Abstracts
The objective of this study was to evaluate the effect of the ethanolic extract of Serjania lethalis leaves and stems on the diaspore germination and seedling growth of wild poinsettia (Euphorbia heterophylla) and barnyardgrass (Echinochloa crus-galli). The crude ethanolic extract was prepared from 100 g of dry plant material dissolved in 500 ml of ethanol. The extracts were solubilized in a buffer solution containing dimethyl sulfoxide (DMSO) at concentrations of 10.0, 7.5, 5.0 and 2.5 mg mL-1. The effect of these extracts was compared with herbicide oxyfluorfen in bioassays. The ethanolic extracts of S. lethalis leaves and stems inhibited the germination and seedling growth of barnyardgrass and wild poinsettia in a concentration-dependent manner. The reduction in the root length of E. heterophylla seedlings might be attributed to the reduced elongation of metaxylem cells. The phytotoxicity of the extracts ranged according to the receptor species, and for some variables, the inhibitory effect was similar, and even superior, to that of the commercial herbicide. Thus, S. lethalis extracts might be a promising alternative for sustainable weed management.
allelopathy; cipó-timbó; Echinochloa crus-galli; Euphorbia heterophylla
O objetivo deste estudo foi avaliar o efeito do extrato etanólico de folhas e caules de Serjania lethalis sobre a germinação dos diásporos e crescimento de plântulas de amendoim-bravo (Euphorbia heterophylla) e capim-arroz (Echinochloa crus-galli). O extrato bruto etanólico foi preparado na proporção de 100 g de material vegetal seco para 500 mL de etanol. A partir deste, os extratos foram solubilizados em solução tampão e dimetil sulfóxido (DMSO) nas concentrações 10,0, 7,5, 5,0 e 2,5 mg mL-1. Nos bioensaios comparou-se o efeito desses extratos com o herbicida oxyfluorfen. Os extratos etanólicos de folhas e caules de S. lethalisexerceram atividade inibitória no processo de germinação e no crescimento das plântulas de capim-arroz e amendoim-bravo, com efeito dependente da concentração. Verificou-se que a redução do crescimento radicular das plântulas de amendoim-bravo pode estar relacionada com a diminuição no alongamento das células do metaxilema. A fitotoxicidade dos extratos variou de acordo com a espécie receptora, sendo que para algumas variáveis o efeito inibitório foi similar e até mesmo superior ao do herbicida comercial. Desta maneira, pode-se dizer que os extratos de S. lethalis podem ser uma alternativa promissora para o manejo sustentável de plantas daninhas.
alelopatia; cipó-timbó; Echinochloa crus-galli; Euphorbia heterophylla
ARTICLES
Effect of Serjania lethalis ethanolic extract on weed control
Efeito do extrato etanólico de Serjania lethalis no controle de plantas daninhas
Grisi, P.U.I; Gualtieri, S.C.J.II; Anese, S.I; Pereira, V.C.I; Forim, M.R.III
IPós-Graduação em Ecologia e Recursos Naturais, Departamento de Botânica, Universidade Federal de São Carlos UFSCar, Rodovia Washington Luiz, Km 235, Caixa Postal 676, 13565-905 São Carlos-SP, Brazil, <patriciaumeda@hotmail.com>
IIProfessora Titular, Dep. de Botânica, UFSCar, São Carlos-SP, Brazil
IIIProfessor Adjunto II, Dep. de Química, UFSCar, São Carlos-SP, Brazil
ABSTRACT
The objective of this study was to evaluate the effect of the ethanolic extract of Serjania lethalis leaves and stems on the diaspore germination and seedling growth of wild poinsettia (Euphorbia heterophylla) and barnyardgrass (Echinochloa crus-galli). The crude ethanolic extract was prepared from 100 g of dry plant material dissolved in 500 ml of ethanol. The extracts were solubilized in a buffer solution containing dimethyl sulfoxide (DMSO) at concentrations of 10.0, 7.5, 5.0 and 2.5 mg mL-1. The effect of these extracts was compared with herbicide oxyfluorfen in bioassays. The ethanolic extracts of S. lethalis leaves and stems inhibited the germination and seedling growth of barnyardgrass and wild poinsettia in a concentration-dependent manner. The reduction in the root length of E. heterophylla seedlings might be attributed to the reduced elongation of metaxylem cells. The phytotoxicity of the extracts ranged according to the receptor species, and for some variables, the inhibitory effect was similar, and even superior, to that of the commercial herbicide. Thus, S. lethalis extracts might be a promising alternative for sustainable weed management.
