com doses de 0, 1/64, 1/32, 1/16, 1/8, 1/4, 1/2, 1 e 2x para CYPIR-S e 0, 1/2, 1, 2, 4, 8, 16, 32, 64 e 128x para CYPIRR, incluindo bispyribac-sodium (50 g i.a. ha-1). As variáveis avaliadas foram: controle visual aos 7, 14, 21 e 28 dias após os tratamentos (DAT) e massa da parte aérea seca (MPAS) aos 28 DAT. Os resultados, ajustados por regressão não linear, comprovaram níveis variados e elevados de resistência cruzada de CYPIR-R aos herbicidas inibidores da ALS do grupo das imidazolinones, sulfonylureas, triazolopyrimidines e pyrimidinyl(thio)benzoates. O bentazone pode ser utilizado como herbicida alternativo, porém não de modo exclusivo, no controle desse biótipo.
Palavras-chave:
Oryza sativa; Cyperus iria; curva de dose-resposta; biótipo resistente
INTRODUCTION
The weed Cyperus iria L. (CYPIR), adapted to wet and flooded environments, is one of the most important cyperaceae that infests irrigated rice cultivations (Lorenzi, 2008Lorenzi H. Plantas daninhas do Brasil: terrestres, aquáticas, parasitas e tóxicas. 4ª.ed. Nova Odessa: Plantarum, 2008. 640p.). It has a prodigious multiplication capacity, due to its short life cycle and to its prolific production of seeds (3-5 thousand seeds per plant). It emerges after the sowing of the rice, flowering in a month, and, thus, it can establish two generations in the same crop (Galinato et al., 1999Galinato, M.I., Moody, K., Piggin, C.M. Upland rice weeds of South and Southeast Asia. Makati City: IRRI, 1999. 156p.); it forms considerable populations in the area where it is established (Moreira and Bragança, 2010Moreira H.J.C., Bragança H.B.N. Manual de identificação de plantas infestantes: arroz. Campinas: FMC, 2010. 854p.).
The occurrence of CYPIR during the rice growing period reduces the productivity of grains of the cereal by 64% (Dhammu and Sandhu, 2002). The rice flatsedge, as it is popularly known, has a C4 photosynthetic mechanism, which, under tropical conditions, gives it a competitive advantage over rice cultivation (C3), such as a greater growth potential and efficient use of light, requiring integrated management practices to control this species (Chauhan and Johnson, 2010Chauhan B.S., Johnson D.E. Responses of rice flatsedge (Cyperus iria) and barnyardgrass (Echinochloa crus-galli) to rice interference. Weed Sci. 2010;58:204-8.).
The chemical method, due to its high efficiency and practicality, is the most used for control of cyperaceae, mainly with herbicides that inhibit the acetolactate synthase (ALS) enzyme (Dal Magro et al., 2010Dal Magro T. et al. Propriedades enzimáticas da enzima ALS de Cyperus difformis e mecanismo de resistência da espécie ao herbicida pyrazosulfuron-ethyl. Ci Rural. 2010;40:2439-45.), also known as acetohydroxy acid synthase (AHAS). The ALS is a key enzyme involved in the branched-chain amino acid biosynthesis (valine, leucine and isoleucine). The inhibition of this enzyme affects the production of these amino acids, resulting in the death of plants (Deng et al., 2014Deng W. et al. Different cross-resistance patterns to AHAS herbicides of two tribenuron-methyl resistant flixweed (Descurainia sophia L.) biotypes in China. Pest Biochem Physiol. 2014;112:26-32.).
The ALS enzyme is the site of action of herbicides of five chemical groups: sulfonylureas (SUs), imidazolinones (IMIs), triazolopyrimidines (TPs), pyrimidinyl(thio)benzoates (PTBs) and sulfonyl-aminocarbonyl-triazolinone (SCT) (Yu and Powles, 2014Yu Q., Powles S.B. Resistance do AHAS inhibitor herbicides: current understanding. Pest Manag Sci. 2014;70:1340-350.). Among their main characteristics, the low toxicity to mammals, the high efficiency and the selectivity are highlighted, controlling the broad spectrum of weeds (Shaner, 1999Shaner D.L. Resistance to acetolactate syntase (ALS) inhibitors in the United States: history, occurrence, detection, and management. J Weed Sci Technol. 1999;44:405-11.). In addition, they are used in low doses (grams per ha-1) (Tranel and Wright, 2002Tranel P., Wright T.R. Resistance of weeds to ALS-inhibiting herbicides: what have we learned? Weed Sci. 2002;50:700-12.), when compared with herbicides of other mechanisms of action.
The ALS inhibitors were first commercialized in 1982 for weed control in cereals (Saari et al., 1994Saari L.L., Cotterman J.C., Thill D.C. Resistance do acetolactate synthase inhibiting herbicides. In: Powles S.B., Holtum J.A.M., editors. Herbicide resistance in plants: biology and biochemistry. Boca Raton: CRC Press, 1994. p.83-139.). In Brazil, the first recorded use in irrigated rice was in 1991 - pyrazosulfuron-ethyl (Sirius®); as others launched in the sequence, they began to be intensely used (Noldin et al., 2009Noldin J.A. Resistência de Fimbristylis miliacea a herbicidas inibidores da ALS. In: Agostinetto D., Vargas L. Resistência de plantas daninhas a herbicidas no Brasil. Passo Fundo: Berthier, 2009. p.215-20.). Nowadays these products represent the largest number of active ingredients for the control of CYPIR in this cultivation, in four groups: SUs, IMIs, TPs and PTBs (AGROFIT, 2016).
