Abstract
Immatures of Acanthocinini (Coleoptera, Cerambycidae, Lamiinae). Larva and pupa of Eutrypanus dorsalis (Germar, 1928), collected in trunks of Pinus elliottii Engelm., and Paratenthras martinsi Monné, 1998, collected in spathes of Scheelea phalerata (Mart. ex Spreng.) Burret, are described and illustrated. Larva and pupa of Lophopoeum timbouvae Lameere, 1884, collected in Hymenaea corbaril L., Enterolobium contortisiliquum (Vell.) Morong and Pterogyne nitens Tul., are redescribed and illustrated. A table with all described immatures of Lamiinae, and a comparison among the immatures of Acanthocinini are presented. Biological notes and new records are also included.
Arecaceae; Cariniana estrellensis; egg; Euterpe edulis; host plants; Insecta
SYSTEMATICS, MORPHOLOGY AND BIOGEOGRAPHY
Immatures of Acanthocinini (Coleoptera, Cerambycidae, Lamiinae)
Sônia A. CasariI; Édson P. TeixeiraII
IMuseu de Zoologia, Universidade de São Paulo, Caixa Postal 42494, 04218970 São Paulo-SP, Brazil. casari@usp.br
IIInstituto Agronômico de Campinas, Caixa Postal 28, 13012970 Campinas-SP, Brazil. edson@iac.sp.gov.br
ABSTRACT
Immatures of Acanthocinini (Coleoptera, Cerambycidae, Lamiinae). Larva and pupa of Eutrypanus dorsalis (Germar, 1928), collected in trunks of Pinus elliottii Engelm., and Paratenthras martinsi Monné, 1998, collected in spathes of Scheelea phalerata (Mart. ex Spreng.) Burret, are described and illustrated. Larva and pupa of Lophopoeum timbouvae Lameere, 1884, collected in Hymenaea corbaril L., Enterolobium contortisiliquum (Vell.) Morong and Pterogyne nitens Tul., are redescribed and illustrated. A table with all described immatures of Lamiinae, and a comparison among the immatures of Acanthocinini are presented. Biological notes and new records are also included.
Keywords: Arecaceae; Cariniana estrellensis; egg; Euterpe edulis; host plants; Insecta.
The subfamily Lamiinae includes about 3,051 genera and 20,652 species separated in 78 tribes, distributed all over the world (Roguet 2013). Thirty-nine tribes are recorded in the Western Hemisphere (Monné & Bezark, 2009).
The tribe Acanthocinini is composed of 150 genera and about 1,045 species, recorded from Western Hemisphere; the genus Eutrypanus Thomson, 1860 is formed by 5 species, Paratenthras Monné, 1998 is monotypic, and Lophopoeum Bates, 1863 includes 15 species (Monné & Bezark 2009).
Considering the large number of the species, the immature forms of these taxa are poorly known (Appendix I Appendix I ). Craighead (1923) treating of the "North American Cerambycidae larvae" characterized the larvae of the family Cerambycidae and included a key to subfamilies. Of the Lamiinae, he presented a characterization of the subfamily and keys to genera and to species for some genera. Also he described the larva and pupa of 44 species and only the larva of other 62 species, belonging to 45 genera.
Böving & Craighead (1931) presented a key to Cerambycidae subfamilies and the illustrations of the head (dorsal and ventral) of each subfamily. Of the Lamiinae, they illustrated Graphisurus LeConte, 1850 and another undetermined species. They characterized the larva of Lamiinae as follows: head oblong, sides parallel or converging behind; epistoma never projecting; tentorial cross-arm internal; epipleurum protuberant on several or all abdominal segments; legs usually absent.
Peterson (1960), who treated the larvae of insects of Nearctic species, described the habits and the larvae of the family Cerambycidae, and included the descriptions and illustrations of Dectes spinosus Say [= Dectes sayi Dillon & Dillon, 1853], Graphisurus sp., prob. fasciata De Geer [from Craighead (1923)], Hippopsis leminiscata (Fabricius, 1801) [from Craighead (1923)], Monochamus scutellatus (Say, 1824) [from Craighead (1923)], Oberea bimaculata Olivier [= O. perspicillata Haldeman, 1847] [from Craighead (1923)], Oberea tripunctata (Swederus, 1787) [from Ruggles (1915)], Saperda calcarata Say, 1824 and Saperda vestita Say, 1824 [from Craighead (1923)]. Lawrence (1991) discussed the relationships of Cerambycidae larvae and presented diagnoses, biology and ecology, descriptions and comments about the family. For Lamiinae, he included a short diagnosis and the same illustrations of Peterson (1960).
The most complete work on immature stages of the Neotropical timber beetles is the book of Duffy (1960) that includes keys to subfamilies, genera and species of the known immatures of the Cerambycidae. For the majority of the studied species he presented, besides the description and illustration of the larva, also those of the pupa. For larvae, he treated 160 species in 123 genera; for pupae, 78 species in 67 genera. A total of eight subfamilies were treated. Regarding the subfamily Lamiinae, he presented the description of at least one stage (egg, mature larva or pupa) of 64 species belonging to twenty tribes. Larval characterization for the majority of the tribes was also presented. The tribes with largest number of described species is Acanthocinini (23) followed by Acanthoderini (12) and Onciderini (6).
Following this earlier work, other studies have described immatures in Lamiinae: seven species were described for Onciderini (Cosarinsky 1996; Giacomel 1992; Marinoni 1969; Marinoni & Silva 1973; Napp 1977; Penteado-Dias 1979; Zajciw 1974), four for Acanthoderini (Zajciw 1964, 1975; Galileo et al. 1993; Mermudes & Monné, 2001), three for Acanthocinini (Costa et al. 1988; Di Iorio 1995; Casari & Martins 2011) and one for Hemilophini (Casari & Martins 2013).
Presently, immatures of 80 species belonging to 49 genera of Acanthocinini were described, including Eutrypanus triangulifer Erichson, 1847 and Lophopoeum timbouvae, eight of which are recorded from Brazil. Eutrypanus incertus was transferred to Neoeutrypanus and is not considered here. Herein, larva and pupa of the Eutrypanus dorsalis (Germar, 1928) and Paratenthras martinsi Monné, 1998 are described and those of the Lophopoeum timbouvae Lameere, 1884 are redescribed.
MATERIAL AND METHODS
The studied material is preserved in alcohol 70ºGL and deposited in the Coleoptera Immature Collection of the Museu de Zoologia da Universidade de São Paulo (MZSP), except for some dried adults of each species that are deposited in the Collection of the Instituto Agronômico de Campinas (IACC).
The material of the three species here described was reared in laboratory. The host plants or fruits with larvae were collected and maintained in cages for observations until the emergence of the adults. The terminology follows Duffy (1960) and the classification of Acanthocinini, that of Monné & Bezark (2009).