Keywords: allelopathy, cipó-timbó, Echinochloa crus-galli, Euphorbia heterophylla.
RESUMO
O objetivo deste estudo foi avaliar o efeito do extrato etanólico de folhas e caules de Serjania lethalis sobre a germinação dos diásporos e crescimento de plântulas de amendoim-bravo (Euphorbia heterophylla) e capim-arroz (Echinochloa crus-galli). O extrato bruto etanólico foi preparado na proporção de 100 g de material vegetal seco para 500 mL de etanol. A partir deste, os extratos foram solubilizados em solução tampão e dimetil sulfóxido (DMSO) nas concentrações 10,0, 7,5, 5,0 e 2,5 mg mL-1. Nos bioensaios comparou-se o efeito desses extratos com o herbicida oxyfluorfen. Os extratos etanólicos de folhas e caules deS. lethalis exerceram atividade inibitória no processo de germinação e no crescimento das plântulas de capim-arroz e amendoim-bravo, com efeito dependente da concentração. Verificou-se que a redução do crescimento radicular das plântulas de amendoim-bravo pode estar relacionada com a diminuição no alongamento das células do metaxilema. A fitotoxicidade dos extratos variou de acordo com a espécie receptora, sendo que para algumas variáveis o efeito inibitório foi similar e até mesmo superior ao do herbicida comercial. Desta maneira, pode-se dizer que os extratos de S. lethalis podem ser uma alternativa promissora para o manejo sustentável de plantas daninhas.
Palavras-chave: alelopatia, cipó-timbó, Echinochloa crus-galli, Euphorbia heterophylla.
INTRODUCTION
Weeds can be controlled using a combination of cultivation practices, such as sowing rates, mechanical weeding, crop rotation and competitive crops (Mortensen et al., 2000). The use of synthetic herbicides is, however, the main method of weed control due to its the high efficiency and practicality. Nevertheless, the intense application of herbicides causes severe environmental damage and the evolution of herbicide resistant weed populations (Pinto et al., 2008). Recent studies have been focused on the application of the allelopathic process in agriculture, with the goal of using allelochemicals as herbicides to develop more sustainable practices (Chon et al., 2003).
Allelopathic interactions are mediated through secondary metabolites released from donor plants into the environment and influence the growth and development in both natural and agro-ecosystems (Inderjit & Duke, 2003). The allelochemicals belong to a diverse chemical group and have different sites and modes of biochemical action (Reigosa et al., 1999). These substances may vary in composition, concentration and location in the plant (Souza Filho, 2006). Typical allelopathic inhibitory effects result from the action of allelochemicals groups that collectively interfere in various physiological processes altering the growth patterns of plants (Gatti et al., 2010). The action of allelochemicals could affect the respiration, photosynthesis, enzyme activity, water relations, stomatal opening, hormone levels, mineral availability, cell division and elongation, and structure and permeability of cell membranes and wall (Reigosa et al., 1999).
The results of allelopathic studies show that allelochemicals are an alternative to preserve the savanna biome (Oliveira et al., 2004), but only a small number of species have been examined. The savanna suffers from the occurrence of fires, has low nutrient availability and is exposed to seasonal water deficit. These characteristics affect the establishment of plant species and make this biome an important source for allelopathic studies (Oliveira et al., 2004).
