Abstract
The objective of the following work is to document the phenotypic expression variability in Best Disease in first-degree relatives. The information was collected by assessing medical notes, interviewing the patient and obtaining photographic record of the diagnostic methods to which the patient was submitted. Data was analyzed along with a thorough review of the literature. A series of cases were reported in which the patient presenting the phenotypic characteristics of the disease has first degree relatives without ophthalmic findings during examination, but present an abnormal pattern on the electro-oculogram (EOG). Our article reveals the importance of electrophysiological exams in the diagnosis of Best vitelliform macular dystrophy, including the prevention of its clinical manifestation (autosomal dominant), providing concrete subsidies for genetic counseling.
Keywords:
Vitelliform macular dystrhohy/genetics; Electrodiagnosis; Electrophysiology; Phenotypic; Case reports
Resumo
O objetivo do presente trabalho é a documentação da variabilidade de expressão fenotípica da Doença de Best em parentes de primeiro grau. As informações foram obtidas por meio de revisão do prontuário, entrevista com o paciente e registro fotográfico dos métodos diagnósticos aos quais os pacientes foram submetidos. Dados foram analisados junto a uma extensa revisão da literatura. Relatamos uma série de casos, no qual o paciente que apresenta as alterações fenotípicas da doença tem familiares de primeiro grau sem alterações ao exame oftalmológico, porém os mesmos apresentam padrão anormal de eletro-oculograma (EOG). O nosso artigo revela a importância dos exames eletrofisiológicos no diagnóstico da distrofia macular viteliforme de Best, inclusive no que se refere à prevenção de sua manifestação clínica (autossômica dominante), fornecendo subsídios concretos para o aconselhamento genético.
Descritores:
Distrofia macular viteliforme/genética; Eletrodiagnóstico; Eletrofisiologia; Fenótipo; Relatos de casos
Introduction
The mutations in the BEST1 gene are a common cause in the hereditary macular disease.11 Khan KN, Islam F, Moore AT, Michaelides M. The fundus phenotype associated with the p.Ala243Val best1 mutation. Retina. 2017:1. doi: 10.1097/IAE.0000000000001569. [Epub ahead of print]
https://doi.org/10.1097/IAE.000000000000...
,22 Boon CJF, Klevering BJ, Leroy BP, Hoyng CB, Keunen JEE, den Hollander AI. The spectrum of ocular phenotypes caused by mutations in the BEST1 gene. Prog Retin Eye Res. 2009;28(3):187-205. In most cases, the gene’s heterozygous variants result in a single vitelliform lesion centered in the macula, the classic phenotype associated with Best Disease (BD).11 Khan KN, Islam F, Moore AT, Michaelides M. The fundus phenotype associated with the p.Ala243Val best1 mutation. Retina. 2017:1. doi: 10.1097/IAE.0000000000001569. [Epub ahead of print]
https://doi.org/10.1097/IAE.000000000000...
,33 Petrukhin K, Wadelius C, Koisti MJ, Bakall B, Li W, Xie G, et al. Identification of the gene responsible for Best macular dystrophy. Nat Genet. 1998;19(3):241-7.,44 Marquardt A. Mutations in a novel gene, VMD2, encoding a protein of unknown properties cause juvenile-onset vitelliform macular dystrophy (Best's disease). Hum Mol Genet. 1998;7(9):1517-25. Most of the patients have a family history consistent with the dominant transmission, even though non-penetrating carriers may occur.55 Burgess R, Millar ID, Leroy BP, Urquhart JE, Fearon IM, De Baere E, et al. Biallelic mutation of BEST1 causes a distinct retinopathy in humans. Am J Hum Genet. 2008;82(1):19-31. Less commonly, the mutations in the BEST1 homozygous and heterozygous composed genes result in a recessive disease.55 Burgess R, Millar ID, Leroy BP, Urquhart JE, Fearon IM, De Baere E, et al. Biallelic mutation of BEST1 causes a distinct retinopathy in humans. Am J Hum Genet. 2008;82(1):19-31.This is generally associated with the accumulation of multifocal sub-retinal deposits, intra retinal and sub-retinal liquids, even in the absence of the choroidal neovascular membrane, short axial length, and narrow iridocorneal angles.11 Khan KN, Islam F, Moore AT, Michaelides M. The fundus phenotype associated with the p.Ala243Val best1 mutation. Retina. 2017:1. doi: 10.1097/IAE.0000000000001569. [Epub ahead of print]
https://doi.org/10.1097/IAE.000000000000...
