Abstracts
OBJECTIVE: Despite cerebral ischemia being a frequent clinical pathologic state, the tolerance of neural tissue to oxygen absence and to reperfusion is controversial. This study aims to evaluate the effects of focal cerebral ischemia/reperfusion, by analyzing the mitochondrial respiration. METHOD: Sixty-four adult rats underwent focal cerebral ischemia by middle cerebral artery occlusion, during 15, 30 and 60 minutes, followed by 10 minutes or 19 hours of reperfusion. The effects of ischemia were analyzed measuring the O2 consumption by mitochondria in the ischemic and non-ischemic areas. RESULTS: There was compromise of the mitochondrial respiration after 30 and 60 minutes of ischemia, followed by 10 minutes of reperfusion but there was no alteration in this function after 19 hours of reperfusion. CONCLUSION: Compromise of the mitochondrial function occurred after 30 minutes of ischemia but, until one hour of ischemia, if the reperfusion was prolonged there was no evidence of ischemic/reperfusion injuries.
focal brain ischemia; reperfusion; mitochondrial respiration
OBJETIVO: Apesar da isquemia cerebral focal ser um estado patológico freqüente, a tolerância do tecido nervoso à ausência de oxigênio e à reperfusão é controversa. Este estudo visou avaliar os efeitos da isquemia/reperfusão cerebrais focais através da respiração mitocondrial. MÉTODO: Sessenta e quatro ratos adultos foram submetidos a isquemia cerebral focal por oclusão da artéria cerebral media durante 15, 30 e 60 minutos, seguida de reperfusão por 10 minutos e 19 horas. Os efeitos da isquemia/reperfusão foram analisados medindo-se o consumo de O2 pelas mitocôndrias nas áreas isquêmicas e não isquêmicas. RESULTADOS: A respiração mitocondrial mostrou-se alterada após 30 e 60 minutos de isquemia seguidos por 10 minutos de reperfusão mas apresentou-se inalterada após 19 horas de reperfusão. CONCLUSÃO: O comprometimento da função mitocondrial ocorreu após 30 minutos de isquemia, porém, até uma hora de isquemia e reperfusão prolongada não foram observadas alterações pós-isquemia/reperfusão.
isquemia cerebral focal; reperfusão; respiração mitocondrial
Functional evaluation of temporary focal cerebral ischemia: experimental model
Avaliação funcional da isquemia cerebral focal temporária em modelo experimental
Sinésio Grace DuarteI; Antônio Dorival CamposII; Benedicto Oscar ColliIII
Laboratory of Surgical Technique and Experimental Surgery - Department of Surgery - Ribeirão Preto Medical School - University of São Paulo (FMRP-USP), Ribeirão Preto SP, Brasil. Part of the requirements for Master of Science Thesis (Dr. Sinésio Grace Duarte)
INeurosurgeon, Head of the Division of Neurosciences, University of Franca, Franca SP, Brasil
IIAssociated Professor, Department of Social Medicine, FMRP-USP
IIIProfessor, Department of Surgery and Head of the Neurosurgical Division, FMRP-USP
ABSTRACT
OBJECTIVE: Despite cerebral ischemia being a frequent clinical pathologic state, the tolerance of neural tissue to oxygen absence and to reperfusion is controversial. This study aims to evaluate the effects of focal cerebral ischemia/reperfusion, by analyzing the mitochondrial respiration.
METHOD: Sixty-four adult rats underwent focal cerebral ischemia by middle cerebral artery occlusion, during 15, 30 and 60 minutes, followed by 10 minutes or 19 hours of reperfusion. The effects of ischemia were analyzed measuring the O2 consumption by mitochondria in the ischemic and non-ischemic areas.
RESULTS: There was compromise of the mitochondrial respiration after 30 and 60 minutes of ischemia, followed by 10 minutes of reperfusion but there was no alteration in this function after 19 hours of reperfusion.
CONCLUSION: Compromise of the mitochondrial function occurred after 30 minutes of ischemia but, until one hour of ischemia, if the reperfusion was prolonged there was no evidence of ischemic/reperfusion injuries.
Key words: focal brain ischemia, reperfusion, mitochondrial respiration.
RESUMO
OBJETIVO: Apesar da isquemia cerebral focal ser um estado patológico freqüente, a tolerância do tecido nervoso à ausência de oxigênio e à reperfusão é controversa. Este estudo visou avaliar os efeitos da isquemia/reperfusão cerebrais focais através da respiração mitocondrial.
MÉTODO: Sessenta e quatro ratos adultos foram submetidos a isquemia cerebral focal por oclusão da artéria cerebral media durante 15, 30 e 60 minutos, seguida de reperfusão por 10 minutos e 19 horas. Os efeitos da isquemia/reperfusão foram analisados medindo-se o consumo de O2 pelas mitocôndrias nas áreas isquêmicas e não isquêmicas.
RESULTADOS: A respiração mitocondrial mostrou-se alterada após 30 e 60 minutos de isquemia seguidos por 10 minutos de reperfusão mas apresentou-se inalterada após 19 horas de reperfusão.
CONCLUSÃO: O comprometimento da função mitocondrial ocorreu após 30 minutos de isquemia, porém, até uma hora de isquemia e reperfusão prolongada não foram observadas alterações pós-isquemia/reperfusão.
Palavras-chave: isquemia cerebral focal, reperfusão, respiração mitocondrial.
