Abstracts
The objective of this work was to evaluate in vitro storage of Piper aduncum and P. hispidinervum under slow-growth conditions. Shoots were stored at low temperatures (10, 20 and 25°C), and the culture medium was supplemented with osmotic agents (sucrose and mannitol - at 1, 2 and 3%) and abiscisic acid - ABA (0, 0.5, 1.0, 2.0 and 3.0 mg L-1). After six-months of storage, shoots were evaluated for survival and regrowth. Low temperature at 20ºC was effective for the in vitro conservation of P. aduncum and P. hispidinervum shoots. In vitro cultures maintained at 20ºC on MS medium showed 100% survival with slow-growth shoots. The presence of mannitol or ABA, in the culture medium, negatively affected shoot growth, which is evidenced by the low rate of recovered shoots.
ex situ conservation; germplasm bank; micropropagation
O objetivo deste trabalho foi avaliar a conservação in vitro de Piper aduncum e P. hispidinervum, em condições de crescimento lento. Os brotos foram armazenados a baixas temperaturas (10, 20 e 25ºC), e o meio de cultura foi suplementado com agentes osmóticos (sucrose e manitol - a 1, 2 e 3%) e ácido abcísico - ABA (0, 0,5, 1,0, 2,0 e 3,0 mg L-1). Após seis meses de armazenamentos os brotos foram avaliados quanto à sobrevivência e à rebrotação. A baixa temperatura de 20ºC foi efetiva para a conservação in vitro de brotos de P. aduncum e P. hispidinervum. Culturas in vitro, mantidas a 20ºC em meio MS apresentaram 100% de sobrevivência com o crescimento lento dos brotos. A presença de manitol ou ABA, no meio de cultura, afetou negativamente o crescimento de brotos, o que foi evidenciado pelo baixo índice de sobrevivência dos brotos.
conservação ex situ; banco do germoplasma; micropropagação
FITOTECNIA
Tatiane Loureiro da SilvaI; Jonny Everson Scherwinski-PereiraII
IUniversidade de São Paulo, Escola Superior de Agricultura Luiz de Queiroz, Avenida Pádua Dias, nº 11, Caixa Postal 9, CEP 13418-900 Piracicaba, SP, Brazil. E-mail: tatianesilva@usp.br
IIEmbrapa Recursos Genéticos e Biotecnologia, Avenida W5 Norte (final), CEP 70770-917 Brasília, DF, Brazil. E-mail: jonny@cenargen.embrapa.br
ABSTRACT
The objective of this work was to evaluate in vitro storage of Piper aduncum and P. hispidinervum under slow-growth conditions. Shoots were stored at low temperatures (10, 20 and 25°C), and the culture medium was supplemented with osmotic agents (sucrose and mannitol - at 1, 2 and 3%) and abiscisic acid - ABA (0, 0.5, 1.0, 2.0 and 3.0 mg L-1). After six-months of storage, shoots were evaluated for survival and regrowth. Low temperature at 20ºC was effective for the in vitro conservation of P. aduncum and P. hispidinervum shoots. In vitro cultures maintained at 20ºC on MS medium showed 100% survival with slow-growth shoots. The presence of mannitol or ABA, in the culture medium, negatively affected shoot growth, which is evidenced by the low rate of recovered shoots.
Index terms: ex situ conservation, germplasm bank, micropropagation.
RESUMO
O objetivo deste trabalho foi avaliar a conservação in vitro de Piper aduncum e P. hispidinervum, em condições de crescimento lento. Os brotos foram armazenados a baixas temperaturas (10, 20 e 25ºC), e o meio de cultura foi suplementado com agentes osmóticos (sucrose e manitol - a 1, 2 e 3%) e ácido abcísico - ABA (0, 0,5, 1,0, 2,0 e 3,0 mg L-1). Após seis meses de armazenamentos os brotos foram avaliados quanto à sobrevivência e à rebrotação. A baixa temperatura de 20ºC foi efetiva para a conservação in vitro de brotos de P. aduncum e P. hispidinervum. Culturas in vitro, mantidas a 20ºC em meio MS apresentaram 100% de sobrevivência com o crescimento lento dos brotos. A presença de manitol ou ABA, no meio de cultura, afetou negativamente o crescimento de brotos, o que foi evidenciado pelo baixo índice de sobrevivência dos brotos.