,66 Yardley J, Leroy BP, Hart-Holden N, Lafaut BA, Loeys B, Messiaen LM, et al. Mutations of VMD2 splicing regulators cause nanophthalmos and autosomal dominant vitreoretinochoroidopathy (ADVIRC). Invest Ophthalmol Vis Sci 2004;45(10):3683-9.
The clinical EOG is a functional integrity test within the photoreceptors and the retinal epithelium pigment. The electroretinogram (ERG) is completely normal, once the electroculoogram (EOG) is common in all cases, representing an important diagnosis tool for this pathology.11 Khan KN, Islam F, Moore AT, Michaelides M. The fundus phenotype associated with the p.Ala243Val best1 mutation. Retina. 2017:1. doi: 10.1097/IAE.0000000000001569. [Epub ahead of print]
https://doi.org/10.1097/IAE.000000000000...
The EOG is essential to detect other family members affected by the disease and characterize the autosomal aspect that is dominant in the disorder. However, other family members that might have been affected may not always be found, since it’s common to find cases of sporadic appearances. The main goal of the following project is to document the rare occurrences of phenotypic expression variability in Best Disease in first-degree relatives.
Case report
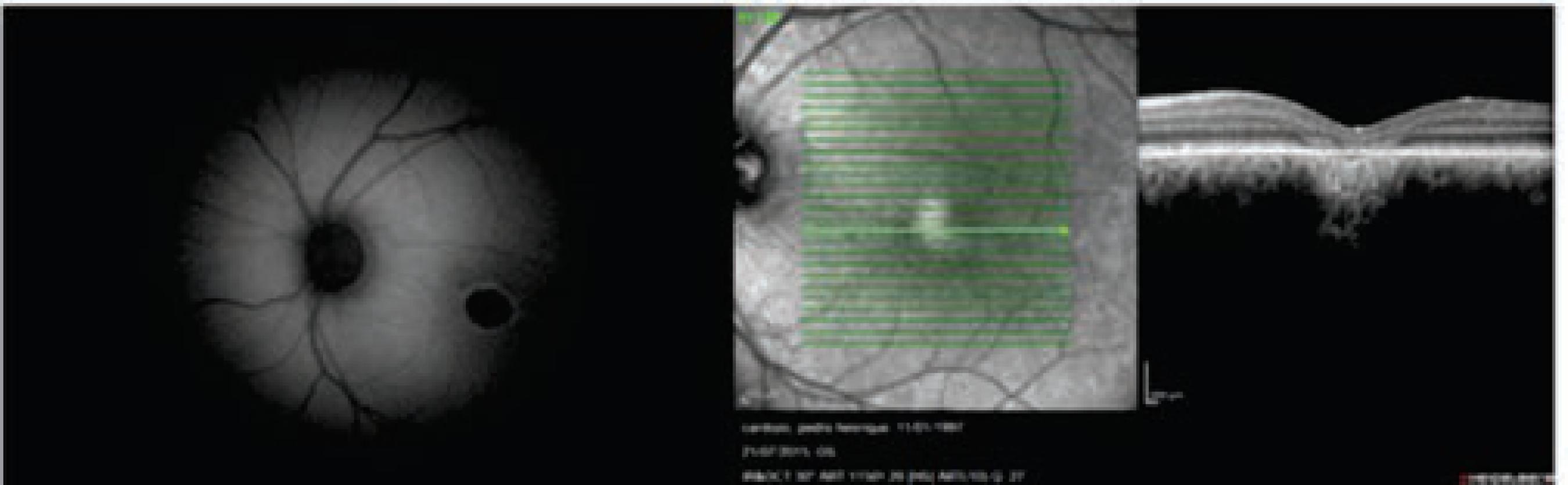
Case 1: P.H.S.C, male, 17 years old, student, AVCC 20/200 in both eyes; Normal reflexes in the pupils; Normal biomicroscopic exam. The fundus of the eye has a round structure with a yolk-like yellow color in both eyes (Figure 1). In the fluorescein angiography, the yellow discharge was presented as highly fluorescent. In the image of the Optical Coherence Tomography (OCT), there is retinal epithelium atropthy, hyperreflectivity above the RPE with fading off deeper retinal layers (Figure 2). The autofluorescence shows a hypofluorescent lesion. (Figure 2) EOG OD 125% and OE 100% - pathological in both eyes, but it’s more accentuated in the left eye. (Arden Barrada and Kelsey index = 180%, normal. L/E x 100%). There is a normal photopic ERG, with the presence of waves a, b1, and b2, with oscillating potentials.The Ishihara color vision tests were normal; Octopus Automized Perimetry 1-2-3: the global indexes (MD and LV) were found to be elaborate and express diffused defects that were shown in the gray scale as central and relative scotoma and in the paracentral, superior and inferior OD scotomas, and as a diffused sensibility depression in OE.