Appendix I Appendix I includes all American species of Lamiinae with described immature forms and represents the current knowledge of the immatures of the subfamily. The data were taken from the description of each species and sometimes are incomplete.
DESCRIPTIONS
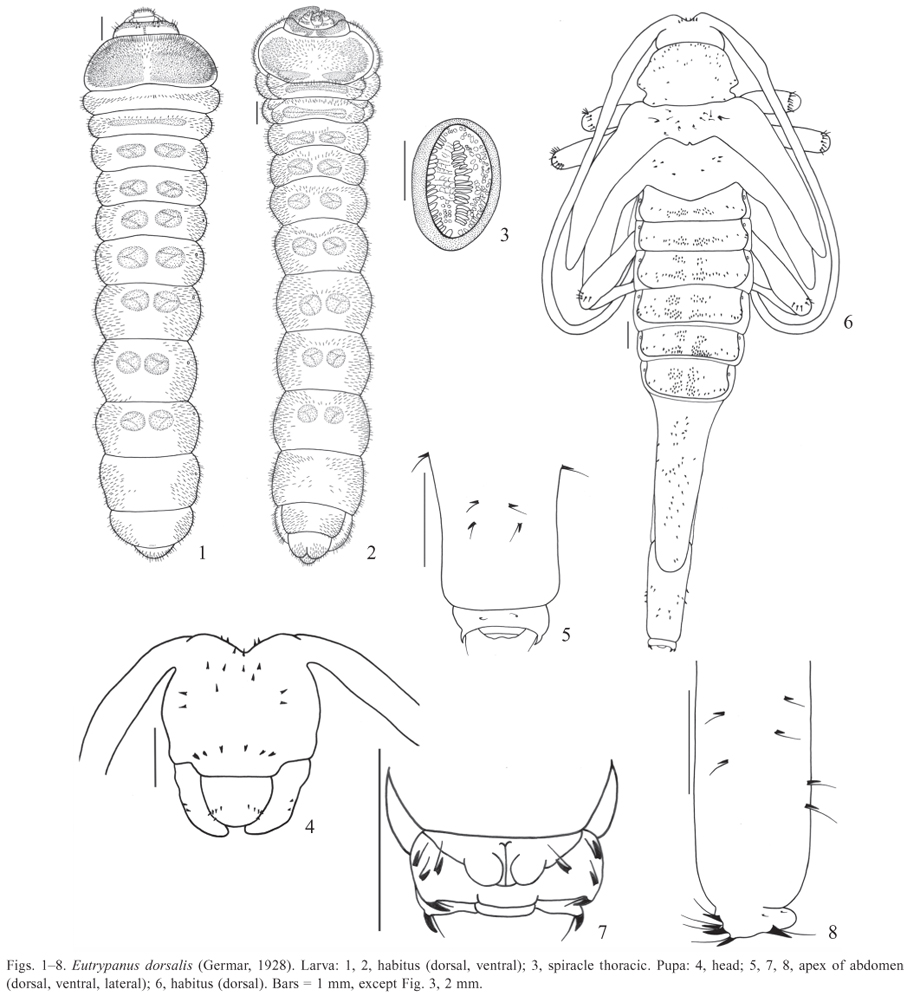
Eutrypanus dorsalis (Germar, 1928)
(Figs. 121, 7577 )
Larva. Length: 2125 mm; width of prothorax: 56 mm.
Elongate, cylindrical and slightly flattened (Figs. 1, 2). General coloration cream; mandibles black; head yellow with anterior narrow dorsal and ventral black band and one longitudinal dorsal carina on each side. Thorax and abdomen with ambulatory ampullae; some areas of ampullae and prothorax covered with micro-spiculi. Prothorax with one dorsal and one ventral yellow patch; ventral patch interrupted at middle and darker near base. Pubescence yellowish-brown, moderately long and dense, more concentrate laterally.
Head (Figs. 910) deeply retracted into prothorax; elongate, prognathous and strongly depressed; sides constricted after middle; retracted portion two-fifths of head length; occipital foramen entire. Head capsule dorsal composed by two epicranial halves; median suture long, continuous with endocarina; one carina each side, not reaching anterior and basal margins. Frontoclypeal suture distinct and almost straight; six epistomal setae; in one specimen, seven; each epicranial half with two setae below epistomal setae and 7 lateral (three short); two tiny setae each side of median suture. Stemmata absent. Ventrally, each side of head capsule with one long seta on dark band, two setae below antenna and six near lateral margin. Gular suture indistinct; gular area triangular with one setae each side. Antennae (Figs. 11, 12) very short, retractable, with two antennomeres: antennomere basal membranous and distal bearing at apex one well developed sensorial membranous appendix with one long seta each side, two short and wide setae dorsal and one small sensorial appendix ventral, latero-internally. Antennal foramen closed posteriorly. Clypeus (Fig. 13) trapezoidal, translucent and glabrous. Labrum (Fig. 13) transverse, semi-elliptical, marginate by band of long setae. Epipharynx (Fig. 14) with fore angles rounded; one elongate sclerite each side on basal twothirds, wider anteriorly; distal third with wide and moderately long setae directed to middle; median region microspined with some setae and some campaniform sensilla; microspined area running like a narrow band internally each sclerite on distal half. Mandibles (Figs. 1821) symmetrical and wide with small rounded dorsal lobe near apex; cutting area wide and grooved; three lateral setae. Maxilla (Figs. 15, 17): cardo and submentum fused; stipes membranous with transversal inclined sclerotized band; long setae near lateral margins, some near sclerotized band; setae and campaniform sensilla at middle, near base. Palpifer ventrally with irregular band of long setae near anterior margin; dorsally with long setae near lateral margin. Maxillary lobe elongate, shorter than palpus with rounded apex; long setae dorsal and ventral, stouter and denser dorsally. Maxillary palpi with three palpomeres: palpomere basal almost as long as wide with band of setae near anterior margin and one campaniform sensillum ventral and some latero-anterior setae dorsal; palpomere median slightly longer than wide, with two long setae and one campaniform sensillum ventral, near anterior margin; palpomere distal elongate with one campaniform sensilla dorsal near base and one short seta latero-external; with peg-like sensilla at apex. Labium (Fig. 17): mentum fused to submentum, translucent, with two pair of setae near anterior margin and one pair below anterior; prementum transverse, partially sclerotized and narrowed at base; palpiger with many long setae, forming an irregular band near apex; ligula wide and membranous with distal margin rounded; bearing many wide long setae near anterior margin and several campaniform sensilla at middle. Labial palpi with two palpomeres: palpomere basal elongate with band of long setae ventral, near anterior margin; palpomere distal elongate with one campaniform sensillum ventral near lateral margin and peg-like sensilla at apex. Hypopharynx (Fig. 16): anterior margin rounded; distal half with many wide and long setae, except at middle; basal half microspined laterally; many campaniform sensilla at middle.