Termos para indexação: conservação ex situ, banco do germoplasma, micropropagação.
Introduction
The family Piperaceae has a great number of species with secondary metabolites and essential oils of great interest for the chemical and pharmaceutical industries (Flores et al., 2009). The species Piper aduncum L. and Piper hispidinervum C. DC., popularly known as spiked pepper and long pepper, respectively, are found in the Amazon Region and possess great economic potential due to their phytochemical properties. The dillapiol rich essential oil, extracted from the branches and leaves of spiked pepper, shows insecticide, fungicide, molluscicidal and larvicidal action (Orjala et al., 1994; Maia et al., 1998; Estrela et al., 2006; Rafael et al., 2008). Spiked pepper is also used by local Amazonian populations for its antiulcer, anti-hemorrhagic and anti-diarrhea properties (Rodrigues & Carvalho, 2001). The essential oil extracted from the branches and leaves of long pepper shows safrole, from which two by-products of great economic importance are obtained: heliotropin, extensively used as a fragrance; and piperonyl butoxide, which is one of the substances in the composition of piretrum-based biodegradable insecticides (Estrela et al., 2006).
Because of the economic potential of these species, associated with their indiscriminate use, measures for germplasm conservation are important strategies to guarantee not only the conservation of the species, but also their sustainable use. Moreover, it is necessary to seek a rational way to preserve the high-yielding accessions of P. aduncum and P. hispidinervum, confirmed by field results through techniques which can keep the material with the same genetic characteristics of the original plants. According to Vieira & Silva (2002), P. aduncum and P. hispidinervum are included in the list of priority species for conservation.
The majority of crop plant germplasm is stored in seed repositories at temperatures varying between -15 and -20ºC. However, there are many problems related to plant germplasm which cannot be stored as seeds, because it either does not form seeds or the seeds are recalcitrant, or it does not represent genetic identity with the parental material because of heterozygosity (Scherwinski-Pereira et al., 2010). At present, the germplasm conservation of P. aduncum and P. hispidinervum is in field collections (Skorupa & Vieira, 2005). Although efficient, this strategy is costly, requires large areas, and can be affected by adverse environmental conditions.
In vitro conservation of plant germplasm has been done using slow growth procedures or cryopreservation (Scherwinski-Pereira & Costa, 2010). Slow growth is usually achieved by reducing the culture temperature, by modifying culture media with supplements of osmotic agents and growth inhibitors, or by removing growth promoters to reduce the cellular metabolism of the material, striving to maximize the time between subcultures (Gonçalves & Romano, 2007; Lata et al., 2010; Scherwinski-Pereira et al., 2010).
Osmotic regulators, such as sucrose and mannitol, act as growth retardants by causing osmotic stress to the material under conservation. When added to the culture medium, these carbohydrates reduce the hydric potential and restrict the water availability to the explants (Fortes & Scherwinski-Pereira 2001; Shibli et al., 2006). Besides temperature and osmotic regulators, growth regulators are also routinely used for in vitro germplasm conservation, with abscisic acid (ABA) being one of the most used (Engelmann, 1998).
Although research on the development of conservation techniques has been done for numerous plant species, up to now there is no report of methodologies for in vitro conservation of P. aduncum and P. hispidinervum.
The objective of this work was to assess in vitro storage of P. aduncum and P. hispidinervum under slow growth conditions, in order to develop an efficient protocol for conservation of the genetic diversity of the species.
Materials and Methods
Seeds of P. aduncum and P. hispidinervum, obtained from the germplasm bank of Embrapa Acre, Rio Branco, AC, Brazil, were used in this study. Donor shoot cultures were obtained through axillary bud proliferation from seed germinated in vitro and subcultured every 30 days on MS medium (Murashige & Skoog, 1962). The pH was adjusted to 5.6-5.7 by the addition of 0.1-1.0 N KOH before autoclaving at 120°C for 15 min. Standard growth conditions were: 25±2°C and a 16-hour-light photoperiod at 32 µmol/ m-2/ s-1 with a Philips 40 W cool-white fluorescent lamps, (Philips do Brasil, Barueri, SP, Brazil).