Weeds evolve in response to disturbances caused by nature and by man (Radosevich et al., 1997Radosevich S.R., Holt J., Ghersa C. Weed ecology: implications for management. 2nd.ed. New York: Wiley, 1997. 589p.). The repeated use of herbicides with the same mechanism of action over a long period results in the selection of individuals with resistance (Lamego et al., 2009aLamego F.P. et al. Cross-resistance of Bidens subalternans to acetolactate synthase inhibitors in Brazil. Weed Res. 2009a;49:634-41.). The weed resistance to herbicides is the inherent and inheritable capacity of a biotype within a given population to survive and reproduce after being exposed to the herbicide registration dose for species control, meeting the application criteria (Gazziero et al., 2008Gazziero D.L.P., Galli A.J.B., Karam D. editores. Critérios para relatos oficiais estatístico de biótipos de plantas daninhas resistente a herbicidas. Campinas: Associação Brasileira de Ação à Resistência de Plantas Daninhas aos Herbicidas no Brasil, 2008. 22p.).
At a global level, there are 159 records of weed species with resistance to ALS-inhibiting herbicides, making up 63% of the cases. In the irrigated rice crop, there are 42 species involved (Heap, 2016). The use of different herbicides with the same mechanism favors the crossresistance selection (Vidal and Merotto Jr., 1999Vidal R.A., Merotto Jr. A. Resistência de amendoim-bravo (Euphorbia heterophylla L.) aos herbicidas inibidores da enzima acetolactato sintase. Planta Daninha. 1999;17:367-74.), which occurs when the genetic trait that makes biotypes of a certain weed species resistant to a herbicide also makes them resistant to others herbicides with the same mechanism of action, however, of different chemical groups (Deng et al., 2014Deng W. et al. Different cross-resistance patterns to AHAS herbicides of two tribenuron-methyl resistant flixweed (Descurainia sophia L.) biotypes in China. Pest Biochem Physiol. 2014;112:26-32.).
Several cases of cross-resistance to ALS inhibitors have been reported in cyperaceae occurring in irrigated rice, such as Cyperus difformis (Galon et al., 2008Galon L. et al. Resistência de Cyperus difformis a herbicidas inibidores da ALS em lavoura de arroz irrigado em Santa Catarina. Planta Daninha. 2008;26:419-27.), Fimbristylis miliacea (Schaedler et al., 2013Schaedler C.E. et al. Globe fringerush (Fimbristylis miliacea) cross resistance to als-inhibitor herbicides under field conditions in irrigated rice in the south of Brazil. Planta Daninha. 2013;31:893-902.), Schoenoplectus juncoides (Sada et al. 2013Sada Y. et al. Characterization of sulfonylurea-resistant Schoenoplectus juncoides having a target-site Asp376Glu mutation in the acetolactate synthase. Pest Biochem Physiol. 2013;107:106-11.) and, more recently, Cyperus iria (Riar et al., 2015Riar D.S. et al. Acetolactate synthase-inhibiting, herbicide- resistant rice flatsedge (Cyperus iria): cross- resistance and molecular mechanism of resistance. Weed Sci. 2015;63:748-57.). It is important to determine the cross-resistance spectrum, since this information may collaborate with technicians/farmers in order to select alternative herbicides for an effective weed management (Deng et al., 2014Deng W. et al. Different cross-resistance patterns to AHAS herbicides of two tribenuron-methyl resistant flixweed (Descurainia sophia L.) biotypes in China. Pest Biochem Physiol. 2014;112:26-32.).
The identification of resistance is crucial for management techniques (Vidal et al., 2006Vidal R.A., Trezzi F.P. Cresce a resistência das plantas daninhas a herbicidas. Visão Agríc. 2006;5:112-4.). For the scientific evidence, it is recommended to conduct studies in which dose-response curves of the biotype suspected of resistance to the herbicide in question are generated, in a wide range of doses under controlled conditions. Thus, the C50 and the GR50 are determined, which indicate, respectively, the dose of herbicide required to control and reduce the shoot dry matter by 50%, of resistant and susceptible biotypes, relative to the control (Gazziero et al., 2008Gazziero D.L.P., Galli A.J.B., Karam D. editores. Critérios para relatos oficiais estatístico de biótipos de plantas daninhas resistente a herbicidas. Campinas: Associação Brasileira de Ação à Resistência de Plantas Daninhas aos Herbicidas no Brasil, 2008. 22p.).
Cyperus iria biotypes remaining from the chemical control with ALS inhibitors were observed in irrigated rice crops in southern Brazil, indicating a possible case of resistance. Nevertheless, there were no official and statistical reports of this herbicide resistant species in Brazil (Heap, 2016). The objective of this research was to evaluate the cross-resistance of CYPIR to ALS-inhibiting herbicides in irrigated rice in Rio Grande do Sul.
MATERIAL AND METHODS
Plant material
In the 2013/2014 agricultural year, Cyperus iria biotypes (CYPIR) remaining of the treatment with ALS-inhibiting herbicides were observed in an irrigated rice crop in the municipality of Itaqui, Western Border of Rio Grande do Sul (28o55’42’ S, 56o11’18" W). The property has been using the ClearField® technology in irrigated rice for eight consecutive years, and herbicides from the group of imidazolinones, sulfonylureas, pyrimidinyl(thio)benzoates and triazolopyrimidines have been commonly used to control this cyperaceae and other weeds occurring in the area.
In March 2014, 25 plants (CYPIR from 1 to 15 of area A and CYPIR, 16 to 25 of area B) were collected with suspected resistance (SR). Biotypes of susceptible CYPIR (S) from the area where these herbicides were never used (29o09’43" S, 56o33’06" W) were also collected for comparison purposes. The seeds were stored in a dry place and at room temperature until the conduction of preliminary study, in a greenhouse.