Serjania lethalis, commonly called cipó-timbó, is a liana often found in the savannas of Brazil (Fernandes & Negreiros, 2001). The species S. lethalis belongs to the family Sapindaceae, which is a rich source of isoprenoids, polyphenols, saponins, triterpenes and diterpenes (Murgu & Rodrigues-Filho, 2006). The phytochemical composition in the leaves and stems of S. lethalis has been well characterized and includes important pharmacological properties (Napolitano et al., 2005; Lima et al., 2006), but few studies have examined the allelopathic potential of this species and its phytotoxic effect on weeds.
Therefore, the aim of this study was evaluate and quantify the phytotoxic effect of the ethanol extract of S. lethalis leaves and stems on the diaspores germination and seedlings growth of wild poinsettia (Euphorbia heterophylla) and barnyardgrass (Echinochloa crus-galli).
MATERIAL AND METHODS
The leaves and stems of S. lethalis were collected in May 2011 from the savanna area on the campus of Universidade Federal de São Carlos (UFSCar) in São Carlos-SP (21º58' a 22º00' S e 47º51' a 47º52' W), Brazil. After collection, the leaves and stems were dried at 40 °C for 72 hours and ground in an industrial mill.
The ethanolic extract was prepared from 100 g of dry plant material in 500 mL of ethanol. The plant material was exhaustively extracted with ethanol in the dark and cold. After 72 hours, the solution was filtered and concentrated in a rotary evaporator under reduced pressure to generate a crude ethanol extract. Subsequently, the extracts were solubilized in buffer solution (10 mM 2-[N-morpholino] ethanesulfonic acid (MES) and 1M NaOH, pH 6) and DMSO (dimethyl sulfoxide, 5 μL mL-1) at concentrations of 10.0, 7.5, 5.0 and 2.5 mg mL-1 (Macías et al., 2010). Two controls were used in the germination and seedling growth tests: a negative control containing only buffer solution and DMSO and a positive control containing the herbicide oxyfluorfen (240 g i.a. L-1), at doses of 1.0 and 2.0 L ha-1.
Germination bioassay
The ethanol extracts of S. lethalis leaves and stems were applied on diaspores of wild poinsettia (Euphorbia heterophylla) and barnyardgrass (Echinochloa crus-galli). Bioassays were conducted in Petri dishes (9 cm in diameter) containing two sheets of filter paper moistened with 5 mL of extract, herbicide or buffer solution and DMSO.
The experimental design was completely randomized, using four replicates of 30 diaspores. The experiment was conducted in a germination chamber at 25 ºC, with a photoperiod of 12 hours (Inoue et al., 2010). The germination criterion was based on embryo protrusion, which was evaluated every 12 hours during the first seven days of the experiment and at intervals of 24 hours thereafter until the stabilization of germination. The germinability, mean germination time, mean germination rate and rate (Maguire's index) were calculated according to Ranal & Santana (2006).
Seedling growth
For the analysis of wild poinsettia and barnyardgrass seedlings growth, the diaspores were previously germinated in distilled water. Only seedlings with roots of 3 mm in length were selected and transferred to transparent plastic boxes (13 x 8 x 3 cm) containing filter paper moistened with 5 mL of buffer solution and DMSO, ethanolic extracts of leaves and stems or herbicide under the same concentrations adopted for the germination test.
The boxes were maintained in a germination chamber at 25 ºC, with a photoperiod of 12 hours. The experimental design was completely randomized, with four replicates of 10 seedlings. After seven days, the shoot and primary root lengths of the seedlings were measured using a caliper.
Examination of metaxylem cells
Wild poinsettia seedlings were grown in negative control solutions and in the ethanol extracts of S. lethalis leaves and stems under the same conditions adopted for the growth bioassay. After four days, the primary root segments of the seedlings were removed and immersed in 70% alcohol (Gatti et al., 2010). The modified Fuchs staining method was used (Kraus & Arduin, 1997), where the roots were immersed in alcohol (70%) for five days and placed in a solution of 25% NaOH at 60 ºC for 48 hours, until the material was clarified.