For the experiments, in vitro shoots of approximately 0.8 cm long, with one lateral bud, were used as source of explants. For all experiments, the shoots were put individually in test tubes (20x150 mm), with 10 mL of MS medium, added with 20 g L-1 sucrose. The pH of the media was adjusted to 5.8±0.1 with 0.1 N KOH, before adding the gelling agent (agar, 6.0 g L-1) and autoclaving at 121°C and 1.3 kg cm-2 for 15 min. Each culture tube received one explant and was closed with a polypropylene cap and covered with parafilm.
In order to standardize growth and suitable conditions for the evaluation, after insertion in the tubes, the shoots were maintained for two weeks at 25±2°C and under a 16-hour photoperiod. After this period, surviving shoots were stored at 10, 20 and 25±2°C with a 12-hour photoperiod with a fluorescent light of approximately 38 µmol s-1 m-2 photon flux. After a six-month storage, these shoots were evaluated and exposed to 25±2°C and a 16-hour photoperiod for regrowth and survival and growth evaluations.
The effects of osmotic agents on the survival and regrowth of the in vitro cultures were observed using MS media supplemented with sucrose and mannitol at concentrations of 1, 2 and 3% w/v. Cultures were maintained for six months in a 12-hour photoperiod with fluorescent light and a photon flux of approximately 38 µmol s-1 m-2 at 25±2°C. At the end of this period, the shoots were evaluated for survival and growth.
In vitro shoots were individually placed in test tubes with MS medium with abiscisic acid (ABA) at 0, 0.5, 1.0, 2.0 and 3.0 mg L-1. The culture medium was sterilized using autoclaving and ABA was sterilized with a 0.22 µm filter before adding to the sterilized medium. All materials were cultured under 25±2°C and 12-hour photoperiod, with approximately 38 µmol s-1 m-2 photon flux.
The experiments were carried out in a completely randomized block design. Data were subjected to analyses of variance (ANOVA), and means were compared by Tukey's test at 5% probability. For ABA concentrations, data were evaluated by regression analysis using Sanest (Zonta & Machado, 1984). Each treatment consisted of at least 12 test tubes with one shoot per tube. General observations of growth conditions were recorded monthly for six months. Due to mortality or excessive growth of the cultures, in some treatments, this evaluation period was considered adequate to select the best treatments within each experiment. Cultures were considered to have survived if at least one green bud or shoot was present.
Results and Discussion
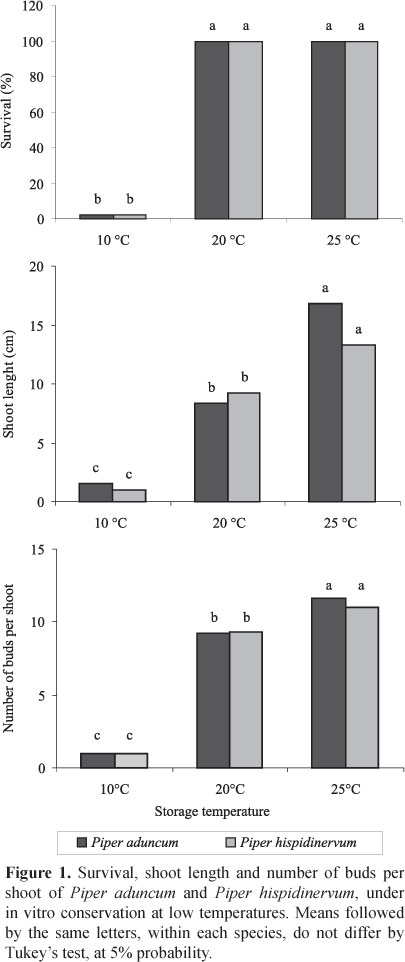
The different temperatures influenced the survival, shoot length and number of buds per shoot of P. aduncum and P. hispidinervum after six months of in vitro conservation. For both species, shoots kept at 10°C resulted in only 2.4% survival, whereas shoots kept at 20°C and 25°C had 100% survival. Significant differences were observed for shoot length and number of buds between temperatures, which increased with the temperature (Figure 1).
