Abstracts
Nemaline myopathy (NM) is a congenital disease that leads to hypotonia and feeding difficulties in neonates. Some cases have a more benign course, with skeletal abnormalities later in life. We analyzed a series of eight patients with NM obtained from a retrospective analysis of 4300 muscle biopsies. Patients were classified as having the typical form in five cases, intermediate form in two cases and severe form in one case. Histochemical analysis showed mixed rods distribution in all cases and predominance of type I fibers in five cases. Immunohistochemical analysis showed abnormal nebulin expression in all patients (four heterogeneous and four absent), homogeneous desmin expression in four cases, strongly positive in three and absent in one, fast myosin expression in a mosaic pattern in six cases and absent in two cases. There was no specific relation between these protein expression patterns and the clinical forms of NM.
nemaline myopathy; nebulin; desmin; myosin; immunohistochemistry
Miopatia nemalínica (NM) é uma doença congênita que leva a hipotonia e dificuldade de sugar em neonatos. Alguns casos possuem uma evolução benigna, com deformidades ósseas tardias. Nós analisamos uma série de oito pacientes com NM obtidos da análise retrospectiva de 4300 biópsias musculares. Os pacientes foram classificados como forma típica em cinco casos, forma intermediária em dois casos e forma severa em um caso. Análise histoquímica mostrou distribuição mista dos rods em todos os casos e predominância de fibras tipo I em cinco casos. Análise imuno-histoquímica mostrou expressão anormal da nebulina em todos os pacientes (quatro heterogênea e quatro ausente), expressão homogenea da desmina em quatro casos, fortemente positiva em tres e ausente em um, expressão da miosina (rápida) com padrão em mosaico em seis casos e ausente em dois casos. Não há relação específica entre a expressão destas proteínas e as formas clínicas da NM.
miopatia nemalínica; nebulina; desmina; miosina; imuno-histoquímica
Nemaline myopathy: clinical, histochemical and immunohistochemical features
Miopatia nemalínica: achados clínicos, histoquímicos e imuno-histoquímicos
Nazah Cherif Mohamad Youssef; Rosana Herminia Scola; Paulo José Lorenzoni; Lineu César Werneck
Neuromuscular Disorders Service, Neurology Division, Internal Medicine Department, Hospital de Clínicas da Universidade Federal do Paraná (UFPR), Curitiba PR, Brasil
ABSTRACT
Nemaline myopathy (NM) is a congenital disease that leads to hypotonia and feeding difficulties in neonates. Some cases have a more benign course, with skeletal abnormalities later in life. We analyzed a series of eight patients with NM obtained from a retrospective analysis of 4300 muscle biopsies. Patients were classified as having the typical form in five cases, intermediate form in two cases and severe form in one case. Histochemical analysis showed mixed rods distribution in all cases and predominance of type I fibers in five cases. Immunohistochemical analysis showed abnormal nebulin expression in all patients (four heterogeneous and four absent), homogeneous desmin expression in four cases, strongly positive in three and absent in one, fast myosin expression in a mosaic pattern in six cases and absent in two cases. There was no specific relation between these protein expression patterns and the clinical forms of NM.
Key words: nemaline myopathy, nebulin, desmin, myosin, immunohistochemistry.
RESUMO
Miopatia nemalínica (NM) é uma doença congênita que leva a hipotonia e dificuldade de sugar em neonatos. Alguns casos possuem uma evolução benigna, com deformidades ósseas tardias. Nós analisamos uma série de oito pacientes com NM obtidos da análise retrospectiva de 4300 biópsias musculares. Os pacientes foram classificados como forma típica em cinco casos, forma intermediária em dois casos e forma severa em um caso. Análise histoquímica mostrou distribuição mista dos rods em todos os casos e predominância de fibras tipo I em cinco casos. Análise imuno-histoquímica mostrou expressão anormal da nebulina em todos os pacientes (quatro heterogênea e quatro ausente), expressão homogenea da desmina em quatro casos, fortemente positiva em tres e ausente em um, expressão da miosina (rápida) com padrão em mosaico em seis casos e ausente em dois casos. Não há relação específica entre a expressão destas proteínas e as formas clínicas da NM.