The previous study consisted of applying the maximum recommended dose of: imazapyr + imazapic (73.5 + 24.5 g a.i. ha-1 - Kifix®) and imazethapyr (106 g a.i. ha-1 - Imazetapir Plus Nortox) from the group of imidazolinones; penoxsulam (36 g a.i. ha-1 - Ricer®), from the triazolopyrimidines; pyrazosulfuron-ethyl (20 g a.i. ha-1, Sirius 250 SC), from the sulfonylureas; and, as an alternative herbicide, the photosystem II inhibitor (PSII), bentazone (960 g a.i. ha-1 - Basagran® 600), in plants with four leaf stage to a tiller.
The visual diagnosis, 28 days after the application (DAT), showed that area B biotypes survived all treatments with ALS-inhibiting herbicides and were totally controlled by bentazone; on the other hand, the susceptible biotype was efficiently controlled with both mechanisms of action. CYPIR-SR biotypes remaining to the treatments were conducted until the end of the cycle, to obtain seeds (used in the dose-response curve experiment), and were called resistant (CYPIR-R).
Dose-response curve to ALS-inhibiting herbicides
Two experiments were conducted between October 2015 and March 2016 to confirm and evaluate levels of cross-resistance of CYPIR-R to ALS-inhibiting herbicides under controlled conditions at the coordinates 29o09’24,65" S and 56o33’11,97" W. The CYPIR-21 biotype of area B, simply referred to as CYPIR-R, was used.
On October 23, 2015, seeds of CYPIR-R and CYPIR-S were seeded in two trays of 128 cells containing sieved (Embrapa, 2013) and sterilized (autoclave at 100 oC for 1 hour) Haplic Plinthosol, conducted in a low tunnel greenhouse, with floating - 10 cm water sheet. Ten days after the emergence (DAE), they were transplanted to 300 mL perforated (capillary irrigation) plastic units (one per vase), containing commercial substrate and sterilized soil (1:1).
The experimental design was completely randomized, in a 2x4x10 factorial scheme, with four replications. The first factor was composed of the CYPIR-R and CYPIR-S biotypes. The second factor was composed of ALS-inhibiting herbicides: imazethapyr (x = 106 g a.i. ha-1), pyrazosulfuronethyl (x = 20 g a.i. ha-1), penoxsulam (x = 36 g a.i. ha-1) and, as an alternative herbicide, bentazone (x = 960 g a.i. ha-1), in which x is the maximum dose recommended by the manufacturer (AGROFIT, 2016). Dash HC 0.5%, Iharol® 0.25%, Veget Oil® 1% and Assist® 1% (v/v) were respectively added to the herbicides. The third factor consisted of the following doses: 0, 1/16, 1/8, 1/4, 1/2, 1, 2, 4, 8 and 16x. The spray volume, as recorded in AGROFIT (2016), was 200 L ha-1 for the imazethapyr; for the others, it was 100 L ha-1.
When the plants reached the stage of up to 3-4 leaves, the herbicide treatments were applied using a CO2 compression pressure sprayer, equipped with nozzles (0.5 m distant) with flat jet, fan type, model XR 110.02, with pressure of 250 kPa and bar placed at 0.4 m from the target. The average temperature during the application was 21.7 oC, and the relative humidity (RH), above 60%. The sprayed plants were taken to a low tunnel greenhouse, where they remained for two days. After this period, they were transferred to a low tunnel greenhouse with floating.
The visual evaluation of control of the plants at 7, 14, 21 (data not shown) and 28 days after the treatment (DAT) was performed, observing the development of chlorosis, compared to the control, using a percentage scale, being 0% attributed when there was no herbicide symptom and 100% when plants died (Burril et al., 1976). At 28 DAT, the remaining plants were collected near the soil and placed in a forced air oven at 60 oC for 72 hours, to determine the shoot dry matter (SDM).
Between January and March 2016, the experiment was repeated following the same previous procedure, evaluating the mentioned variables, however, the factorial scheme was 2x5x10. The herbicidal doses were: 0, 1/64, 1/32, 1/16, 1/8, 1/4, 1/2, 1 and 2x for the CYPIR-S biotype and 0, 1/2, 1, 2, 4, 8, 16, 32, 64 and 128x for the CYPIR-R biotype. Bispyribac-sodium (50 g a.i. ha-1, Nominee 400 SC), from the pyrimidinyl(thio)benzoate group, was added with 0.25% v/v Iharol and 200 L ha-1 spray volume. For the second experiment, the average temperature was 27.3 oC and the RH, between 77 and 85%, at the beginning and at the end of the application of the treatments.
Statistical analysis
The obtained data were analyzed for normality and homogeneity and submitted to the analysis of variance, ANOVA (p≤0.05), to determine interactions among biotypes x herbicides x doses. When a significant difference was detected, the data were analyzed using nonlinear adjustment models of dose-response curve. Thus, the control and dry matter variables were adjusted (equation 1) according to the model proposed by Streibig et al. (1988).
in which Y corresponds to the control or shoot dry matter (SDM), in percentage of the control (%); X is the herbicidal dose in g a.i. ha-1; a is the maximum asymptote; X0 is the required herbicidal dose that provides 50% of response of the control variable (C50) or reduces the shoot dry matter by 50% (GR50) of CYPIR-R and CYPIR-S biotypes; and b is the slope of the curve around X0. In both variables, the Sigma Plot 10.0 program was used for regression analysis and curve fitting.
The resistance factor (RF), calculated by dividing the C50 or GR50 of the resistant biotype by the correspondents to the susceptible biotype, was also determined. The RF expresses the number of times that the herbicide dose to obtain the C50 or GR50 of the resistant biotype is higher than the C50 or GR50 of the susceptible biotype (Hall et al., 1998Hall L.M. et al. Resistance to acetolactate sintase inhibithors and quinclorac in a biotypes of false clover (Gallium sourium). Weed Sci. 1998;46:390-6.).