Surviving shoots at 10°C had no new leaves or buds. Shoots under 25°C had length and number of buds or shoots larger than those kept at 20°C, which shows that the latter temperature was more effective for maintenance of P. aduncum and P. hispidinervum in vitro.
Growth reduction is achieved by modifying the environmental conditions and the culture medium. The reduction of incubation temperature has been shown to be very effective in prolonging the subculturing cycle by reducing the growth rate (Westcott, 1981; Divakaran et al., 2006; Engelmann, 2011). The results of the present work are also in accordance with those reported by Bekheet (2000) in his study on Asparagus officinalis L. Negash et al. (2001) observed the maintenance viability of banana genotypes for 15 months at 15°C and 18°C and, all genotypes remained viable to supply new explants when they were kept at 15°C. Furthermore, the recommended temperature regimes differ from crop to crop. Some crops are more cold-tolerant than others, and the cultures can be maintained at very low temperatures, as are the cases of temperate crops (Islam et al., 2003). Thus, for gene bank purposes, it is essential to standardize and simplify the culture conditions for plant conservation, due to the high costs involving the decrease in temperature, mainly if the gene banks maintain several tropical and temperate species with different temperature requirements (Engelmann, 1998).
The osmotic regulators sucrose and mannitol significantly affected survival and growth in both species, and the interaction between carbohydrates and concentration was not significant (p>0.05) (Table 1). Microplant survival was 100% for both species, with no differences in the normal growth and development of shoots at different sucrose concentrations. Independently of the species, the cultures grew faster and filled the culture vessel within 90-120 days, and this overgrowth resulted in the drying up of the cultures. Therefore, with sucrose and full-strength MS salts, the cultures could be stored for a maximum of 120 days with 100% survival. However, addition of mannitol (1-3%) reduced growth, but above 1% mannitol, microshoot survival did not surpass 20% and practically ceased at 3%.
Carbohydrates strongly affect growth and physiology of plants in all in vitro culture phases, including conservation, as they serve both as carbon sources for cultured tissues and as osmotic regulators in the medium (Pruski et al., 2000). Sucrose is almost universally used as the most suitable energy source for plant micropropagation. Mannitol has often been added to culture media to mimic osmotic stress, as it is assumed to be only occasionally metabolized by in vitro cultured woody plants (George, 1993).
High mannitol concentrations may be harmful and cause plant death. Sarkar & Naik (1998) reported that 2 or 4% mannitol could enhance survival of plant germplasm conserved in vitro. However, the lethal concentration seems to be species dependent. Lata et al. (2010) reported that 2 to 4% mannitol was not adequate for in vitro conservation of Podophyllum peltatum L., an important medicinal plant. Fortes & Scherwinski-Pereira (2001) observed that mannitol caused a reduction of shoot growth and number of buds in potato in comparison to sucrose.
Depending on the concentration, ABA affected the survival, shoot length and number of buds per shoot for both species (Figure 2). In general, for P. aduncum a decrease in all values was observed with the increase of ABA concentrations. The survival of shoots at 0, 0.5 and 1.0 mg L-1 ABA was 100%; but, from 2.0 mg L-1 ABA, there was a decrease of shoot survival. A linear tendency of decrease in shoot length and number of buds per shoot, was observed with the increase of the ABA concentrations. For P. hispidinervum, ABA concentrations from 1 to 2 mg L-1 showed no significant inhibitory effect on survival, shoot length and buds per shoot. At these concentrations, shoot survival reached 100%, and the shoot length was between 11 and 13 cm with 9 to 10 buds per shoot. However, at 3 mg L-1 ABA, all parameters were negatively affected, reaching 34.5% shoot survival, 8.8 cm shoot length and 6.8 buds per shoot, respectively.
The abscisic acid generally acts as an endogenous growth retardant and has been used for growth reduction of in vitro cultures (Gopal et al., 2004). Jarret & Gawel (1991) succeeded in conserving the nodal segments of sweet potato for over 12 months by addition of 10 mg L-1 ABA. In apple, a woody species, ABA has also been used successfully at 0.5 or
1 mg L-1 concentrations, with improved plant ratings at 21 months (Kovalchuk et al., 2009). In the present study, ABA at 2 and 3 mg L-1 effectively depressed shoot growth in both species, and the survival was negatively affected, reaching very low and dangerous percentages.