Then, the root segments were immersed in safranin (C20H19N4C1) and caustic soda (10% NaOH) for 24 hours at 60 ºC. After staining, the material was mounted on glass slides in Apathy's syrup (Kraus & Arduin, 1997), with the roots, for observation under an optical microscope (Olympus-BX41) coupled to a camera (Sony CCD-IRIS). Four primary roots of wild poinsettia seedlings grown in different concentrations of the extracts and control solutions were used. Half of the length of each root from the central region upward was photographed. From each photograph, 15 central cells of the metaxylem were measured at 20X magnification (Image Pro Plus program) (Gatti et al., 2010).
Statistical analysis
The data were subjected to normality (Shapiro-Wilk) and homogeneity (Levene) tests. When these two assumptions were met, an analysis of variance (ANOVA) was applied followed by Tukey's test at a significance level of 0.05. The linear or quadratic regression models were adjusted when the ANOVA F was significant. The goodness of the models was evaluated by the coefficient of determination (R2). For the variables that showed no significant differences between treatments, the means were represented in the figures with their standard deviations.
The data were subjected to conjoint analyses because the ratio between the larger and smaller residual mean square was not greater than 7 (Pimentel-Gomes, 1990).
RESULTS AND DISCUSSION
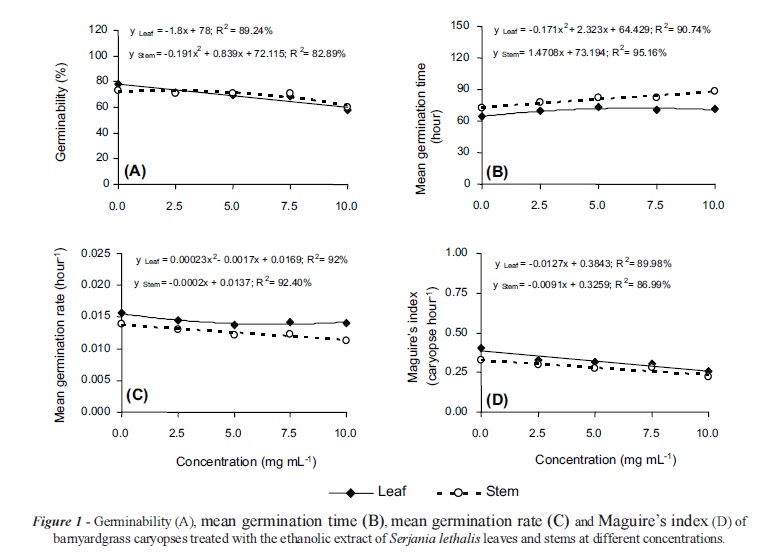
The ethanol extracts of S. lethalis leaves and stems significantly inhibited the germination process of barnyardgrass caryopses. For caryopses seeded with leaf extract, a linear decrease in the germinability and Maguire's index (1.08% and 0.0127 caryopses hour-1 for each addition of 1 mg mL-1 of extract) was observed, where as the mean germination time and mean germination rate reached its maximum (72.32 hours) and minimum (0.0137 hours-1) at estimated concentrations of 6.79 and 3.69 mg mL-1, respectively. When the extract from the stem was applied, the mean germination time increased linearly (1.47 hours for each addition of 1 mg mL-1 of extract) and, as a consequence, there was a linear decrease in the mean germination rate and Maguire's index (0.0002 hours-1 and 0.0091 caryopses hour-1 for each 1 mg mL-1 of extract). The germinability of barnyardgrass was minimal (59%) with 10 mg mL-1 of stem extract (Figure 1).
The barnyardgrass seedlings grown in leaf extracts had the lowest shoot (21.47 mm) and root (4.05 mm) lengths at concentrations of 9.44 and 7.29 mg mL-1, respectively. The application of the stem extracts did not significantly affect the shoot development of these seedlings, but the lowest root length (4.90 mm) was recorded at an estimated concentration of 6.98 mg mL-1 (Figure 2). Grisi et al. (2012) reported that aqueous extracts of Sapindus saponaria leaves also inhibited the germination and seedling development of E. crus-galli, showing the phytotoxicity of other species of Sapindaceae in the barnyardgrass control.