RESULTS AND DISCUSSION
The obtained results show a significant difference between resistant biotype (CYPIR R) and susceptible biotype (CYPIR-S) of Cyperus iria, in response to the doses and to the different used herbicides (p≤0.05). The dose-response curve showed that CYPIR-R is insensitive to the labelled dose of ALS herbicides belonging to the group of imidazolinones (IMIs), sulfonylureas (SUs), triazolopyrimidines (TPs) and pyrimidinyl(thio)benzoates (PTBs).
The variables evaluated at 28 DAT in the two experiments (I-2015 and II-2016) indicated that the herbicide dose to control and/or reduce shoot dry matter in 50% of CYPIR R is higher than the maximum recommended - for control of the species in the irrigated rice crop (Tables 1, 2, 3 and 4). The regression model used to adjust the data from the dose-response curve showed that in both biotypes the level of control increases and the dry matter decreases due to the increase in the herbicide dose. Nevertheless, for the CYPIR-R biotype, the level of control and the reduction of the dry matter is smaller and gradual compared to the ones of the susceptible biotype (Figures 1, 2, 3 and 4).
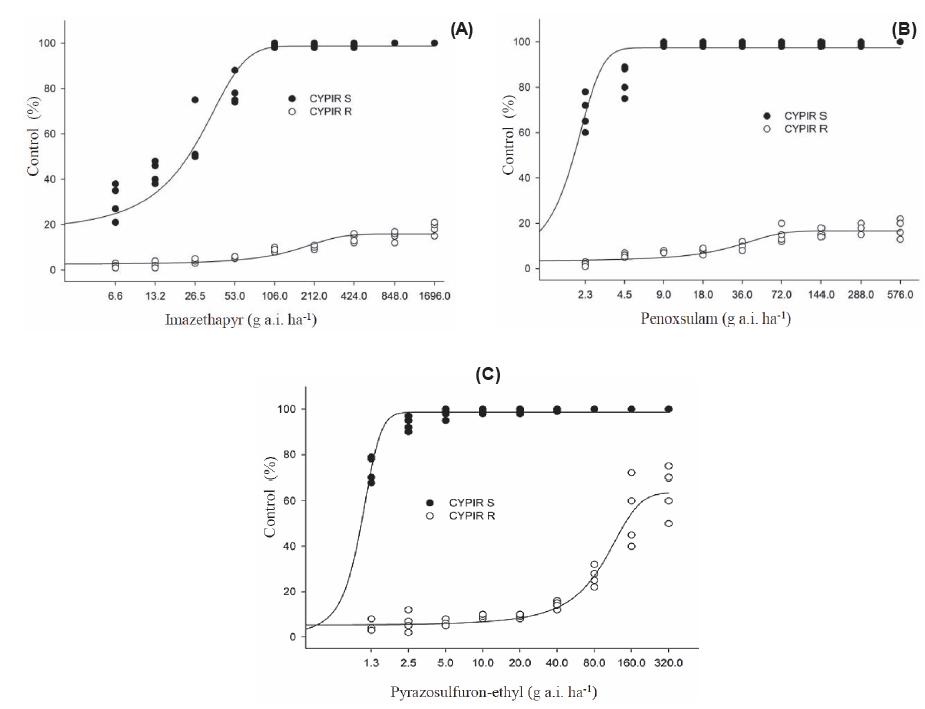
Observed and adjusted control values (% in relation to the control), 28 days after the treatment, of biotype of resistant (○ CYPIR-R) and susceptible Cyperus iria (● CYPIR-S) to the herbicides imazethapyr (A), penoxsulam (B), and pyrazosulfuron-ethyl (C). Experiment I.
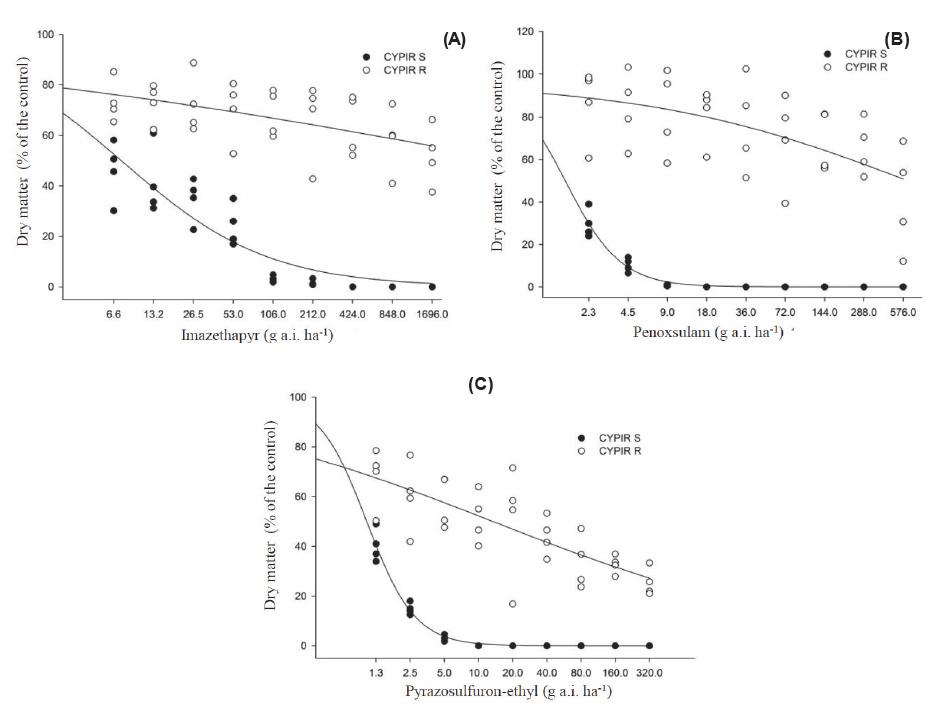
Observed and adjusted shoot dry matter values (% in relation to the control), 28 days after the treatment, of biotype of resistant (○ CYPIRR) and susceptible Cyperus iria (● CYPIR-S) to the herbicides imazethapyr (A), penoxsulam (B), and pyrazosulfuron-ethyl (C). Experiment I.