Palavras-chave: miopatia nemalínica, nebulina, desmina, miosina, imuno-histoquímica.
Nemaline myopathy (NM) is a congenital myopathy first described in 1963 by Shy and Conen, characterized by hypotonia and general muscle weakness predominantly in facial muscles, cervical and trunk flexor muscles, foot dorsiflexor muscles and finger extensor muscles1,2. Patients may also exhibit dysphagia, respiratory insufficiency, foot deformities, arch palate, scoliosis, chest deformities and superior and inferior limbs contractures1-7. Currently, NM is classified into six different forms: severe congenital, typical, intermediate congenital, juvenile, adult and other forms associated with cardiomyopathy, ophthalmoplegia and the presence of intranuclear nemaline bodies on muscle biopsy8. Adult forms are known as sporadic late-onset nemaline myopathies (SLONM) and are associated with HIV and monoclonal gammopathy9,10.
An NM diagnosis is made with a muscle biopsy in which modified Gomori-trichrome (MGT) staining shows the presence of nemaline bodies in muscle fibers in the subsarcolemmal or intermyofibrilar region1,2,5,11,12. The histochemistry also reveals features in NM such as a predominance of type I fibers and an increase in acid phosphatase activity5,12-14. An immunohistochemical study of muscle proteins shows the nature of the structural failures in NM. Several studies have shown negative and positive expression of nebulin, myosin, desmin, titin and e dystrophin in muscle fiber and rods5,15-18. Nemaline myopathy remains genetically heterogeneous. To date, defects in six distinct genes have been identified in cases of NM. These include mutations in α-tropomyosin (TPM3), beta-tropomyosin (TPM2), nebulin (NEB), α-actin (ACTA1), cofilin2 (CFL2) and troponin T (TNNT1)4,17-28.
This study describes the clinical manifestations of nemaline myopathy using histochemical analysis along with expression analysis of nebulin, desmin and myosin proteins with immunohistochemistry.
METHOD
A retrospective analysis of 4300 muscle biopsies performed between January 1978 and July 2007 identified 15 patients with a diagnosis of NM, but seven cases were excluded because there was not enough material from the muscle biopsy for immunohistochemical analysis. Relevant data, including the clinical evaluation, serum enzymes levels, needle electromyography (EMG), and histological-immunohistochemical aspects of the each muscle biopsy, were collected. Consent to analyze the muscle biopsies was obtained in the out-patient clinic or during hospital admission for diagnostic investigation.
Clinical evaluation
Patients were classified as typical form, intermediate form or severe form, according to criteria established by the International Nemalin Myopathy Consortium8. Particular attention was paid to obstetrical history, pregnancy complications, muscle weakness and tonus, breastfeeding difficulties, respiratory failures, dysphagia, and facial and skeletal deformities (dysmorphisms). Other relevant data were also collected including age, gender, course of disease and family history.
Laboratory analysis
The levels of creatine kinase (CK), aldolase (AL) and lactate dehydrogenase (LDH) were recorded as a proportion reflecting their increase above normal limits.
Electromyographic findings
The electromyography (EMG) pattern was classified as normal, myopathic, denervation or mixed (myopathic with denervation findings) according to standard procedures29.
Muscle biopsy
Muscle biopsies were frozen in liquid nitrogen at -170ºC and cryostat sections were stained histologically, according to standard procedures7,11. The predominance of type I fibers with an ATPase reaction (pH 9.4, 4.6 and 4.3), moth-eaten fibers with NADH-tetrazolium redutase, focal increases in acid phosphatase, and the frequency and distribution (subsarcolemmal, intermyofibrilar or mixed) of fibers with rods on MGT were determined by counting approximately 300 to 500 muscle fibers of each specimen.