The ethanol extracts of S. lethalis leaves and stems exerted phytotoxic effects on the germination process of wild poinsettia seeds. The application of the stem extract caused a linear reduction of the germinability, mean germination rate and Maguire's index (1.83%, 0.0008 hours-1 and 0.0277 seeds hour‑1 for each addition of 1 mg mL-1 of extract) and, conversely, there was linear increase in the mean germination time (3.47 hours for each 1 mg mL-1 of extract) of these seeds. Minimum values in the germinability (6.75%), mean germination rate (0.0187 hour-1) and Maguire's index (0.2543 seeds hour-1) were observed for the leaf extracts at estimated concentrations of 6.75, 10.0 and 7.61 mg mL‑1, respectively. At a concentration of 7.70 mg mL‑1 of leaf extract, the highest mean germination time (55.56 hours) for wild poinsettia seeds was recorded (Figure 3).
For all parameters evaluated in the growth test, the ethanol extracts of S. lethalis leaves and stems exerted inhibitory effects on the development of wild poinsettia seedlings. The shoot (19.98 mm) and root (8.91 mm) lengths were lowest at concentrations of 7.24 and 7.15 mg mL-1 of leaf extract, respectively. The seedlings grown in stem extracts showed lower shoot and root lengths at estimated concentrations of 7.98 and 8.67 mg mL-1, respectively (Figure 4).
Kern et al. (2009) reported that E. heterophylla seeds showed high resistance to various known allelochemicals, such as rutin, quercetin, aconitic acid, ferulic acid, coumaric acid, vanillic acid and eucalyptol. The wild poinsettia is an aggressive species that is typically difficult to control; therefore, more studies in search of alternatives for managing this weed are needed.
Germination and seedling growth bioassays are important and necessary for determining the responses of the tested substances. However, it is extremely important to use several variables to analyze the germination process, since only the germination percentage does not include the physical and temporal factors involved in this process (Ferreira & Áquila, 2000). The mean germination time is an important factor in the survival of weeds because plants that germinate more slowly might exhibit a reduced size, lower competition for resources and less chance of establishment in the environment. Similarly, root and shoot damage could probably delay, or even prevent, seedling development, which in turn would increase seedling vulnerability and competitive ability, decreasing the chances of a seedling to survive, grow and reach maturity (Souza et al., 2010). Thus, weed control is recommended, particularly during the early stages of development.
Comparing the effects of the extracts from the leaves and stems of S. lethalis with oxyfluorfen herbicide, we observed that the germinability of the wild poinsettia seeds was more affected with the leaf extract; however, the application of herbicide followed by stem extract caused a greater delay in seed germination. The extracts of S. lethalis and herbicide exerted similar effects on the germinability of barnyardgrass, but caryopses treated with herbicide showed higher  , with lower
, with lower  and RATE values (Table 1). The effects on weed growth were similar between the extracts. For both species, the shoot elongation was more affected with herbicide; however, the leaf and stem extracts more strongly inhibited root growth than did the herbicide (Table 1).
and RATE values (Table 1). The effects on weed growth were similar between the extracts. For both species, the shoot elongation was more affected with herbicide; however, the leaf and stem extracts more strongly inhibited root growth than did the herbicide (Table 1).
These results are in agreement with the finding that extracts of allelopathic plants generally have more pronounced effects on radicle rather than hypocotyl growth (Inoue et al., 2010; Grisi et al., 2012). Root growth is characterized by high metabolic rates, and for this reason roots are highly susceptible to environmental stresses, such as allelochemicals in substrate (Cruz-Ortega et al., 1998). At the cellular level, the allelochemical induces lipid peroxidation, affects certain enzymatic activities and rapidly depolarizes the root cell membrane, resulting in a generalized increase in membrane permeability, thus blocking plant nutrient uptake (Santos et al., 2008). According to Souza Filho & Alves (2000), the effect of extracts on root development prevents weed establishment, thus favoring the growth of crop species.