Conclusions
1. Piper aduncum and P. hispidinervum in vitro cultures can be conserved for six months in MS medium, at 20°C, without losing regeneration capacity and without apparent morphological changes.
2. For P. aduncum and P. hispidinervum, the presence of mannitol (from 1 to 3%) or ABA (from 1 to 3 mg/ L-1), in the culture medium, negatively affects shoot growth, which is evidenced by the rate of recovered shoots after storage.
Acknowledgements
To Conselho Nacional de Desenvolvimento Científico e Tecnológico, for financial support and fellowships.
Received on November 19, 2010 and accepted on April 18, 2011
- BEKHEET, S.A.; TAHA, H.S.; SAWIRES, E.S.; EL-BAHR, M.K. Salt stress in tissue cultures of Asparagus officinalis Egyptian Journal of Horticulture, v.27, 275-187, 2000.
- DIVAKARAN, M.; BABU, K.N.; PETER, K.V. Conservation of Vanilla species, in vitro. Scientia Horticulturae, v.110, p.175-180, 2006.
- ENGELMANN, F. In vitro germplasm conservation. Acta Horticulturae, n.461, p.41-47, 1998.
- ENGELMANN, F. Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cellular and Development Biology - Plant, v.47, p.5-16, 2011.
- ESTRELA, J.L.V.; FAZOLIN, M.; CATANI, V.; ALÉCIO, M.R.; LIMA, M.S. de. Toxicidade de óleos essenciais de Piper aduncum e Piper hispidinervum em Sitophilus zeamais Pesquisa Agropecuária Brasileira, v.41, p.217-222, 2006.
- FLORES, N.; JIMÉNEZ, I.A.; GIMÉNEZ, A.; RUIZ, G.; GUTIÉRREZ, D.; BOURDY, G.; BAZZOCCHI, I.L. Antiparasitic activity of prenylated benzoic acid derivatives from Piper species. Phytochemistry, v.70, p.621-627, 2009.
- FORTES, G.R. de L.; SCHERWINSKI-PEREIRA, J.E. Preservação in vitro de batata com ácido acetilsalicílico e duas fontes de carboidrato. Pesquisa Agropecuária Brasileira, v.36, p.1261-1264, 2001.
- GEORGE, E.F. Plant propagation by tissue culture 2nd ed. Exegetic: Edington, 1993. 547p.
- GONÇALVES, S.; ROMANO, A. In vitro minimum growth for conservation of Drosophyllum lusitanicum Biologia Plantarum, v.51, p.795-798, 2007.
- GOPAL, J.; CHAMAIL, A.; SARKAR, D. In vitro production of microtubers for conservation of potato germplasm: effect of genotype, abscisic acid, and sucrose. In Vitro Cellular and Developmental Biology - Plant, v.40, p.485-490, 2004.
- ISLAM, M.T.; LEUNUFNA, S.; DEMBELE, D.P.; KELLER, E.R.J. In vitro conservation of four mint (Mentha spp.) accessions. Plant Tissue Culture, v.13, p.37-46, 2003.
- JARRET, R.L.; GAWEL, N. Abscisic acid-induced growth inhibition of sweet potato (Ipomoea batatas L.) in vitro. Plant Cell, Tissue and Organ Culture, v.24, p.13-18, 1991.
- KOVALCHUK, I.; LYUDVIKOVA, Y.; VOLGINA, M.; REED, B.M. Medium, container and genotype all influence in vitro cold storage of apple germplasm. Plant Cell, Tissue and Organ Culture, v.96, p.127-136, 2009.
- LATA, H.; MORAES, R.M.; BERTONI, B.; PEREIRA, A.M.S. In vitro germplasm conservation of Podophyllum peltatum L. under slow growth conditions. In Vitro Cellular and Developmental Biology-Plant, v.46, p.22-27, 2010.