The autofluorescence shows a hypofluorescent lesion; OCT shows retinal epithelium atropthy, hyperreflectivity above the RPE with fading off deeper retinal layers
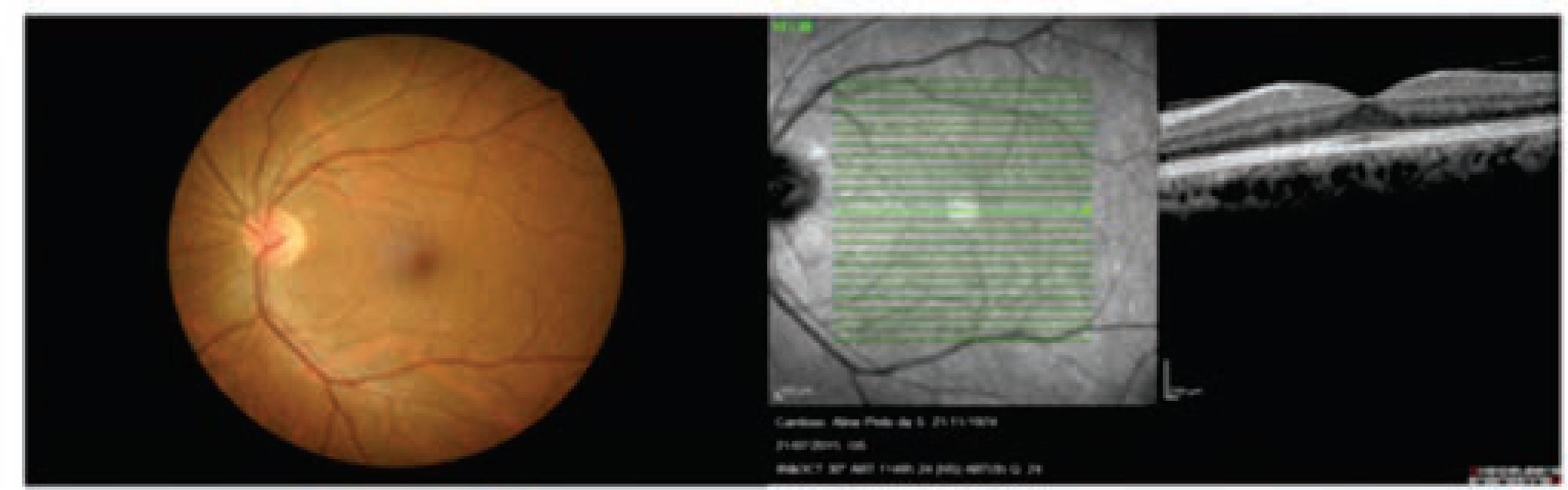
Case 2: F.H.P.C.J., male, 19 years old, student, AVCC 20/20 in both eyes; The reflexes of the pupils are normal; The biomicroscopic exam is normal; Fundus of the eye is normal and OCT normal (Figure 3); EOG OD 150% and OE 125% - pathological in both eyes. (Anden Barrada and Kelsey indexes = 180%, normal. L/E x 100%). There is a normal photopic ERG, with the presence of a, b1, b2 waves with oscillating potentials.
Case 3. A.P.S.C, female, 41 years old, homemaker, AVCC 20/20 in both eyes; The reflexes of the pupils are normal; The biomicroscopic exam is normal; Fundus of the eye is normal and OCT normal (Figure 4); EOG OD 125% and OE 120% - pathological in both eyes. (Anden Barrada and Kelsey indexes = 180%, normal. L/E x 100%). There is a normal photopic ERG, with the presence of a, b1, b2 waves with oscillating potentials.