Prothorax wider than long, gradually narrowed anteriad; pronotum with long setae more concentrated anteriorly; basal two-thirds micro-spiculate. Setae coarser and bristle ventrally; presternum and eusternum indistinct; sternellum with one elliptical area each side, covered with micro-spiculi. Mesoand metathorax transverse, band-like. Mesothorax with transversal median band of setae dorsal and with one transverse narrow ampulla ventral; each side with one ventral elliptical spiracle (Figs. 2, 3) near anterior margin. Metathorax with one transversal median narrow ampulla dorsal and one ventral.
Abdomen: epipleurum protuberant on segments VIIVIII. Segments IVIII with one dorsolateral elliptical spiracle each side, smaller than thoracics; segments I-VII dorsal- and ventrally slightly grooved longitudinal-medially, with two elliptical or rounded ampullae at middle of each segment; ampullae with large and micro-spiculate verrucae; segment
Pupa. Length: 2425 mm; width of pronotum: 5 mm (Figs. 48).
General coloration cream. Integument with small spines dorsal, each with one basal seta (Fig. 4 (setae not represented); Fig. 6 (setae represented only on mesonotum and legs). Spines of clypeal and labral areas and those of mesonotum very small. Glabrous ventrally, except by crown of spines with basal long seta at apex of femura.
Head (Figs. 4, 6) partially visible dorsally, deeply excavate between antennal tubercles; three pairs of spines between antennal insertions (one pair visible ventrally), each with one long basal seta; ventrally spines more concentrate at median anterior region and in one transversal row at clypeal area; each mandible with two lateral spines, each with one longer than hind angle of pronotum; spines, each with one basal seta; labrum with five spines each side. Antennae gla-basal seta, more concentrate at anterior third; two spines, each brous. with one basal seta, each lateral lobe and two inner near basal
Pronotum wider than long, constricted at hind angles base, margin. Mesonotum narrow with posterior margin promiforming one lobe before hind angles; ante-proximal lobe nent and emarginated at middle; small spines, each with one basal seta, more concentrate near middle and one larger each side. Ventrally one well developed elliptical spiracle laterally on mesothorax. Metanotum longer than mesonotum, with posterior margin prominent at middle and four spines each side, each with one basal seta. Femura of all legs with crown of small spines near apex, each with one long basal seta.
Abdomen with segments IVI transverse, band-like with elliptical dorsolateral spiracles, first larger; segments III with one transverse band of spines, each with basal seta, interrupted at middle; segments IIIVI with two transversal bands interrupted at middle, anterior band shorter; segments VII VIII narrow and moderate or extremely elongate in female (Fig. 6), VII longer than VIII, with dorsal spines with basal setae, larger near apex; segment IX (Figs. 58) short and apical, with two small dorsal spines, each with basal seta; apex with one pair of dorsal well developed inwardly directed spines, each with one basal seta, and one pair lateral (Figs. 5,7); ventrally, three well developed spines, each with one basal seta, each side; segment X (Fig. 7) ventral with bilobed apex.
We examined two female pupae: one with abdominal segments VIIVIII extremely elongate (Fig. 6) and other with these segments moderately elongate, like the females of the Figs. 76 and 77 .
Material examined. Brazil. São Paulo: Mogi Guaçu (Est. Ecológica Mogi Guaçu) [22º15'S 47º00'W; 600-730 m.], 23.IX.77, E. P. Teixeira col.; Host: Pinus elliottii Engelm, 4 larvae and 3 pupae fixed (MZSP), 18 pinned adults (IACC).
Biological Notes. The larvae and pupae were collected in a decayed trunk inside a plantation of Pinus elliottii Engelm. The trunk, collected in September, was kept in laboratory and the adults emerged in November and December. The emergence of the adults was first noted by presence of the exit holes (Fig. 73) in the trunk.
The larvae feed on dead trees. The larval galleries and the pupal cells (Fig. 74) agree with the description of Duffy (1960) for Eutrypanus incertus [now included in Neoeutrypanus by Monné & Bezark 2009]: "entirely subcortical, the latter consisting of a shallow concavity (in the inner bark or outer sapwood), lined with an oval barricade or interlaced fibrous shreds".
Besides the material reared in Pinus elliottii, there are in the IACC collection some adults reared in trunks of a live tree of Cariniana estrellensis (Raddi) Kuntze ("jequitibá branco") with symptoms of wasting, collected in Campinas, São Paulo (Chácara São Quirino, VII.1989, J. L. Silva col.).
Remarks. According to Duffy (1960), the larvae of Eutrypanus Thomson, 1860, Chaetanes Bates, 1864 and Carphina Bates, 1862 "are distinguishable from the remainder of the Acanthocinini by the presence of a large, broad, sclerotized process on abdominal tergite 9, which may possible act as stridulatory organ". He included also Eutrypanus incertus (currently placed in Neoeutrypanus). In E. dorsalis, here described, this sclerotized process is very small, like a narrow transverse sclerotization.
Comparing Eutrypanus dorsalis with E. triangulifer [described as Acanthocinus triangulifer] it was verified that the head is elongate with sides feebly constricted, after the middle in E. dorsalis and before the middle in E. triangulifer; antennal foramen closed in both species; gular region indiscernible, except in E. triangulifer with gula triangular; pronotum with basal two-thirds micro-spiculate in both species and spiracles with peritreme circular, except elliptical in E. dorsalis. Abdomen: pleural tubercles with a pair of sclerotized pits, absent in E. dorsalis; ampullae with large moniliform micro-spiculate verrucae in E. dorsalis and velvety microspiculate, non tuberculate in E. triangulifer; tergite IX with transverse sclerotized process very small in E. dorsalis and absent in E. triangulifer.
Comparing both species of Eutrypanus with Neoeutrypanus incertus, several differences were observed. Differently from the species of Eutrypanus, this species exhibits: head with sides feebly constricted medially; stemmata present; pronotum with basal two-thirds micro-pubescent; abdomen with ampullae with larger, oval, shining moniliform tubercles; tergite IX with well developed transverse sclerotized process.
The larva of Eutrypanus dorsalis is characterized especially by head with one longitudinal carina on each side of median suture, not reaching the base, absence of sclerotized pits on pleural tubercles and spiracles with elliptical peritreme.