- MAIA, J.G.S.; ZOHHBI, M.G.B.; ANDRADE, E.H.A.; SANTOS, A.S.; SILVA, M.H.L.; LUZ, A.I.R.; BASTOS, C.N. Constituents of the essential oil of Piper aduncum L. growing wild in the Amazon region. Flavour and Fragrance Journal, v.13, p.269-272, 1998.
- MURASHIGE, T.; SKOOG, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum, v.15, p.473-497, 1962.
- NEGASH, A.; KRENS, F.; SCHAART, J.; VISSER, B. In vitro conservation of enset under slow-growth conditions. Plant Cell, Tissue and Organ Culture, v.66, p.107-111, 2001.
- ORJALA, J.; WRIGHT, A.D.; BEHRENDS, H.; FOLKERS, G.; STICHER, O.; RÜEGGER, H.; RALI, T. Cytotoxic and antibacterial dihydrochalcones from Piper aduncum Journal of Natural Products, v.57, p.18-26, 1994.
- PRUSKI, K.; KOZAI, T.; LEWIS, T.; ASTAKIE, T.; NOVAK, J. Sucrose and light effects on in vitro cultures of potato, chokecherry and Saskatoon berry during low temperature storage. Plant Cell, Tissue and Organ Culture, v.63, p.215-221, 2000.
- RAFAEL, M.S.; HEREIRA-ROJAS, W.J.; ROPER, J.J.; NUNOMURA, S.M.; TADEI, W.P. Potential control of Aedes aegypti (Diptera: Culicidae) with Piper aduncum L. (Piperaceae) extracts demonstrated by chromosomal biomarkers and toxic effects on interphase nuclei. Genetics and Molecular Research, v.7, p.772-781, 2008.
- RODRIGUES, V.E.G.; CARVALHO, D.A. de. Levantamento etnobotânico de plantas medicinais no domínio do Cerrado na Região do Alto Rio Grande - Minas Gerais. Ciência e Agrotecnologia, v.25, p.102-123, 2001.
- SARKAR, D.; NAIK, P.S. Factors affecting minimal growth conservation of potato microplants in vitro. Euphytica, v.102, p.275-280, 1998.
- SCHERWINSKI-PEREIRA, J.E.; COSTA, F.H.S. Conservação in vitro de recursos genéticos de plantas: estratégias, princípios e aplicações. In: BARRUETO CID, L.P. (Org.). Cultivo in vitro de plantas Brasília: Embrapa Informação Tecnológica, 2010. p.177-234.
- SCHERWINSKI-PEREIRA, J.E.; COSTA, F.H.S.; CAMILLO, J.; SILVA, D.B.; ALVES, R.B.N.; VIEIRA, R.F. Tissue culture storage of Brazilian medicinal plants germplasm. Acta Horticulturae, n.860, p.211-214, 2010.
- SHIBLI, R.D.; SHATNAWI, M.A.; SUBAIH, W.S.; AJLOUNI, M.M. In vitro conservation and cryopreservation of plant genetic resources: a review. World Journal of Agricultural Sciences, v.2, p.372-382, 2006.
- SKORUPA, L.A.; VIEIRA, R.F. Coleta de germoplasma de plantas medicinais. In: WALTER, B.M.T.; CAVALCANTI, T.B. (Org.). Fundamentos para a coleta de germoplasma vegetal Brasília: Embrapa Recursos Genéticos e Biotecnologia, 2005. p.435-468.
- VIEIRA, R.F.; SILVA, S.R. (Coord.). Estratégias para conservação e manejo de recursos genéticos de plantas medicinais e aromáticas: resultados da 1Ş reunião técnica. Brasília: Embrapa Recursos Genéticos e Biotecnologia, 2002. 184p.
- WESTCOTT, R.J. Tissue culture storage of potato germplasm. 1. Minimal growth storage. Potato Research, v.24, p.331-342, 1981.
- ZONTA, E.P.; MACHADO, A.A. SANEST - sistema de análise estatística para microcomputadores Pelotas: Universidade Federal de Pelotas, 1984. 138p.
In vitro conservation of Piper aduncum and Piper hispidinervum under slow-growth conditions
Publication Dates
-
Publication in this collection
27 June 2011 -
Date of issue
Apr 2011
History
-
Accepted
18 Apr 2011 -
Received
19 Nov 2010