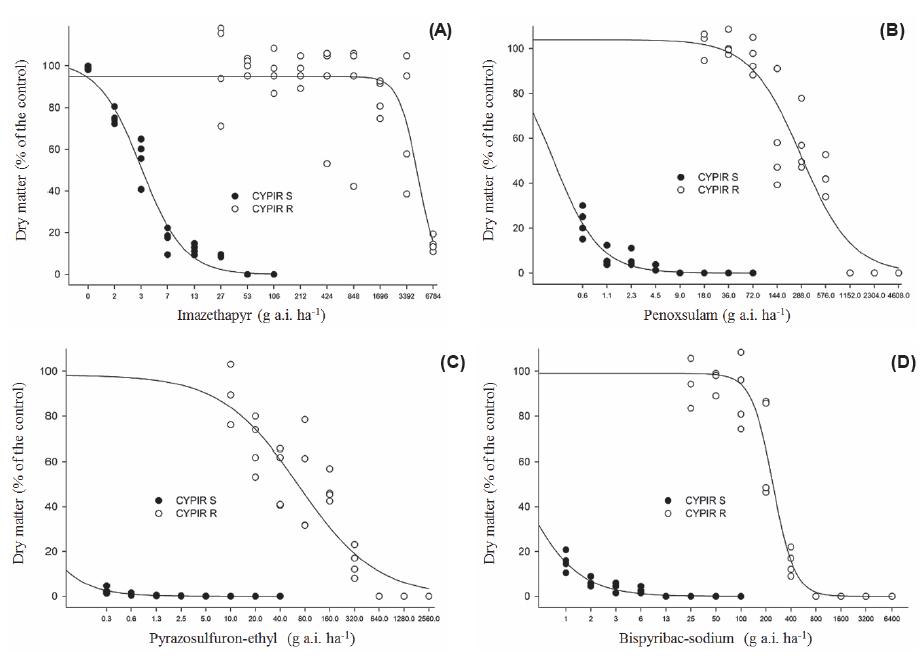
Observed and adjusted control values (% in relation to the control), 28 days after the treatment, of biotype of resistant (○ CYPIR-R) and susceptible Cyperus iria (● CYPIR-S) to the herbicides imazethapyr (A), penoxsulam (B), pyrazosulfuron-ethyl (C) and bispyribac-sodium (D). Experiment II.
Observed and adjusted shoot dry matter values (% in relation to the control), 28 days after the treatment, of biotype of resistant (○ CYPIRR) and susceptible Cyperus iria (● CYPIR-S) to the herbicides imazethapyr (A), penoxsulam (B), pyrazosulfuron-ethyl (C) and bispyribac-sodium (D). Experiment II.
Estimated parameters a, b, R² and C50 by nonlinear regression equation(1), based on the control (in relation % of the control) in bioassay of the whole plant, for resistant biotype and susceptible biotype of Cyperus iria, affected by ALS-inhibiting herbicides 28 days after the treatment. Experiment I
Estimated parameters a, b, R² and GR50 by nonlinear regression equation(1), based on the shoot dry matter (in relation % of the control) in bioassay of the whole plant, for resistant biotype and susceptible biotype of Cyperus iria, affected by ALS-inhibiting herbicides 28 days after the treatment. Experiment I
Estimated parameters a, b, R² and C50 by nonlinear regression equation(1), based on the control (in relation % of the control) in bioassay of the whole plant, for resistant biotype and susceptible biotype of Cyperus iri a, affected by ALS-inhibiting herbicides 28 days after the treatment. Experiment II
Estimated parameters a, b, R² and GR50 by nonlinear regression equation(1), based on the shoot dry matter (in relation % of the control) in bioassay of the whole plant, for resistant biotype and susceptible biotype of Cyperus iria, affected by ALS-inhibiting herbicides 28 days after the treatment. Experiment II
In experiment I, for the CYPIR-R, the model estimated that doses of imazethapyr and penoxsulam higher than 16X were required to obtain the C50 and the GR50 (Tables 1 and 2; Figures 1A,B, 2A,B), and it was not possible to accurately quantify these values to calculate the resistance factor. In experiment II, however, it was possible to determine the C50 and the GR50 for all tested herbicides (Tables 3 and 4). The CYPIR-S biotype was fully controlled up to the registration dose, at both times.
For imazethapyr (IMI), the control of 50% of the CYPIR-R was obtained with a dose 505 times higher than the one of the CYPIR-S, i.e., the C50 was obtained with 4,980 g a.i. ha-1, equivalent to 46.99X (x = 106 g a.i. ha-1) and 9.86 g a.i. ha-1, respectively (Table 3 and Figure 3A). Regarding the reduction of 50% in the dry matter, the one of the CYPIR-R was obtained with a dose 1,366 times higher than the one of the CYPIR-S, i.e., with 9,060 g a.i. ha-1 (equivalent to 85.47X) and 6.63 g a.i. ha-1 (Table 4 and Figure 4A).
The higher herbicidal activity on the susceptible biotype observed in experiment II may be a consequence of relative humidity (RH), about 60% in the first experiment and ranging from 77 to 85% in the second one. Researches, such as the one carried out by Kent (1991Kent L.M. et al. Influence of ammonium-sulfate, imazapyr, temperature, and relative humidity on the absorption and translocation of imazethapyr. Weed Sci. 1991;39:412-6.) using labeled carbon ALS-inhibiting herbicides (C-imazethapyr), indicates that high RH increases the activity of these herbicides. Similar results were observed with penoxsulam.