Comparing the pupae of Eutrypanus dorsalis with that of E. triangulifer it was observed (the head was not compared because in E. triangulifer it was too mutilated and was not described): mesonotum with six pairs of spines, each with one with basal seta in E. dorsalis and glabrous in E. triangulifer; metanotum with four spines on each side in E. dorsalis and two oblique rows in E. triangulifer, each row with six large and two or three smaller spines; elytra without spines; legs: femora of all legs with row of spines near apex in both species; hind femora with long, slender tuberculate process near base in E. triangulifer. Abdomen: segments VI VIII extremely elongate and strongly produced posteriorly in both species; posterior half of tergites IVI bearing groups of spines directed posteriorly and anterior half of tergites III VI with similar but less numerous spines in both species; segment IX with two small dorsal spines and at apex, one pair of well developed dorsal spines inwardly directed, one pair of small spines lateral, and ventrally, three well developed spines on each side in E. dorsalis; four pairs of stout, inwardly curved spines in E. triangulifer. Functional spiracles on segments IVI in both species.
Comparing the pupae of the two species of Eutrypanus with that of Neoeutrypnus incertus, it was observed the following differences in N. incertus: clypeal area with two pairs of setae; mesonotum glabrous (like in E. triangulifer); metanotum with two oblique row of spines (like in E. triangulifer); elytra with one pair of spines; tergites IVI with spines with basal setae arranged more or less in two transverse rows; tergite VII with numerous (more than 25) spines.
Paratenthras martinsi Monné, 1998
(Figs. 2239, 78 , 79 )
Larva. Length: 59.5 mm; width of prothorax: 23 mm.
Elongate, cylindrical, slightly flattened; slightly narrowed apicad; segments VIIVIII widened laterally (Figs. 22, 25). General coloration cream; mandibles black; head yellow with dorsal and ventral anterior narrow black band. Densely se-tose; setae yellowish-white long and thin, denser laterally. Thorax and abdomen with ambulatory ampullae covered with shining glabrous moniliform tubercles.
Head (Figs. 30, 33) deeply retracted into prothorax; slightly elongate, prognathous and strongly depressed; retracted portion about one-third of head length; occipital foramen entire. Head capsule dorsally composed by two epicranial halves; median suture long, continuous with endocarina and almost reaching frontoclypeal suture. Frontoclypeal suture distinct almost straight; six epistomal setae; each epicranial half with three long setae (one below epistomal setae and two near lateral lobe) and four short setae: three forming an inclined row starting near median suture and one near stemma; one lateral long setae above stemma; tiny setae (not represented) sparse on exposed portion. Each side with one translucent stemma below antenna. Head capsule with four ventral setae each side. Gula indistinct; gular area with seven setae. Antenna (Fig. 31) very short and retractile; with two antennomeres; basal antennomere almost totally retractile; distal antennomere bearing at apex one well developed sensorial membranous appendix and four wide setae: one long and three short. Antennal foramen closed posteriorly. Clypeus (Fig. 32) trapezoidal, translucent and glabrous. Labrum (Fig. 32) semielliptical, densely setose, except basal region. Epipharynx (Fig. 35) with anterior margin rounded, narrowed at basal half; one elongate sclerite each side of basal half; anterior and lateral margins with irregular band of long setae of varied sizes; a wide microspined median band interrupted at middle on basal two-thirds and continuous running like a narrow band internally each sclerite; many campaniform sensilla more concentrate in a median glabrous area on basal two-thirds. Mandible (Figs. 36, 37) symmetrical, narrow with acute apex and subapical rounded tooth; two lateral long setae. Maxilla (Figs. 34, 39): cardo and submentum fused; stipes membranous with sclerotized in transversal narrow band at base (continuous with submentum) and inclined band below palpifer; several long setae more concentrate latero-externally and many campaniform sensilla more concentrate latero-externally near base. Palpifer ventrally slightly sclerotized with many campaniform sensilla and irregular band of long setae near anterior margin; dorsally with some long setae latero-anteriorly, a band of microsetae transverse near anterior margin and continuous longitudinally near middle; several campaniform sensilla irregularly distributed. Maxillary lobe elongate with rounded apex, shorter than palpus; ventrally with several stout and many piliform setae and three campaniform sensilla near base; dorsally with stout setae, some very wide. Maxillary palpi with three palpomeres: palpomere basal elongate, ventrally with one campaniform sensillum near base and four long setae and three campaniform sensilla near apex, and dorsally with two long lateroexternal setae; palpomere median as long as wide, ventrally with one campaniform sensillum near base and four setae and one campaniform sensillum near apex, and dorsally with one lateroexternal long seta; palpomere distal with one ventral campaniform sensillum lateroexternal, one long dorsal seta latero-internally and peg-like sensilla at middle of apex. Labium (Fig. 39): mentum fused to submentum, translucent with transverse sclerotized narrow basal band, continuous with that of maxilla; many long setae and campaniform sensilla on distal third; prementum slightly sclerotized with several campaniform sensilla more concentrate at base; palpiger with transverse band of long setae near anterior margin; ligula densely microspined at middle with several long setae. Labial palpi with two palpomeres: palpomere basal elongate with five long setae and one or two campaniform sensilla near apex; palpomere distal elongate, narrower than basal with one campaniform sensillum near middle of lateroexternal margin and some peg-like sensilla at middle of apex. Hypopharynx (Fig. 38) with fore angles rounded, marginate by short setae; each side with wide microspined band on distal third; microspined band starting on fore angles, wider and convergent at middle; many moderately long setae and campaniform sensilla between two lateral microspined bands on anterior third; campaniform sensilla at median region, behind microspined band.
Prothorax wider than long, strongly narrowed anteriorly; more strongly convex dorsally; ventrally with transverse groove parallel posterior margin. Meso- and metathorax bandlike, similar in length; both together shorter than prothorax dorsal and longer ventral. Mesothorax ventrally with one median ampulla and anteriorly with one well developed elliptical spiracle (Fig. 23) each side. Metathorax with one transverse ampulla dorsal and one ventral.
Abdomen: segments IVII with one elliptical ampullae dorsal and one ventral; ampullae covered with shining glabrous moniliform tubercles; segments IVIII with one elliptical spiracle each side, smaller than those of thorax; epipleurum protuberant on segments VIIVIII. Segment IX (Fig. 24) short; segment X with trilobed apex.
Pupa. Length: 812 mm; width of prothorax: 1.52.5 mm (Figs. 26, 29).
Coloration cream; black crown of spines at apex of abdomen. Pubescence ferrugineous, long and dense. Abdomen dorsally with unciform spines of varied sizes; each spine with one long seta at base (setae not represented on abdomen in Fig. 26).
Head (Figs. 26, 29) partially visible dorsally with many long setae; ventrally, two setae below antennal insertion and two laterally near base of mandible; labrum densely setose; each mandible with two setae near base. Antennae glabrous.