Discussion
Up to this moment, more than 200 mutations were described in BEST1, most of which were heterozygous and associated with a macular dystrophy in the vitelliform. A DB, in its classic form is a condition that occurs in the start of childhood, characterized by the accumulation of subretinal deposits centered in the fovea.11 Khan KN, Islam F, Moore AT, Michaelides M. The fundus phenotype associated with the p.Ala243Val best1 mutation. Retina. 2017:1. doi: 10.1097/IAE.0000000000001569. [Epub ahead of print]
https://doi.org/10.1097/IAE.000000000000...
,55 Burgess R, Millar ID, Leroy BP, Urquhart JE, Fearon IM, De Baere E, et al. Biallelic mutation of BEST1 causes a distinct retinopathy in humans. Am J Hum Genet. 2008;82(1):19-31.
These mutations may, howerver, harm a specific aspect of the EPR function. Although the responsible genetic mechanisms are present in all of the EPR cells, the clinic phenotype is problably influenced by the regional variations, metabolic activities, and the diferences in the group of EPR cells, even inside the macula.11 Khan KN, Islam F, Moore AT, Michaelides M. The fundus phenotype associated with the p.Ala243Val best1 mutation. Retina. 2017:1. doi: 10.1097/IAE.0000000000001569. [Epub ahead of print]
https://doi.org/10.1097/IAE.000000000000...
Initially, the vitelliform distrophy is presented with characteristics of an “egg yolk” and its diagnosis is fairly simple to attain. In old scars, or when a fundus of the eye with a normal appearance is seen, the final diagnosis may be made based of the abnormal findings of the EOG.
The clinical EOG is a functional integrity test among the photoreceptors of the EPR.88 Arden GB, Barrada A, Kelsey JH. New clinical test of retinal function based upon the standing potential of the eye. Br J Ophthalmol. 1962;46(8):449-67.
9 Constable PA. A Perspective on the Mechanism of the light-rise of the electrooculogram. Invest Opthalmol Visual Sci. 2014;55(4):2669.-1010 Constable PA, Ngo D, Quinn S, Thompson DA. A meta-analysis of clinical electro-oculography values. Doc Ophthalmol. 2017;135(3):219-32. The LP:DT relation of the EOG is affected in some diffused disfunctions of the EPR and of the retinal photoreceptor layer, including acquired retinopathy and retinal dystrophies characterized by the disfunction of the shaft or atrophy of the choroid of the retina. In most of these disturbances, the EOG abnormalities are proportional to the severity of the disease. Notable exceptions include BEST1 gene disfunctions. Inside of the DB standards, the complete field ERG is generally normal and the LP:DT relation in regarding the EOG is abnormal.
Specifically, a severe loss of potential to light occurs. All of the affected individuals who have or have not presented manifestations towards the fundoscopy, show a light-dark reason (or Aden’s) of less than 1,5 and frequently close to 1,1. For this reason, the EOG is used to evaluate individuals that present a macular lesion that is not clearly defined. Electroretinographic studies only show that a reduced “C” wave which is normal in all other aspects. This regards the only disease that present electroretinographic results that are relatively normal associated with the abnormal findings in the EOG.
The ophthalmologic exam applied on the P.H.S.C ‘s mother and brother appear to be normal, therefore, with an abnormal EOG. Such findings may suggest that the nature of the family members’ disease with the possibility of having occurred generation alternations in the manifestation of the disease.66 Yardley J, Leroy BP, Hart-Holden N, Lafaut BA, Loeys B, Messiaen LM, et al. Mutations of VMD2 splicing regulators cause nanophthalmos and autosomal dominant vitreoretinochoroidopathy (ADVIRC). Invest Ophthalmol Vis Sci 2004;45(10):3683-9. An EOG that is considerably abnormal appears to be pathognomonic of the vitelliform macular dystrophy of Best even in and member that traces back to the fundus of the eye and is proved to be normal.77 Chowers I, Tiosano L, Audo I, Grunin M, Boon CJF. Adult-onset foveomacular vitelliform dystrophy: A fresh perspective. Progr Retin Eye Res. 2015;47:64-85. In a family inheritance study, the identification of heterozygous individuals were reported through small modifications in the EOG, without presenting any sign of or symptom of the disease.88 Arden GB, Barrada A, Kelsey JH. New clinical test of retinal function based upon the standing potential of the eye. Br J Ophthalmol. 1962;46(8):449-67.