Immunohistochemical analysis
The cryostat sections (4 um) of muscle biopsies were used for immunohistochemistry with specific primary antibodies and secondary antibody (anti-IgG) conjugated to fluorescein for nebulin, desmin and myosin proteins, according to standard procedures. The primary antibodies used were an anti-nebulin monoclonal antibody (Sigma, dilution 1:50), an anti-desmin monoclonal antibody (Sigma, dilution 1:20) and a fast anti-myosin monoclonal antibody (Sigma, dilution 1:10). The secondary antibody conjugated to fluorescein was used at a dilution 30:500. Afterwards, an immunofluorescence analysis was performed using an epifluorescence microscope. The immunofluorescent classification was modified based on the descriptions of Gurgel-Giannetti et al.15 and Imoto et al.16: (1) nebulin: absent expression, heterogeneous expression (diffused imperfection), or homogeneous expression; (2) desmin: absent expression, homogeneous expression, or strongly positive expression; and (3) fast myosin: absent expression or mosaic-type expression.
RESULTS
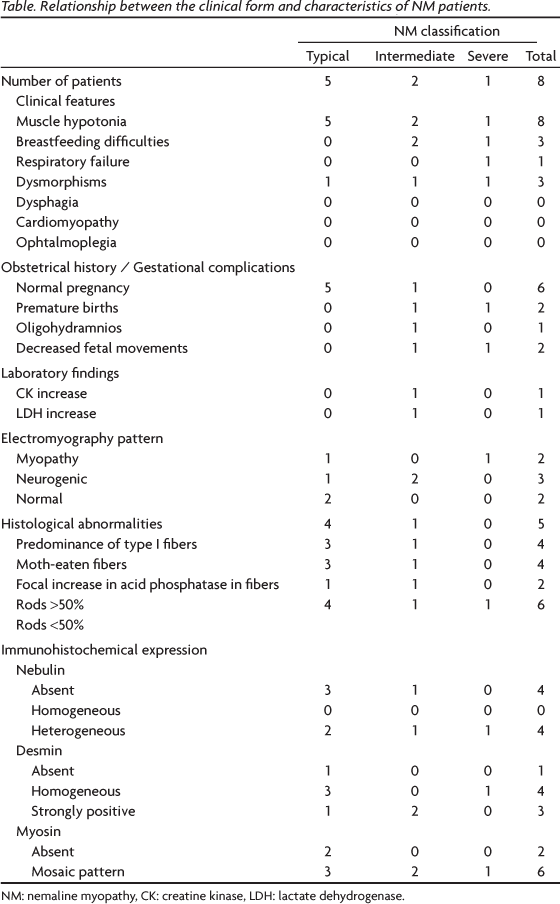
There were eight patients (six female and two male) aged 2 months to 6 years, with a mean age of 22 months. The patients were classified as having the typical form in five cases, the intermediate form in two cases and severe form in one case. Muscle hypotonia since birth was present in all cases. The other clinical findings reported were skeletal or facial dysmorphisms, breastfeeding difficulties and respiratory failure (Table). Dysphagia, cardiomyopathy and ophtalmoplegia were not observed. No obstetric abnormalities were observed in six cases, but gestational complications such as a decreased fetal movements, oligohydramnios and premature birth were observed in two cases (intermediated form and severe form). No family history was observed (Table).
Only one case (case 4) had serum CK and LDH levels increased above the normal limits (Table).
The EMG pattern was observed in seven patients and was myopathic in two cases, neurogenic in three cases and normal in the remainder. The EMG revealed abnormalities in four cases of the typical form, two of the intermediate form and in the one of the severe congenital case (Table).
A muscle histological analysis was performed in all cases and the most common abnormalities found in muscle biopsies were rods in all cases, type 1 fiber predominance in five cases, moth-eaten fibers in four cases and a focal increase in fibers of acid phosphatase activity in four cases (Fig 1 / Table). A frequency of rods above 50% with MGT staining was found in two patients, while a frequency below 50% was found in six patients (Table). The distribution of rods was mixed (subsarcolemmal and intermyofibrilar) in all cases (Fig 1).
Immunohistochemical analysis showed abnormal results for nebulin, myosin and desmin muscle proteins in all cases (Table). Nebulin showed heterogeneous expression in four cases and was absent in the other four cases (Fig 2 / Table). We never observed homogeneous expression of nebulin. Desmin expression was homogeneous in four cases, strongly positive in three cases, and absent in one case (Fig 2 / Table). Fast myosin expression showed a mosaic pattern in six cases and was absent in two cases (Fig 2 / Table). The relationship between the clinical form of NM and nebulin, desmin and fast myosin expression on muscle biopsy is shown in Table.