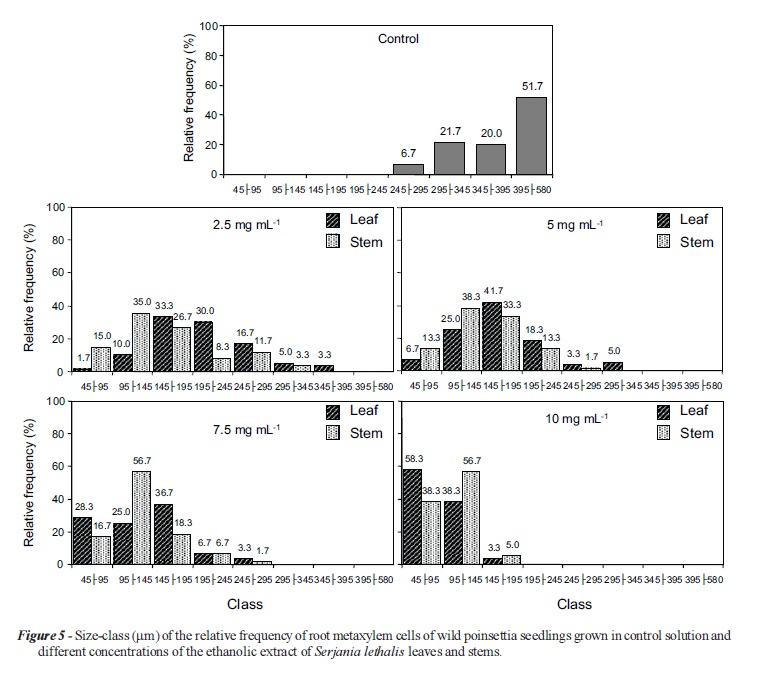
The anatomical study of wild poinsettia roots allowed better visualization of the phytotoxic effects of extracts at the cellular level. The data on the percentage of cells distributed in size classes showed that the control group had a homogenous cell size distribution, with the highest percentage (51.7%) observed for cells between 395 580 μm in length. There were no cells smaller than 245 μm in the control group. The metaxylem cells of seedlings grown under the influence of the leaf extracts at concentrations of 2.5, 5.0 and 7.5 mg mL-1 showed predominance in size between 145 195 μm, whereas at a concentration of 10 mg mL-1, 58.3% of the cells had sizes smaller than 95 μm. For all concentrations of the stem extract, most of the metaxylem cells showed sizes between 95‑145 μm (Figures 5 and 7 ).
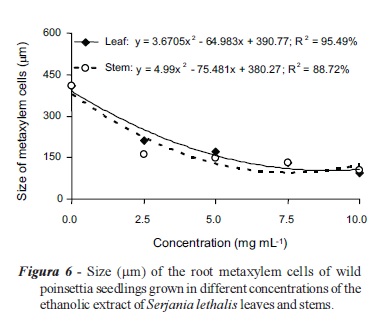
There was no significant difference between the effects of the leaf and stem extracts of S. lethalis on the metaxylem cell length in wild poinsettia roots (Table 1); however, this effect was concentration dependent. The lowest length of these cells was recorded using estimated concentrations of 8.85 and 7.55 mg mL-1 of leaf (95.18 μm) and stem (95.34 μm) extracts, respectively (Figure 6).
The reduction in the growth of wild poinsettia seedlings roots treated with S. lethalis extracts might be associated with inhibition of metaxylem cell elongation, suggesting the potential interference of allelochemicals in the concentrations of hormones, including cytokinins and auxins. These hormones are important in root development, vascular differentiation and gravitropism in plants. Some studies also suggest that these two hormones, combined with ethylene, regulate the initiation of lateral root growth (Aloni et al., 2006). According to Al-Wakeel et al. (2007), the inhibition of cell elongation might be associated with the direct action of allelochemicals through the interference in process of cell division, thus altering the hormone balance.