Atypically, in our case, a lack of a positive family history, there are two explanations:11 Khan KN, Islam F, Moore AT, Michaelides M. The fundus phenotype associated with the p.Ala243Val best1 mutation. Retina. 2017:1. doi: 10.1097/IAE.0000000000001569. [Epub ahead of print]
https://doi.org/10.1097/IAE.000000000000...
occurrences in sporadic cases, that were previously reported;22 Boon CJF, Klevering BJ, Leroy BP, Hoyng CB, Keunen JEE, den Hollander AI. The spectrum of ocular phenotypes caused by mutations in the BEST1 gene. Prog Retin Eye Res. 2009;28(3):187-205. a lessened penetration that could mask the genetic standard of the family.44 Marquardt A. Mutations in a novel gene, VMD2, encoding a protein of unknown properties cause juvenile-onset vitelliform macular dystrophy (Best's disease). Hum Mol Genet. 1998;7(9):1517-25.,66 Yardley J, Leroy BP, Hart-Holden N, Lafaut BA, Loeys B, Messiaen LM, et al. Mutations of VMD2 splicing regulators cause nanophthalmos and autosomal dominant vitreoretinochoroidopathy (ADVIRC). Invest Ophthalmol Vis Sci 2004;45(10):3683-9.,99 Constable PA. A Perspective on the Mechanism of the light-rise of the electrooculogram. Invest Opthalmol Visual Sci. 2014;55(4):2669.,1010 Constable PA, Ngo D, Quinn S, Thompson DA. A meta-analysis of clinical electro-oculography values. Doc Ophthalmol. 2017;135(3):219-32. We believe, however, that the family members may be carriers of the disease even if it is not manifested. However, to obtain this confirmation, it would be important to map the family’s genetics.
Our article reveals the importance of the electrophysiological exams regarding the diagnosis of BD, including the prevention of its clinical manifestation (autosomal dominant), and supplying concrete contributions to its genetic counseling.
-
Institution: Instituto Benjamin Constant, Rio de Janeiro, RJ, Brazil.
References
-
1Khan KN, Islam F, Moore AT, Michaelides M. The fundus phenotype associated with the p.Ala243Val best1 mutation. Retina. 2017:1. doi: 10.1097/IAE.0000000000001569. [Epub ahead of print]
» https://doi.org/10.1097/IAE.0000000000001569 -
2Boon CJF, Klevering BJ, Leroy BP, Hoyng CB, Keunen JEE, den Hollander AI. The spectrum of ocular phenotypes caused by mutations in the BEST1 gene. Prog Retin Eye Res. 2009;28(3):187-205.
-
3Petrukhin K, Wadelius C, Koisti MJ, Bakall B, Li W, Xie G, et al. Identification of the gene responsible for Best macular dystrophy. Nat Genet. 1998;19(3):241-7.
-
4Marquardt A. Mutations in a novel gene, VMD2, encoding a protein of unknown properties cause juvenile-onset vitelliform macular dystrophy (Best's disease). Hum Mol Genet. 1998;7(9):1517-25.
-
5Burgess R, Millar ID, Leroy BP, Urquhart JE, Fearon IM, De Baere E, et al. Biallelic mutation of BEST1 causes a distinct retinopathy in humans. Am J Hum Genet. 2008;82(1):19-31.
-
6Yardley J, Leroy BP, Hart-Holden N, Lafaut BA, Loeys B, Messiaen LM, et al. Mutations of VMD2 splicing regulators cause nanophthalmos and autosomal dominant vitreoretinochoroidopathy (ADVIRC). Invest Ophthalmol Vis Sci 2004;45(10):3683-9.
-
7Chowers I, Tiosano L, Audo I, Grunin M, Boon CJF. Adult-onset foveomacular vitelliform dystrophy: A fresh perspective. Progr Retin Eye Res. 2015;47:64-85.
-
8Arden GB, Barrada A, Kelsey JH. New clinical test of retinal function based upon the standing potential of the eye. Br J Ophthalmol. 1962;46(8):449-67.
-
9Constable PA. A Perspective on the Mechanism of the light-rise of the electrooculogram. Invest Opthalmol Visual Sci. 2014;55(4):2669.
-
10Constable PA, Ngo D, Quinn S, Thompson DA. A meta-analysis of clinical electro-oculography values. Doc Ophthalmol. 2017;135(3):219-32.
Publication Dates
-
Publication in this collection
Mar-Apr 2018
History
-
Received
28 Sept 2017 -
Accepted
27 Nov 2017