DISCUSSION
NM is a rare form of congenital myopathy. The small number of cases found in our study is illustrative of the low incidence of NM of 1 case per 500000 live births25. We identified a higher number of female patients than male patients (six female and two male), although the literature does not suggest any gender predominance.
In the series described by Gommans et al. and Wallgren-Pettersson, there was a higher incidence of the typical form of NM3,6. Although this is a rare disease, the typical form has the highest incidence, followed by the intermediate congenital form and the severe congenital form.
In this study, all of the patients showed mild to severe hypotonia from birth onward, confirming reports of other series of patients with NM1-3, 6,7,13.
With regard to muscle enzymes, CK was normal or slightly elevated (up to five times the normal value), and there was no muscle necrosis or muscle regeneration pattern detected in biopsies of NM patients. This has also been observed in most other cases5,6.
The EMG examination typically shows diverse features in NM patients monitored throughout the course of the disease6,29. Before the age of 3 years, the EMG usually shows mild abnormalities and a myopathic pattern (the motor unit potential (MUP) has a short duration, low amplitude, increased recruitment and short polyphasic potentials) can be found in a few patients. Between the ages of 3 and 10 years, there is an increase in the incidence of abnormalities, although there are many normal MUPs, myopathic MUPs are predominant. After the age of 11 years, there are few normal MUPs and a neurogenic pattern (MUPs with long duration, high amplitude, decreased recruitment and long polyphasic potentials) can occur. In adults with NM, these features are primarily seen in distal muscles such as the tibialis anterior. Spontaneous muscle activity such as fibrillation and positive waves are rare in NM. These abnormalities suggest a progressive form of primary myopathy probably due to motor unit remodeling leading to secondary neuropathy changes. In our cases, the patients with normal EMG were under 2 years old, consistent with the literature. The patients with myopathic and neurogenic patterns, however, were between the ages of 2 months and 3.5 years, suggesting premature EMG abnormalities in our group6,24.
Nemalin bodies or rods are a sign of NM, and the best technique for detecting them is the MGT staining of biopsy sections1,2,5,11. With other staining or histochemical techniques, such as ATPase staining, the results are often not distinct or can show a mild negative image. Nemaline bodies are distributed randomly along the muscle fiber, but have a tendency to be attached below the sarcolemma (subsarcolemmal location), or appear as difused spots in the interior of the fiber (intermyofibrilar location). Mixed patterns can also occur. Intranuclear rods have been described in severe neonatal and adult forms26,30. Rods were identified in less than 50% of muscle fibers in six of our patients and more than 50% in two of our patients via MGT staining. All cases showed a mixed location pattern, regardless of the NM clinical form.
The predominance of type I fibers is a common feature in NM, and some patients can have exclusively type I fibers or poor differentiation between fibers5,12-14,. Infrequently, there is a focal increase in acid phosphatase activity in fibers, which is associated with cases with a fast evolution. This suggests a degenerative process, inducing lysosomal enzyme activation and celular destruction13,14. Our cases showed a predominance of type I fibers and increased acid phosphatase activity, regardless the NM clinical form.
The analysis of nebulin protein expression using immunohistochemical began in the last decade and there are descriptions of normal and abnormal expression patterns in patients with NM, mainly for the typical form15-17. Nebulin acts as a molecular ruler for thin filaments and is important for the assembly and integration of Z discs with the sarcomere15,17. In our study, the typical form of NM was associated with abnormal nebulin expression, as were the severe and intermediate congenital forms, which is similar to reports in other studies15-17. For desmin protein expression, the presence of areas of accumulation in muscle fibers is a pathological finding and may represent immaturity or a muscle fiber regeneration process. Van der Ven described strongly positive desmin expression in cases of severe congenital NM, while in mild cases, there was not a distinct expression pattern18. In our study, both the severe congenital form and the typical and intermediate congenital forms showed strongly positive desmin expression in muscle fibers. Fast myosin expression was absent in two patients, while positive expression with a mosaic pattern was observed in the majority of patients.