Pronotum wider than long, narrowed anteriad; constricted laterally near base of hind angles, forming a rounded lobe, densely setose; setae long, more concentrate laterally and sparser near base. Mesonotum band-like with posterior margin prominent at middle and many setae each side of scutellar area; each elytron with four setae near base. Mesothorax ventrally with a lateral well developed elliptical spiracle, near anterior margin. Metanotum longer than mesonotum with many setae near middle. Legs: distal half of femura, tibiae and tarsi densely setose; setae very long.
Abdomen: segments IVI with one dorsolateral elliptical spiracle each side; spiracles smaller than thoracic. Segments IVI transverse, band-like; segment I dorsally with transverse band of unciform spines near base, each spine with one basal seta (not included in Fig. 26); densely setose laterally. Segments IIVI dorsally with two transverse bands of unciform spines each: one band near anterior margin with smaller spines and other near basal margin with larger spines; each spine with one basal seta (not included in Fig. 26); densely setose laterally. Segments IVI ventrally with irregular band of setae of varied sizes. Segment VII elongate, narrower than VI, slightly narrowed apicad; dorsally with many unciform spines, each spine with one basal seta (not represented in Fig. 26); spines forming a median basal rounded patch, a transversal median band and some sparse near apex; distal margin bilobate; each lobe with one well developed spine, each with one long seta at base; setose laterodorsally and lateroventrally. Segment VIII (Figs. 2629) short, like a nar-row band; dorsally with a crown formed by 8 well developed apical spines, upwardly directed, each with one long seta at base; densely setose ventrally, forming a band near distal margin, interrupted at middle. Segment IX (Figs. 2729) reduced, ventral, surrounded by segment VIII, forming three lobes: one dorsal and one each side; densely setose laterally; two small rounded lobes ventral, at middle of basal margin; anal opening transverse.
Material examined. Brazil. São Paulo: Campinas (IAC- Sta Elisa) [22º51'47"S 47º5'6"W], 08.VI.10; Hosp. Scheelea phalerata ("espata"), E. P. Teixeira col., 12 larvae, 6 pupae (3 with last larval exuvia), 18 adults fixed and 6 pinned (MZSP), 39 pinned adults (IACC).
Biological Notes. On November 27, 2006, several emergence holes, apparently made by beetles, were observed in one spathe of Scheelea phalerata (Mart. ex Spreng.) Burret. This spathe was collected and kept in laboratory inside a nylon cage (Fig. 72), and the emergence of the adults of Paratenthras martinsi occurred from November, 2006 to June, 2007, with peak of emergence in November, December and January. This period of emergence was the same observed in field conditions, during four years, in three palm trees. The larvae, pupae and adults here studied, were obtained from one spathe collected in June, 2010, from the same plant of the first collected in 2006 and kept in laboratory.
It was observed that the larvae feed on the internal part of the spathe and the pupation takes place in the shallow cell, very close to the outer wall (Fig. 68). To separate the larvae and pupae, the spathe was tore with a knife.
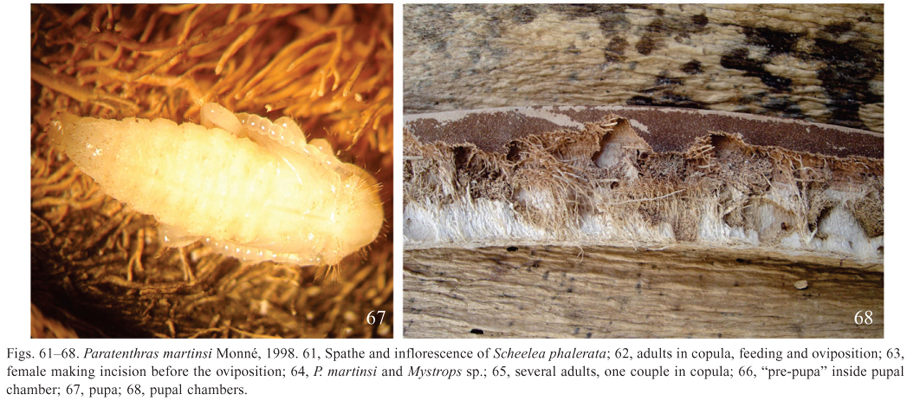
The female lays the eggs on the internal face of the spathe (Figs. 61, 62, 64, 65) and before the oviposition, a jaw incision (Fig. 63) is performed. This operation lasts a few seconds. The males attempt to copulate, even during the oviposition (Fig. 65), and the copulation always occurred in the spathe.
Based on the emergence holes, it was verified that hundreds of adults emerged from one spathe, as observed on March, 25, 2008 (Fig. 70). In another spathe 115 cm long observed on April, 05, 2012, a total of 808 "exit" holes were found in the external face, 41 on the internal and 13 in the lateral face. To facilitate the counting, the emergence holes were surrounded with a ballpoint pen (Fig. 71). These holes were located from thirty centimeters from the base to 10 cm before the apex. The spathes remain on the plant after the emergence of the insects (Fig. 69). The number of adults reared in each spathe depends on the period that the spathe opens. If it opens in the high peak of emergence of the adults, more adults will be attracted and more chance for reproduction. When the spathe opens in low peak of emergence, few adults will be available to be attracted and to reproduce.
During field observations on March 25, 2008, it was verified that the highest activity of the beetles, feeding and copulation, occurred in the morning immediately after the opening of the spathe. This intense activity lasted for three days and after that no activity was observed and few flowers remained in the inflorescence. It is likely that the activity of the beetles, including oviposition, is conditioned on tender spathes. It was not observed adults feeding on pollen. It is likely that they feed on the spathe tissues.
According to Monné (1998), there is a very sharp chromatic variation in both sexes of P. martinsi, which corroborates with the data obtained in this study (Figs. 78 , 79 ). The majority of the males observed had dark-brown elytra, except for the yellow apex. On April 18, 2008, in three couples observed in copulation, one of the males had elytra with a small center-median yellow spot, not forming the cross track.
Along with the adults of the Cerambycidae, it was found on the spathe adults of Mystrops sp. (Coleoptera, Nitidulidae) (Fig. 64), occurring in larger number in the inflorescence, where they feed on pollen. It is probably that this species also feed on the spathe. Besides Mystrops sp., Trigona sp. (Hymenoptera, Meliponini) was observed in the inflorescence.
This is the first record of Paratenthras martinsi in the state of São Paulo and also for Scheelea phalerata as the host plant of this species. In addition, this is the second record of Lamiinae species in Brazil associated with palm spathes. Based on the distribution of this species presented by Monné, 1998 (Mato Grosso and Mato Grosso do Sul) and on the distribution of the palm (São Paulo, Minas Gerais, Goiás, Tocantins, Pará, Amazonas, Acre, Rondônia, Mato Grosso and Mato Grosso do Sul) (Lorenzi et al. 2004) it is supposed that the distribution of the beetle follows the distribution of the palm.