The herbicide penoxsulam (TP) indicated, in experiment I, that doses above 576 g a.i. ha-1 (16X) were required to obtain the C50 and/or the GR50 of the CYPIR-R (Tables 1 and 2). In experiment II, however, the C50 of the resistant biotype was obtained with 120 g a.i. ha-1 (3.34X, x = 36 g a.i. ha-1) and the GR50 with 287 g a.i. ha-1 (7.97X), while the corresponding values of the CYPIR-S were estimated at 0.44 and 0.24 g a.i. ha-1, respectively. Thus, considering the second experiment, the C50 of the resistant biotype was obtained with a dose of 273 and the GR50 with 1,195 times higher than the one of the susceptible biotype (Tables 3 and 4).
As for the herbicide pyrazosulfuron-ethyl (SU), experiment I indicated values of 91.6 g a.i. ha-1 (4.58X, x = 20 g a.i. ha-1) and 13.61 g a.i. ha-1 (0.68x) to obtain the C50 and the GR50 of the CYPIRR, respectively. The estimated correspondents for the CYPIR-S were 1.03 g a.i. ha-1 and 1.03 g a.i. ha-1 (Tables 1 and 2). In experiment II, however, showing an inverse behavior to the previously evaluated herbicides, there was an increase in the dose required to obtain both the C50 (118 g a.i. ha-1) and the GR50 (67.55 g a.i. ha-1) of the CYPIR R. The correspondents of the CYPIR-S were obtained with 0.25 g a.i. ha-1 and 0.024 g a.i. ha-1, respectively. The RF was 475 to control and 2,814 times higher to reduce the dry matter by 50% (Tables 3 and 4).
The group of imidazolinones (IMIs), sulfonylureas (SUs) and triazolopyrimidines (TPs), chemically different, are widely used in rice fields. The adoption, use (in large scale) and persistence of these herbicides led to the selection of resistant biotypes (Powles and Preston, 2016). Nonetheless, herbicides belonging to the sulfonylurea group bind closer to the active site of ALS than herbicides from other groups, which explains their higher activity (Yu and Powles, 2014Yu Q., Powles S.B. Resistance do AHAS inhibitor herbicides: current understanding. Pest Manag Sci. 2014;70:1340-350.), as evidenced by the high resistance factor related to the SDM reduction.
It should be considered that cross-resistance patterns are extremely complex (Deng et al., 2014Deng W. et al. Different cross-resistance patterns to AHAS herbicides of two tribenuron-methyl resistant flixweed (Descurainia sophia L.) biotypes in China. Pest Biochem Physiol. 2014;112:26-32.), which may also be evidenced in the CYPIR-R biotype in response to the herbicide bispyribacsodium, from the pyrimidinyl(thio)benzoate group (PTBs). C50 and GR50 values of the CYPIR-R were obtained with 246.6 g a.i. ha-1 (4.93X, x = 50 g a.i. ha-1) and 243.7 g a.i. ha-1 (4.87X), respectively. The respective correspondents of the CYPIR-S were obtained with 0.60 g a.i. ha-1 and 0.20 g a.i. ha-1, resulting in a resistance factor of 410.9 and 1,218 times higher (Tables 3 and 4).
Characteristics such as continuous use in agriculture, high efficacy, soil residual activity, high ecological adaptability of the resistant biotype and point mutations may confer resistance of a weed to one or more of the ALS-inhibiting herbicides (Tranel and Wright, 2002Tranel P., Wright T.R. Resistance of weeds to ALS-inhibiting herbicides: what have we learned? Weed Sci. 2002;50:700-12.). In fact, most of the reported cases of resistance to these herbicides are due to a change in the enzyme resulting from a point mutation in the ALS gene. This mutation causes amino acid substitution, which alters the structure of the ALS enzyme and, consequently, its binding site to the herbicides (Ntoanidou et al., 2016Ntoanidou S. et al. Molecular basis of Cyperus difformis cross-resistance to ALS-inhibiting herbicides. Pest Biochem Physiol. 2016;127:38-45.).
Thus, a point mutation or combination of two separate mutations at the site of action may cause resistance to one or more classes of ALS herbicides, and various cross-resistance patterns can be observed (Yu and Powles, 2014Yu Q., Powles S.B. Resistance do AHAS inhibitor herbicides: current understanding. Pest Manag Sci. 2014;70:1340-350.). Generally, mutations in the Asp376 and Trp574 of the enzyme gene confer resistance to the four tested chemical groups (Tranel et al., 2016). For the CYPIR biotypes with high cross-resistance to ALS-inhibiting herbicides, Riar et al. (2015Riar D.S. et al. Acetolactate synthase-inhibiting, herbicide- resistant rice flatsedge (Cyperus iria): cross- resistance and molecular mechanism of resistance. Weed Sci. 2015;63:748-57.) identified the Trp-574 alteration in the enzyme gene. In a biotype of Bidens subalternans, this mutation was also responsible for the cross-resistance to ALS inhibitors, conferring a high level of resistance (Lamego et al., 2009bLamego F.P. et al. Molecular basis of resistance to ALS-inhibitor herbicides in greater beggarticks. Weed Sci. 2009b;57:474-81.). Brosnan et al. (2015) observed that the substitution of the amino acid alanine (Ala-205-Fen) also confers a broad spectrum of resistance to ALS inhibitors.