Six NM-related genes, all encoding components of the thin filament of the sarcomere, have been described. Studies of several families with severe, intermediate and typical NM forms have identified mutations in α-tropomyosin (TPM3) and beta-tropomyosin19-21. A recent study described an Australian familly with a mutation in the TPM3 gene22. Several mutations were identified in the nebulin (NEB) gene due to small deletions or insertions22,23. Mutations in the α-actin gene (ACTA1) may be related to congenital fiber type disproportion24,25, and have been reported to be exclusively associated with intranuclear rods26. Troponin T gene (TNNT1) mutations were described in an isolated group of old order Amish NM patients4, 24. A sixth gene, CFL2, encoding the actin-binding protein muscle cofilin-2, was reported in two siblings with congenital myopathy27. Our patients could have had any of these genetic alterations. Our patients had abnormal nebulin expression by immunohistochemistry suggesting mutations in nebulin. Mutations in CFL2, ACTA1 and TNNT1 are less probable to occur in our patients because we had only one case of the congenital form of NM, and there were no cases with intranuclear rods or Amish patients. Unfortunately, mutations in many of these genes do not predict the severity or prognosis of the disease and do not determine patterns of inheritance31.
In the present study, we focused on protein expression analysis in patients with NM and concluded that regardless of the NM clinical form, nebulin may have heterogeneous or absent protein expression, and fast myosin protein expression may be positive with a mosaic pattern in the majority of patients. In addition, the desmin protein, which is related to the maturity level of the muscle, may show normal or abnormal expression in NM patients.
Received 20 February 2009, received in final form 1 July 2009. Accepted 17 July 2009.
Dra. Rosana Herminia Scola - Serviço de Doenças Neuromusculares / Hospital de Clínicas da UFPR - Rua General Carneiro 181 / 3º andar - 80060-900 Curitiba PR - Brasil. E-mail: scola@hc.ufpr.br
- 1. Shy GM, Engel WK, Somers JE, Wanko T. Nemaline myopathy: a new congenital myophaty. Brain 1963;86:793.
- 2. Conen PE, Murphy EG, Donohue WL. Light and electron microscopic studies of myogranules in a child with hypotonia and muscle weakness. Can Med Assoc J 1963;89:983.
- 3. Gommans IMP, Van Engelen BGM, Ter Laak HJ, et al. A new phenotype of autosomal dominant nemaline myophathy. Neuromusc Disord 2002;12:13-18.
- 4. Johnstson JJ, Kelley RI, Crawford TO, et al. A novel nemaline myophaty in the Amish caused by mutation in troponin T1. Am J Hum Gent 2000;67:814-821.
- 5. North KL, Laing NN, Wallgren-Pettersson C. Nemaline myophathy: current concepts. J. Med Genet 1997;34:705-713.
- 6. Wallgren-Pettersson C. Congenital nemaline myophathy: a clinical follow-up study of twelve patients. J Neurol Sci 1989;98:1-14.
- 7. Werneck LC, Jamur MC, Silvado CE, et al. Miopatia nemalínica: relato de um caso com estudo histoquímico e microscopia eletrônica. Arq Neuropsiquiatr 1983;41:199-207.
- 8. Wallgren-Pettersson C, Laing NG. 70th ENMC International workshop: nemaline myopathy. Neuromusc Disord 2000;10:299-306.
- 9. Santics JT, Cumbo-Nacheli G, Dobbie D, Baumgartner D. HIV-associated nemaline rod myopathy: role of intravenous immunoglobulin therapy in two persons with HIV and AIDS. AIDS Read 2008;18:90-94.
- 10. 1Chahin N, Selcen D, Engel AG. Sporadic late onset nemaline myopathy. Neurology 2005;65:1158-1164.
- 11. Werneck LC. The value of muscle biopsy in neurology: a study of 290 biopsies. Rev Bras Clin Ter 1981;10(Suppl):S2-S24.
- 12. Gurgel-Giannetti J, Reed UC, Marie SK, et al. Rod distribution and muscle fiber type modification in the progression of nemaline myopathy. J Child Neurol 2003;18:235-240.