Remarks. The larva and especially the pupa of Paratenthras martinsi is very setose, with setae longer and denser than any other studied species. It presents elliptical ambulatory ampullae bearing shinning glabrous moniliform tubercles. The pupa of this species presents, at apex of abdomen, a crown of well-developed black spines upwardly directed.
Lophopoeum timbouvae Lameere, 1884
(Figs. 4060, 80 )
Larva. Length: 911 mm; width of prothorax: 2.53.0 mm.
Body elongate, cylindrical, slightly flattened and narrowed apicad; segments VIIVIII widest (Figs. 40, 41). General coloration cream; pronotum with one yellowish median area; head yellow with band on anterior margin (dorsal and ventral) and mandibles black; ventroanterior black band of head with one projection on each side of gular suture; spiracles brown. Pubescence thin, long, dense, pale yellow (almost invisible under alcohol); longest setae brownish. Micro-spiculate areas on basal region of pronotum and anterior region of mesonotum. Thorax and abdomen with ambulatory ampullae covered with shining glabrous moniliform small tubercles.
Head (Figs. 49, 50) deeply retracted into prothorax; elongate, prognathous and strongly depressed; retracted portion almost half of head length; occipital foramen entire. Dorsally, head capsule composed by two epicranial halves; median suture long, continuous with endocarina almost reaching suture, three laterally outside each darker projection and two frontoclypeal suture. Frontoclypeal suture distinct, almost near lateral margin. One well-developed stemma each side, straight; six epistomal setae; each epicranial half with two below antenna (at apex of a small projection). Antenna (Figs. moderately long setae below epistomal setae and six more lat-52, 56) very short and retractile with two antennomeres; basal eral, all on/near darker band; two tiny setae near median su-antennomere membranous; distal antennomere slightly wider ture. Gular suture present. Three setae on each side of gular than long, bearing one ventral campaniform sensillum near base and at apex, one well-developed sensorial membranous appendix and five setae of varied sizes, two very wide. Antennal foramen opened posteriorly. Clypeus (Fig. 51) trapezoidal, translucent and glabrous. Labrum (Fig. 51) semielliptical, setose near distal margin; campaniform sensilla irregularly distributed on basal two-thirds (not represented in Fig. 51). Epipharynx (Fig. 53) with fore angles rounded; each side with one triangular elongate sclerite on basal two-thirds; distal forth with stout setae directed to middle; median area with microspines directed to middle and some campaniform sensilla; microspined median band bifurcate and following internal margin of each sclerite; basal half between sclerites with four setae and many campaniform sensilla. Mandible (Figs. 5860) symmetrical with wide apex; internal margin emarginate dorsally near apex forming small lobes; two long lat-near band, one seta longest. Palpifer ventrally partially scleroeral setae. Maxilla (Figs. 54, 55): cardo and submentum fused; tized, with campaniform sensilla near base and irregular dense stipes membranous with sclerotized transversal inclined band, band of setae near apex; dorsally with three campaniform senbearing several campaniform sensilla; about eight long setae silla and some microspines at middle and long setae and one campaniform sensillum near lateral margin. Maxillary lobe elongate, shorter than palpus, with apex rounded and slightly narrowed internally near base; many long dorsal and ventral setae, more concentrate and wider dorsally. Maxillary palpi with three palpomeres: basal palpomere almost as long as wide, with three moderately short setae and three campaniform sensilla ventral, and dorsally, one moderately long lateral seta and microspiculi near apex; median palpomere as long as wide with one seta and two campaniform sensilla ventrally, and two setae dorsally: one lateroexternal long and one laterointernal short; distal palpomere elongate, dorsally with one short laterointernal seta and one wide seta parallel to external margin; apex with several peg-like sensilla. Labium (Fig. 54): mentum fused to submentum, translucent with two setae near apex and a transverse row with four setae near middle, and many campaniform sensilla; prementum transverse and narrowed at base; palpiger with some campaniform sensilla and transverse row of long setae near apex; ligula wide and membranous with distal margin rounded, bearing many long setae at middle, near apex. Labial palpi with two palpomeres: basal elongate, bearing ventrally one campaniform sensillum and a row of five setae near anterior margin; distal palpomere narrower with one ventral campaniform sensillum and many peglike sensilla at apex. Hypopharynx (Fig. 57) with distal margin rounded; a semicircular band of stout setae at anterior half; microspines forming band at lateral margins, converging at middle; many campaniform sensilla before and below transverse microspined band.
Prothorax more convex dorsally; ventrally with transverse groove parallel posterior margin; pronotum wider in basal third, gradually narrowed anteriad. Meso- and metanotum transverse, together almost as long as pronotum. Mesothorax ventrally with one elliptical spiracle (Fig. 42) each side. Mesothorax with a transverse tuberculate ampulla ventral; metathorax with a transverse tuberculate ampulla dorsal and two rounded ventral.
Segments IVII with two rounded tuberculate ampullae dorsal and two ventral; ventral ampullae narrower. Segments IVIII with one elliptical dorsolateral spiracle on each side, each with subcontigous marginal chambers; pleural tubercles each with a pair of sclerotized pits. Epipleurum protuberant on segments VIIVIII. Segments VIIIIX transverse and se-tose. Segment X (Fig. 44) reduced, distal, partially visible dorsally; trilobed.
Pupa (Figs. 4548). Length: 11 mm; width of prothorax: 2.5 mm.
Coloration cream. Integument with small tubercles with spines of varied sizes, each with one seta at base; seta at least two times spine length.
Head (Figs. 45, 48) hypognathous, partially visible dorsally; three well-developed dorsal spines at base of each antenna, each with one seta. Spines smaller ventrally, sometimes indistinguishable, seeming only seta; each side of frontal area with eight spines with seta each side: a group of three near antennal base, a group of two below anterior, one near middle and two near clypeal area; labrum with three spines each side, each with a basal seta each side; each mandible with two spines, each with a seta.
Pronotum wider than long, prominent laterally forming a rounded lobe each side, near middle; each side with tiny spines, each with a seta, forming a row with seven near anterior and lateroanterior margins; one on each lateral tubercle and one below it; four spines each side, each with a basal seta, forming irregular long row from basal third to posterior margin. Mesonotum with 45 tiny spines each side, each with a basal seta; several spines almost invisible and only setae well visible. Metanotum longer than mesonotum with a small round carina each side of scutellar area; four tiny spines with basal setae each side, forming inclined row: a group of three forming inclined row and one near middle. Legs with tiny spines and long setae on apex of femura and tibiae.