The effects of hydroalcoholic extracts of Annona crassiflora leaves and stems on the germinability and mean germination rate of wild poinsettia seeds were similar, but the stem extracts more strongly inhibited shoot and root growth than did the leaf extracts (Inoue et al., 2010). Li & Jin (2010) also reported that the stem extracts of Mikania micrantha showed higher inhibitory activity than the leaf extracts. However, the leaves appear to be the most consistent source of chemicals involved in phytotoxicity, producing the greatest inhibitory effects on target species (Souza et al., 2010; Khan et al., 2011).
Phytotoxic activity depends on the concentration of the extracts and the plant organ from which they were extracted (Li & Jin, 2010). Different plant tissues, such as leaves, stems and roots, can release different amounts of allelochemicals into the surrounding environment (Souza Filho, 2006). Nevertheless, the leaves and stems of S. lethalis, although having different physiological functions, showed no significant differences in most bioassay tests.
The ethanol extracts of S. lethalis leaves and stems exerted inhibitory activity on the germination process and seedling growth of barnyardgrass and wild poinsettia with concentration-dependent effect. The reduction in the root growth of wild poinsettia seedlings might be associated with the inhibition of metaxylem cell elongation. The phytotoxicity of the extracts ranged according to the receptor species, and for some variables, the inhibitory effects were similar, and even superior, to the commercial herbicide. Thus, the crude or purified extracts of S. lethalis might be a promising alternative for sustainable weed management. However, further studies are required to isolate and identify the compounds responsible for the phytotoxic activity.
ACKNOWLEDGEMENTS
The authors would like to thank Mrs. Maristela Imatomi for assistance in collecting plant materials and the CAPES and CNPq for financial support.
LITERATURE CITED
Recebido para publicação em 20.11.2012 e aprovado em 19.2.2013.
- ALONI, R. et al. Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann. Bot., v. 97, n. 5, p. 883-893, 2006.
- AL-WAKEEL, S. A. M. et al. Allelopathic effects of Acacia nilotica leaf residue on Pisum sativum L. Allelopathy J., v. 19, n. 2, p. 411-422, 2007.
- CHON, S. U.; KIM, Y. M.; LEE, J. C. Herbicidal potential and quantifi cation of causative allelochemicals from several Compositae weeds. Weed Res., v. 43, n. 6, p. 444-450, 2003.
- CRUZ-ORTEGA, R. et al. Effects of allelochemical stress produced by Sicyos deppei on seedling root ultrastructure of Phaseolus vulgaris and Cucurbita ficifolia J. Chemical Ecol., v. 24, n. 12, p. 2039-2057, 1998.
- FERNANDES, G. W.; NEGREIROS, D. The occurrence and effectiveness of hypersensitive reaction against galling herbivores across host taxa. Ecol. Entomol., v. 26, n. 1, p. 46‑55, 2001.
- FERREIRA, A. G.; ÁQUILA, M. E. A. Alelopatia: uma área emergente da ecofisiologia. R. Bras. Fisiol. Vegetal, v. 12, n. 1, p. 175-204, 2000.
- GATTI, A. B. et al. Allelopathic effects of aqueous extracts of Aristolochia esperanzae O.Kuntze on development of Sesamum indicum L. seedlings. Acta Bot. Bras., v. 24, n. 2, p. 454-461, 2010.
- GRISI, P. U. et al. Allelopathic potential of Sapindus saponaria L. leaves in the control of weeds. Acta Sci. Agron., v. 34, n. 1, p. 1-9, 2012.
- INDERJIT, I.; DUKE S. O. Ecophysiological aspects of allelopathy. Planta, v. 217, n. 4, p. 529-539, 2003.
- INOUE, M. H. et al. Avaliação do potencial alelopático de substâncias isoladas em sementes de araticum (Annona crassiflora). Planta Daninha, v. 28, n. 4, p. 735-741, 2010.