- 13. Nonaka I, Ishiura S, Sasaki M, et al. Progression in nemaline myopathy. J Neurol Sci 1989;98:100.
- 14. Nonaka I, Ishiura S, Arahata K, et al. Progression in nemaline myophathy. Acta Neuropathol 1989;78:484-491.
- 15. Gurgel-Gianetti J, Reed U, Bang M, et al. Nemalin expression in patients with nemaline myopathy. Neuromusc Disord 2001;11:154-162.
- 16. Imoto C, Kimura S, Kawai M. Nebulin is normally expressed in nemaline myopathy. Acta Neuropathol 1999;97:433-436.
- 17. Sewry CA, Brown SC, Pelin K, et al. Abnormalities in the expression of nebulin in chromosome-2 linked nemaline myopathy. Neuromusc Disord 2000;11:146-153.
- 18. Van Der Ven PFM, Jap PHK, Laak HJ, et al. Immunophenotyping of congenital myopathies: disorganization of sarcomeric, cytoskeletal and extracellular matrix proteins. J Neurol Sci 1995;129:199-213.
- 19. Laing NG, Wilton SD, Akkari PA, et al. Mutation in the alfa-tropomiosin gene TPM3 associated with autossomal dominant nemaline myopathy. Nat Genet 1995;9:75-79.
- 20. Donner K, Ollikainen M, Ridanpaa M, et al. Mutations in the beta-tropomyosin (TPM2) gene: a rare cause of nemaline myopathy. Neuromusc Disord 2002;12:151-158.
- 21. Clarke FN, Kolski H, Dye ED, et al. Mutations in TPM3 are a common cause of congenital fiber type disproportion. Ann Neurol 2008;63: 329-337.
- 22. Ilkovski B, Mokbel N, Lewis R, et al. Disease severity and thin filament regulation in M9R TPM3 nemaline myopathy. J Neuropathol Exp Neurol 2008;67:867-877.
- 23. Pelin K, Hilpela P, Donner K, et al. Mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Proc Natl Acad Sci USA 1999;96:2305-2310.
- 24. Wallgren-Pettersson C, Laing NG. Workshop report: 138th ENMC Workshop: Nemaline myopathy, 20-22 may 2005, Naarden, The Netherlands. Neuromusc Disord 2006;3:273-275.
- 25. Nowak KJ, Wattanasirichaigoon D, Goebel HH, et al. Mutations in the skeletal muscle alfa-actin gene in patients with actin myopathy and nemaline myopathy. Nat Genet 1999;23:208-212.
- 26. Koy A, Ilkovski B, Laing N, et al. Nemaline myopathy with exclusively intranuclear rods and a novel mutation in ACTA1 (Q139H). Neuropediatrics 2007;38:282-286.
- 27. Agrawal PB, Greenleaf RS, Tomczak KK, et al. Nemaline myopathy with minicores caused by mutation on the CLF2 gene encoding the skeletal muscle actin-binding protein, cofilin2. Am J Hum Genet 2007;80:162-167.
- 28. Wallgren-Pettersson C, Kaariainen H, Rapola J, et al. Genetics of congenital nemaline myopathy: a study of 10 families. J Med Genet 1990;27:480-487.
- 29. Amato AA, Dumitru D. Hereditary Myopathies. In: Dumitru D, Amato AA, Zwarts MJ (Eds). Electrodiagnostic medicine, second edition. Philadelphia: Hanley & Belfus 2002:1320-1322.
- 30. Goebel HH, Warlo I. Nemaline myopathy with intranuclear rods-intranuclear rod myopathy. Neuromusc Disord 1997;7:13-19.
- 31. Sanoudou D, Beggs A. Clinical and genetic heterogeneity in nemaline myopathy: a disease of skeletal muscle thin filaments. Trends Mol Med 2001;8:362-368.
Publication Dates
-
Publication in this collection
13 Oct 2009 -
Date of issue
Sept 2009
History
-
Accepted
17 July 2009 -
Reviewed
01 July 2009 -
Received
20 Feb 2009