Bentazone, active ingredient of the benzothiadiazinone group, photosystem II inhibitor (PSII) (HRAC, 2016), controlled 100% CYPIR-R and CYPIR-S biotypes up to the recommended dose (data not shown). For the occurrence of CYPIR biotypes with cross-resistance to ALS-inhibiting herbicides, Riar et al. (2015Riar D.S. et al. Acetolactate synthase-inhibiting, herbicide- resistant rice flatsedge (Cyperus iria): cross- resistance and molecular mechanism of resistance. Weed Sci. 2015;63:748-57.) used 2,4-D (synthetic auxin mimic), bentazone and propanil (PSII) in the alternative chemical control. The control index was considered efficient, i.e., above 93%. In Brazil, C. difformis resistant to pyrazosulfuron-ethyl was 100% controlled with carfentrazoneethyl, bentazone and propanil (Agostinetto et al., 2011Agostinetto D. et al. Resistência de Cyperus difformis L. ao herbicida pyrazosulfuron-ethyl e alternativas de controle. Semina Ci Agr. 2011;32:839-48.).
It is important to emphasize that the exclusive use of an alternative herbicide may cause new selection pressure. When resistance to a group of herbicides is found, it is not enough to replace this group of products with alternative ones. This only tends to select biotypes with multiple resistance (Vidal and Trezzi, 2006Vidal R.A., Trezzi F.P. Cresce a resistência das plantas daninhas a herbicidas. Visão Agríc. 2006;5:112-4.). Therefore, it is advisable to make a reservation regarding the control of CYPIR-R solely with the herbicide bentazone - requiring differentiated products and adoption of integrated management practices, such as crop rotation.
The adoption of integrated management with crop rotation in which soybean, maize or sorghum prevail, for example, allows the exchange of mechanisms of herbicidal action and brings a series of positive aspects to the crop as a whole. The cropping scenario used in the study, with reports of eight years of exclusive adoption of a weed control technology, shows the unsustainability of this management strategy. Previous planning is fundamental, observing the cost-benefit relation, the exchange of mechanisms of herbicidal action and the adoption of strategies not dependent on the chemical control, aiming to delay the evolution of new cases of resistance.
This is the first official and statistical report of cross-resistance of C. iria to the ALS-inhibiting herbicides in Brazil (Heap, 2016). Thus, a judicious use of herbicides of other mechanisms of action is suggested, such as 2,4-D, bentazone and propanil (Riar et al., 2015Riar D.S. et al. Acetolactate synthase-inhibiting, herbicide- resistant rice flatsedge (Cyperus iria): cross- resistance and molecular mechanism of resistance. Weed Sci. 2015;63:748-57.), to decrease the selection pressure and the evolution of resistance in other C. iria biotypes to the ALS inhibitors. The high resistance factor found in the resistant biotype (Tranel et al., 2016), compared to the CYPIR-S, makes it impossible to control the tested herbicides, which may indicate changes in the ALS enzyme resulting from mutations (Devine and Shukla, 2000Devine M.D., Shukla A. Altered target sites as a mechanism of herbicide resistance. Crop Protec. 2000;19:881-9.).
Various levels of cross-resistance of the CYPIR-R biotype to ALS-inhibiting herbicides belonging to the chemical group of imidazolinones; sulfonylureas; triazolopyrimidines; and pyrimidinyl(thio)benzoates were verified. Similar results are plausible with other herbicides with the same mechanism of action. Resistant biotypes often present cross-resistance to herbicides belonging to the same mechanism and may present different patterns to other ALS groups (Manley et al., 1999Manley B.S. et al. Imidazolinone resistance in smooth pigweed (Amaranthus hybridus) is due to an altered acetolactate synthase. Weed Technol. 1999;13:697-705.). Researches with alternative herbicides, detection of the resistance mechanism involved and analysis of the adaptive value of the biotypes should be carried out in sequence.
ACKNOWLEDGMENTS
To the Research Support Foundation of the State of Rio Grande do Sul (FAPERGS) and to the Tutorial Education Program, by granting scholarship to the first and third author, respectively.
REFERENCES
- Agostinetto D. et al. Resistência de Cyperus difformis L. ao herbicida pyrazosulfuron-ethyl e alternativas de controle. Semina Ci Agr. 2011;32:839-48.
- Sistema de consulta a agrotóxicos registrados no Brasil - AGROFIT. [accessed on: 11 jan. 2016]. Available at: www.agricultura.gov.br/agrofit
» www.agricultura.gov.br/agrofit - Brosnan J.T. et al. A new amino acid substitution (Ala-205-Phe) in acetolactato synthase (ALS) confers broad spectrum resistance to ALS-inhibiting herbicides. Planta. 2016;243:149-59.
- Burrill L.C., Cardenas J.C., Locatelli E. Field manual for weed control research. Corvallis: International Plant Protection Center, 1976. 59p.
- Chauhan B.S., Johnson D.E. Responses of rice flatsedge (Cyperus iria) and barnyardgrass (Echinochloa crus-galli) to rice interference. Weed Sci. 2010;58:204-8.
- Christoffoleti P.J. Curvas de dose-resposta de biótipos resistente e suscetível de Bidens pilosa L. aos herbicidas inibidores da ALS. Sci Agric. 2002;59:513-19.
- Devine M.D., Shukla A. Altered target sites as a mechanism of herbicide resistance. Crop Protec. 2000;19:881-9.
- Dhammu, H.S., Sandhu, K.S. Critical period of Cyperus iria L. competition in transplanted rice. In: 13th Australian Weeds Conference: Weeds “Threats now and forever?” Perth: 2002. p.79-82.
- Dal Magro T. et al. Propriedades enzimáticas da enzima ALS de Cyperus difformis e mecanismo de resistência da espécie ao herbicida pyrazosulfuron-ethyl. Ci Rural. 2010;40:2439-45.