- KHAN, M. et al. Allelopathic effects of Rhazya stricta decne on seed germination and seedling growth of maize. Afr. J. Agric. Res., v. 6, n. 30, p. 6391-6396, 2011.
- KERN, K. A. et al. The phytotoxic effect of exogenous ethanol on Euphorbia heterophylla L. Plant Physiol. Biochem., v. 47,n. 11-12, p. 1095-1101, 2009.
- KRAUS, J. E.; ARDUIN, M. Manual básico de métodos em morfologia vegetal Seropedica, EDUR, 1997. 198 p.
- LI, J.; JIN, Z. Potential allelopathic effects of Mikania micrantha on the seed germination and seedling growth of Coix lacryma-jobi Weed Biol. Manag., v. 10, n. 3, p. 194‑201, 2010.
- LIMA, M. R. F. et al. Anti-bacterial activity of some Brazilian medicinal plants. J. Ethnopharm., v. 105, n. 1, p. 137-147, 2006.
- MACÍAS, F. A. et al. Isolation and phtytotoxicity of terpenes from Tectona grandis J. Chem. Ecol., v. 36, n. 4, p. 396-404, 2010.
- MORTENSEN, D. A.; BASTIAANS L.; SATTIN, M. The role of ecology in the development of weed management systems: an outlook. Weed Res., v. 40, n. 1, p. 49-62, 2000.
- MURGU, M.; RODRIGUES-FILHO, E. Dereplication of glycosides from sapindus saponaria using liquid chromatography-mass spectrometry. J. Braz. Chem. Soc., v. 17, n. 7, p. 1281-1290, 2006.
- NAPOLITANO, D. R. et al. Down-modulation of nitric oxide production in murine macrophages treated with crude plant extracts from the Brazilian Cerrado. J. Ethnopharm., v. 99, n. 1, p. 37-41, 2005.
- OLIVEIRA, S. C. C.; FERREIRA, A. G.; BORGHETTI, F. Efeito alelopático de folhas de Solanum lycocarpum A. St.‑Hil. (Solanaceae) na germinação e crescimento de Sesamum indicum L. (Pedaliaceae) sob diferentes temperaturas. Acta Bot. Bras., v. 18, n. 3, p. 401-406, 2004.
- PIMENTEL-GOMES, F. P. Curso de estatística experimental 13.ed. Piracicaba: Nobel, 1990. 477 p.
- PINTO, J. J. O. et al. Controle de capim-arroz (Echinochloa spp.) em função de métodos de manejo na cultura do arroz irrigado. Planta Daninha, v. 26, n. 4, p. 767-777, 2008.
- RANAL, M. A.; SANTANA, D. G. How and why to measure the germination process? R. Bras. Bot., v. 29, n. 1, p. 1-11, 2006.
- REIGOSA, M. J.; SANCHEZ-MOREIRAS, A.; GONZALES, L. Ecophysiological approach in allelopathy. Crit. Rev. Plant Sci., v. 18, n. 5, p. 577-608, 1999.
- SANTOS, W. D. et al. Soybean (Glycine max) root lignification induced by ferulic acid. the possible mode of action. J. Chem. Ecol., v. 34, n. 9, p. 1230-1241, 2008.
- SOUZA FILHO, A. P. S. Proposta metodológica para análise da ocorrência de sinergismo e efeitos potencializadores entre aleloquímicos. Planta Daninha, v. 24, n. 3, p. 607-610, 2006.
- SOUZA FILHO, A. P. S.; ALVES, S. M. Potencial alelopático de plantas acapu (Vouacapoua americana): efeitos sobre plantas daninhas de pastagem. Planta Daninha, v. 18, n. 3, p. 453-441, 2000.
- SOUZA, F. M. et al. Allelopathic potential of bark and leaves of Esenbeckia leiocarpa Engl. (Rutaceae). Acta Bot. Bras., v. 24, n. 1, p. 169-174, 2010.
Publication Dates
-
Publication in this collection
17 Apr 2013 -
Date of issue
June 2013
History
-
Received
20 Nov 2012 -
Accepted
19 Feb 2013