Abdomen dorsally (Figs. 47, 48) with spines, each with one seta at base (setae partially represented in Fig. 45); spines larger laterally, on apex of segment VII and segment VII. Segments IVI transverse, band-like, with dorsal and lateral spines, each with a basal seta; dorsal spines increasing in number and size to apex direction; lateral spines larger; laterally each segment with one lateral elliptical spiracle (1st largest); segment VII with lateral not sclerotized and not functional spiracle. Segment VII longer, wider than long, slightly narrowed apicad, with distal margin rounded; spines larger, each with a basal seta. Segment VIII narrow, dorsally with 10 large spines, each with a basal seta, and ventrally with four tiny spines, each with a basal seta. Segment IX (Figs. 4548) short, like a narrow band, dorsally with two welldeveloped spines at apex, each with a basal seta. Segment X reduced, surrounded by segment IX.
Material examined. Brazil. São Paulo: Teodoro Sampaio (Parque Estadual do Morro do Diabo) [22º27'S 52º10'W], 28.VIII.1997, E. P. Teixeira col., 4 larvae (1 dissected), 1 pupa and 1 adult fixed (MZSP).
Biological Notes. Duffy (1960) studied the larva and pupa of this species collected in fruits of Gleditsia amorphoides from Paraguay.The larvae here studied were collected in fruits of Hymenaea corbaril L., Enterolobium contortisiliquum (Vell.) Morong and Pterogyne nitens Tul. The pupae and adults were obtained from fruits of Hymenaea corbaril in laboratory. This is the first record of L. timbouvae collected in fruits of Pterogyne nitens.
In addition to the material used in the redescription, there is in the IACC collection, some adults of this species reared in seeds of Euterpe edulis Mart., collected in Pariquera-Açu, São Paulo (Estação Experimental Pariquera-Açu, VI.1988, M.L.A. Bovi col.). This is another new record of host plant for L. timbouvae.
Remarks. Duffy (1960) considered the spiracles of the larva with peritreme rounded, here considered as elliptical. Besides the description of the larva, he presented the illustrations of the habitus of the larva and the apex of abdomen of the pupa, and included a comparison of the pupa of L. timbouvae with that of Ozineus prolixus Melzer. Differently from the other studied species, the larva of L. timbouvae presents a well-defined gular suture, and spiracles with subcontigous marginal chambers.
Comparison of known immature forms of Acanthocinini
As stated by Duffy (1960), the larvae of the tribe Acanthocinini are remarkably diverse and very difficult to characterize. A comparison among the known immatures based on the descriptions (Appendix I Appendix I ), and the studied material is presented below. It was not possible to compare all structures for all species because several of them are randomly described, lacking the information about structures taxonomically important.
Only the egg of Alcidion deletum has been described and it is elongate without any ornamentation. The larvae have the body of variable shapes, but it is usually cylindrical and slightly depressed with ambulatory ampullae on the thorax and the abdomen. Head retracted, moderately to very strongly depressed and moderately to strongly elongate, with sides usually constricted at middle, before or after the middle; median suture long, continuous with endocarina; each epicranial half usually with 715 setae (including long and tiny setae), sometimes 25; six epistomal setae present. One pair of stemmata present, except for Cosmotoma, Acanthocinus triangulifer [nowadays Eutrypanus triangulifer] and Eutrypanus dorsalis. Antennal foramen open or closed posteriorly; antennae very short and retractile with two antennomeres; distal antennomere, with one membranous appendix and some setae of varied sizes at apex. Clypeus translucent and trapezoidal, glabrous, except Cosmotoma with two setae each side [probably are frontal setae]. Labrum transverse with fore angles rounded and setae on distal half or third. Mandible usually with apex wide, rounded or emarginate forming two lobes or teeth; two lateral setae (Eutrypanus dorsalis with three). Cardo and submentum fused. Maxilla with galea with rounded apex; maxillary palpi usually with three palpomeres. Mentum fused to submentum. Gula usually indistinct; gular area with 27 setae. Thorax with ampullae on metanotun and meso- and metasternum; abdomen with ampullae on tergites and sternites IVII. Ampullae tuberculate or non-tuberculate, micro-spiculate or glabrous. Anus trilobed. Spiracle oval or circular, usually very thick and strongly raised above cuticle; marginal chambers present in Acanthocinus obliquus, Lepturges sejunctimaculata and Lophopeum timbouvae.
The pupae have the head partially visible from above, strongly deeply or moderately excavate between antennal tubercles. The majority of species bear short spines (each with a basal seta) behind each antenna; long setae in Exocentrus sp., Eutrypanus incertus [nowadays Neoeutrypanus incertus] and Cosmotoma setifer; in Lepturges sejunctimaculata the spines or setae are absent. Frons with varied number of spines (each with a basal seta). Occular area feebly convex and glabrous, except Lepturges sejunctimaculata and Paratenthras martinsi, with one or two setae. Clypeal area lacking or with 14 spines (each with a basal seta) or setae near each basal angle. Mandibles each with one or two setae near middle of outer face. Pronotum: each side with one stout tubercle, rounded or acutely pointed; numerous short spines (each with a basal seta) and/or setae; Lophopoeum timbouvae with one long seta and Alcidion bispinum [now Neoalcidion bispinum, in Monné & Bezark 2009] with a few conical papillae (each with a small spine and long seta). Elytra: usually glabrous, Paratenthras martinsi with four setae on each elytron. Abdomen: tergites IVI with median oval tuberculate spinose protuberance or only groups of spines (curved inwards or straight) with or without basal seta, usually arranged in one or two rows; tergites VIIVIII some-times extremely elongate, usually with stout incurved spines, sometimes similar to those of anterior tergites; tergite IX very short, truncate apically, bearing 16 large incurved spines. Legs: each femur with a crown of spines near apex; sometimes tibiae with a row of spines; sometimes femora, tibiae and tarsi with numerous scattered setae. Spiracles: functional spiracles on segments 16, sometimes vestigial spiracles on segments 78.
ACKNOWLEDGEMENTS
To Antonio Santos-Silva (MZSP) for helping in the identification of pupa of Eutrypanus dorsalis and for separating the adults of the three species to photograph; Carlos Campaner (MZSP) for identification of Nitidulidae genus and for mounting the adult of Eutrypanus dorsalis to photograph; Ubirajara R.M. de Souza for identification of the longhorned beetles; Luiz Antonio Ferraz Matthes (IAC) for the identification of the palm; Gabriel Biffi (MZSP) for taking the photographs of the adults and preparing the plate corresponding to Figs. 75 80 ; Juares Fuhrmann (MZSP) for diagramming the first plate.