- Deng W. et al. Different cross-resistance patterns to AHAS herbicides of two tribenuron-methyl resistant flixweed (Descurainia sophia L.) biotypes in China. Pest Biochem Physiol. 2014;112:26-32.
- Empresa Brasileira de Pesquisa Agropecuária - Embrapa. Sistema brasileiro de classificação de solos. 3ª.ed. Brasília: 2013. 353p.
- Galinato, M.I., Moody, K., Piggin, C.M. Upland rice weeds of South and Southeast Asia. Makati City: IRRI, 1999. 156p.
- Galon L. et al. Resistência de Cyperus difformis a herbicidas inibidores da ALS em lavoura de arroz irrigado em Santa Catarina. Planta Daninha. 2008;26:419-27.
- Gazziero D.L.P., Galli A.J.B., Karam D. editores. Critérios para relatos oficiais estatístico de biótipos de plantas daninhas resistente a herbicidas. Campinas: Associação Brasileira de Ação à Resistência de Plantas Daninhas aos Herbicidas no Brasil, 2008. 22p.
- Hall L.M. et al. Resistance to acetolactate sintase inhibithors and quinclorac in a biotypes of false clover (Gallium sourium). Weed Sci. 1998;46:390-6.
- Heap I. The International Survey of Herbicide Resistant Weeds. [accessed on: 16 Feb. 2016]. Available at: www.weedscience.org
» www.weedscience.org - Herbicide Resistance Action Committee - HRAC. World of herbicides map. [accessed: on: 16 Feb. 2016]. Available at: www.hracglobal.com/pages/world%20of%20herbicides%20map.aspx
» www.hracglobal.com/pages/world%20of%20herbicides%20map.aspx - Kent L.M. et al. Influence of ammonium-sulfate, imazapyr, temperature, and relative humidity on the absorption and translocation of imazethapyr. Weed Sci. 1991;39:412-6.
- Lamego F.P. et al. Cross-resistance of Bidens subalternans to acetolactate synthase inhibitors in Brazil. Weed Res. 2009a;49:634-41.
- Lamego F.P. et al. Molecular basis of resistance to ALS-inhibitor herbicides in greater beggarticks. Weed Sci. 2009b;57:474-81.
- Lorenzi H. Plantas daninhas do Brasil: terrestres, aquáticas, parasitas e tóxicas. 4ª.ed. Nova Odessa: Plantarum, 2008. 640p.
- Manley B.S. et al. Imidazolinone resistance in smooth pigweed (Amaranthus hybridus) is due to an altered acetolactate synthase. Weed Technol. 1999;13:697-705.
- Moreira H.J.C., Bragança H.B.N. Manual de identificação de plantas infestantes: arroz. Campinas: FMC, 2010. 854p.
- Noldin J.A. Resistência de Fimbristylis miliacea a herbicidas inibidores da ALS. In: Agostinetto D., Vargas L. Resistência de plantas daninhas a herbicidas no Brasil. Passo Fundo: Berthier, 2009. p.215-20.
- Ntoanidou S. et al. Molecular basis of Cyperus difformis cross-resistance to ALS-inhibiting herbicides. Pest Biochem Physiol. 2016;127:38-45.
- Powles S.B., Preston C. Herbicide cross resistance and multiple resistance in plants. [accessed on: 15 Feb. 2016]. Available at: www.hracglobal.com/pages/herbicidecrossresistanceandmultipleresistance.aspx
» www.hracglobal.com/pages/herbicidecrossresistanceandmultipleresistance.aspx - Radosevich S.R., Holt J., Ghersa C. Weed ecology: implications for management. 2nded. New York: Wiley, 1997. 589p.
- Riar D.S. et al. Acetolactate synthase-inhibiting, herbicide- resistant rice flatsedge (Cyperus iria): cross- resistance and molecular mechanism of resistance. Weed Sci. 2015;63:748-57.
- Saari L.L., Cotterman J.C., Thill D.C. Resistance do acetolactate synthase inhibiting herbicides. In: Powles S.B., Holtum J.A.M., editors. Herbicide resistance in plants: biology and biochemistry. Boca Raton: CRC Press, 1994. p.83-139.
- Sada Y. et al. Characterization of sulfonylurea-resistant Schoenoplectus juncoides having a target-site Asp376Glu mutation in the acetolactate synthase. Pest Biochem Physiol. 2013;107:106-11.
- Schaedler C.E. et al. Globe fringerush (Fimbristylis miliacea) cross resistance to als-inhibitor herbicides under field conditions in irrigated rice in the south of Brazil. Planta Daninha. 2013;31:893-902.
- Shaner D.L. Resistance to acetolactate syntase (ALS) inhibitors in the United States: history, occurrence, detection, and management. J Weed Sci Technol. 1999;44:405-11.
- Streibig J.C. Herbicide bioassay. Weed Res. 1998;28:479-84.
- Tranel P., Wright T.R. Resistance of weeds to ALS-inhibiting herbicides: what have we learned? Weed Sci. 2002;50:700-12.
- Tranel P.J. et al. Mutations in herbicide-resistant weeds to ALS inhibitors. [accessed on: 21 Feb. 2016]. Available at:www.weedscience.com
» www.weedscience.com - Vidal R.A., Merotto Jr. A. Resistência de amendoim-bravo (Euphorbia heterophylla L.) aos herbicidas inibidores da enzima acetolactato sintase. Planta Daninha. 1999;17:367-74.
- Vidal R.A., Trezzi F.P. Cresce a resistência das plantas daninhas a herbicidas. Visão Agríc. 2006;5:112-4.
- Yu Q., Powles S.B. Resistance do AHAS inhibitor herbicides: current understanding. Pest Manag Sci. 2014;70:1340-350.
Publication Dates
-
Publication in this collection
2017
History
-
Received
25 July 2016 -
Accepted
01 Sept 2016