Received 10 January 2014;
accepted 13 May 2014
Associate Editor: Marcela L. Monné
Appendix
Clique para ampliar Appendix I
- Böving, A.G. & Craighead, F.C. 1931. An illustrated synopsis of the principal larval forms of the order Coleoptera. Entomologica Americana 11: 1352.
- Bruch, C. 1941. Misceláneas entomológicas, VII. Notas del Museo de La Plata 50: 355359.
- Casari, S.A. & Martins, U.R. 2011. Larva of Nealcidion bicristatum (Bates, 1863) (Cerambycidae, Lamiinae, Acanthocinini). Papéis Avulsos de Zoologia 51: 499504.
- Casari, S.A. & Martins, U.R. 2013. Immatures of Phoebemima ensifera Tippmann, 1960 (Cerambycidae, Lamiinae, Hemilophini). Papéis Avulsos de Zoologia 53: 295299.
- Chapuis, M.F. & Candèze, M.E. 1853. VIII. Catalogue des Larves des Coléoptères. connues jusqu'à ce jour avec la description de plusieurs espèces nouvelles. Mémoires de la Société Royale de Sciences de Liège 8: 347653.
- Cosarinsky, M. 1996. Redescription of the immature stages of Oncideres germari Thomson, 1868 (Coleoptera, Cerambycidae, Lamiinae, Onciderini). Giornale Italiano di Entomologia 8: 167176.
- Costa, C., Vanin, S.A. & Casari-Chen, S.A. 1988. Larvas de Coleoptera do Brasil São Paulo, Museu de Zoologia, Universidade de São Paulo, 282 p.
- Craighead, F.C. 1923. North American cerambycid larvae: a classification and the biology of North American cerambycid larvae. Canada Department of Agriculture Technical Bulletin 27: 3239.
- Di Iorio, O.R. 1995. Lophopoeum timbouvae Lameere, 1884 and L. bruchi Monné & Martins, 1976 (Coleopetera, Cerambycidae, Lamiinae, Acanthocinini): their relation to fruits of Leguminosae. Giornale Italiano di Entomologia 7: 231245.
- Duffy, E.A.J. 1960. A monograph of the Immature Stages of Neotropical Timber Beetles (Cerambycidae). London, British Museum of Natural History, 327 p.
- Galileo, M.H.M., Martins, U.R. & Moura, L.A. 1993. Sobre o comportamento, ontogenia e morfologia do aparelho reprodutor de Hedypathes betulinus (Klug, 1825) (Coleoptera, Cerambycidae, Lamiinae, Acanthoderini) a broca da erva-mate. Revista Brasileira de Entomologia 37: 705715.
- Giacomel, F. 1992. Morfologia e comportamento das formas imaturas de Psylotoxus melanospidus Giacomel (Coleoptera, Cerambycidae, Lamiinae). Revista Brasileira de Zoologia 9: 3945.
- Lawrence, J. F. 1991. Cerambycidae (Chrysomeloidea), p. 556561. In: Stehr, F.W. (ed.), Immature insects v. 2, Dubuque, Kendall/Hunt, 975 p.
- Lorenzi, H., Moreira de Souza, H., Medeiros-Costa, J.T., Coelho de Cerqueira, L.S. & Ferreira, E. 2004. Palmeiras Brasileiras e Exóticas Cultivadas Nova Odessa, Editora Plantarum Ltda, 432 p.
- Marinoni, R.C. 1969. Sôbre a biologia e ontogenia de Oncideres dejeanii Thomson, 1868 (Coleoptera, Cerambycidae). Boletim da Universidade Federal do Paraná 3: 193201.
- Marinoni, R.C. & Silva, I. 1973. Sobre o desenvolvimento ontogenético de Oncideres saga saga (Dalman, 1823) (Coleoptera, Cerambycidae). Revista Brasileira de Entomologia 17: 18.
- Mermudes, J.R.M. & Monné, M.L. 2001. Descrição da larva e pupa de Acathoderes (Psapharochrus) melanostica White, 1855 (Coleoptera, Cerambycidae, Lamiinae, Acanthoderini). Revista Brasileira de Entomologia 45: 331334.
- Monné, M.A. 1998. Notas sobre Acanthocinini Neotropicais (Coleoptera, Cerambycidae, Lamiinae): gênero e espécies novas. Revista Brasileira de Entomologia 41: 289295.
- Monné, M.A. & Bezark, L.G. 2009. Checklist of the Cerambycidae of the Western Hemisphere. Available at: http://www.cerambycoidea.com/titles/monnebezark2009.pdf (Acessed 1 September 2013).
- Napp, D.S. 1977. Sobre a ontogenia de Oncideres guttulata Thomson, 1868 (Coleoptera, Cermabycidae, Lamiinae). Revista Brasileira de Entomologia 21: 1923.
- Penteado-Dias, A.M. 1979. Alguns aspectos da biologia e ontogenia de Midanus hecabe (Coleoptera, Cerambycidae, Lamiinae, Onciderini). Revista Brasileira de Entomologia 23: 914.
- Peterson, A. 1960. Larvae of Insects.An introduction of Nearctic species. Part II. Coleoptera, Diptera, Neuroptera, Siphonaptera, Mecoptera, Trichoptera Columbus, Edwards Brothers, 416 p.
- Roguet, J.P. 2013. Lamiaires du Monde (Coleoptera Cerambycidae Lamiinae). Available at: http://www.lamiinae.org (acessed 1 September 2013).
- Ruggles, A.G. 1915. The history of Oberea tripunctata Swederus (Cerambycidae). Journal of Economic Entomology 8: 7985.
- Vitali, F. 2001. Description de la larve de deux longicornes de Guadeloupe: Chaetanes fleutiauxi Villiers, 1980 et Leptostyloides assimilis (Gahan, 1895) (Coleoptera, Cermabycidae, Lamiinae, Acanthocinini). L'Entomologiste 57: 151156.
- Zajciw, D. 1964. Descriptions of larva and pupa of "Acanthoderes juno" Fisher, 1938 (Coleoptera, Cerambycidae, Lamiinae). Revista Brasileira de Biologia 24: 229233.
- Zajciw, D. 1974. Descriptions of larva and pupa of Psyllotoxus griseocinctus Thomson, 1868 (Coleoptera, Cerambycidae, Lamiinae). Revista Brasileira de Biologia 34: 339396.
- Zajciw, D. 1975. Descriptions of larva and pupa of Macropophora accentifer (Olivier, 1795) (Coleoptera, Cerambycidae, Lamiinae). Anais da Academia Brasileira de Ciências 47: 347350.
Appendix I


Publication Dates
-
Publication in this collection
14 July 2014 -
Date of issue
June 2014
History
-
Received
10 Jan 2014 -
Accepted
13 May 2014