Despite many experimental studies and great clinical experience, the treatment of cerebral ischemia is still a difficult problem.1 The ideal therapeutic reperfusion after some time of ischemia can contribute to worsen tissue conditions, a phenomenon called "reperfusion injury"2,3. This situation is commonly seen in the neurosurgical practice during temporary clipping of vessels, when short-period of ischemia followed by reperfusion are produced. There is some controversy about the tolerance time of cerebral tissue to the absence of oxygen and glucose supply and to the role of the reestablishment of blood flow on the tissue lesion. Attempts have been made to establish time parameters to temporary ischemia without injuries to the brain using clinical and experimental studies4-6. Among several methods for evaluating the effects of the ischemic and reperfusion insults to the brain, the metabolic evaluation such as the mitochondrial respiration or measures of involved enzymatic activity, can give precocious information about the energetic state of the neuron and the functional capacity after reperfusion6-10. Experimental studies showing the mitochondrial response to short period focal ischemia followed by reperfusion could not be found in the literature.
The maintenance of a reduced blood flow in the periphery of the ischemic area allows the occurrence of cellular dysfunction without imminent death, that permits the cellular recuperation depending on the time and intensity of ischemia11-17. This territory, known as penumbra zone, is the goal of recuperation attempts in definitive ischemias and probably the place where metabolic changes that can cause the reperfusion injury. There are no histopathological changes in the presence of short period ischemia11,13-15. Deterioration of the energetic state and metabolic changes occurs earlier and can be seen about 5 minutes after the ischemia; they are more pronounced in the central zone than in the penumbra zone and it seems that it does not progress in the first 30 minutes16.
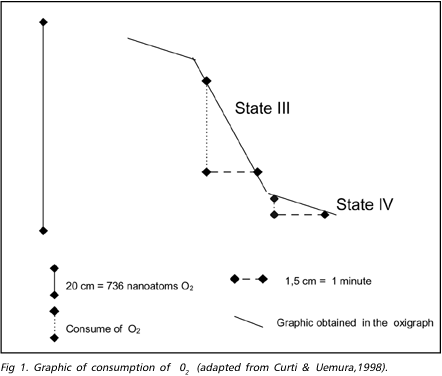
During the ischemic process the mitochondrial respiration is inhibited by the association of phenomena as Ca++ excess, lactic acidosis overload and degradative enzymes action, which delay the provision of ATP to the cell12,17,18. The restoration of O2 and glucose supply to the cell allows the restablishment of the oxidative phosphorylation, but can trigger a series of biochemical reactions that affects the recuperation of the mitochondria, and acts against the beneficial effect of the reperfusion, producing the "reperfusion injury"2,15,17-19. The mitochondrial respiration can be measured through the consumption of O2 during the procedure, either in the presence of substratum (state 3) or in rest (state 4). The consumption of O2 can be registered in a graphic and calculated for a given time (Fig. 1). There is a decrease of about 50% and 75% after 15 and 30 minutes of ischemia, respectively, in the consumption of O2 (state 3). In the state 4, the consumption of O2 continues stable during ischemia lasting less than 1 hour and it is reduced after longer periods of ischemia15. These changes indicate that in early stage the mitochondrial mechanism of respiration coupling is compromised, and after 1 hour or more of ischemia, the mitochondrial respiration is affected even in resting. After short periods of reperfusion (30 minutes or less), the mitochondrias of the areas completely affected tend to recover its function in full. After longer periods of ischemia, recovery the mitochondria function is partial and the consumption of O2 remain lower than those in the pre-ischemic period8,9,18. In incomplete ischemic areas, with the same duration, the consumption of O2 continue below the values obtained in the mitochondria which were not affected by ischemic aggression17. Studies in which similar periods of ischemia were used showed that, after periods of reperfusion, there is a deterioration in the consumption of O2 indicating degradation of the mitochondria, probably due to structural or functional changes induced by enzymatic action.
The aim of this study is to evaluate the effects of focal ischemia followed by reperfusion in the cerebral cortex of rats, analysing the mitochondrial function.
METHOD
The experiments were performed using 64 male, adult Wistar rats, each weighing between 282 and 305g, which were kept in cages in the pre-surgical period with free access to food and water. Four groups of 10 animals and 3 of 8 animals each were included in this study: animals of groups 1 to 3 were submitted to ischemia of 15, 30 and 60 minutes respectively, and reperfusion of 10 minutes. The fourth group was a control (sham). Animals of groups 5 to 7 were submitted to ischemia of 15, 30 and 60 minutes respectively, and reperfusion of 19 hours.
Induction of Ischemia - Anesthesia was induced through inhalation of halothane in a glass campanula and kept with a vaporizer linked to a mechanical respirator after endotracheal intubation. Mean blood pressure was monitored through a PE 50 catheter inserted in the tail's artery and arterial blood samples were periodically collected for gases analysis. The mean arterial pressure was kept between 80 and 90 mmHg and the pCO2 between 34-42mmHg, adjusting the parameters of the respirator. Body temperature was continuously recorded by a rectal term-resistor linked to a biological monitor, and it was kept between 37 and 38º C using a 220v lamp next to the animal. The cervical vessels were exposed through a median ventral cervical incision in the skin, under the surgical microscope. The obstruction of the middle cerebral artery was performed by inserting a 4-0 nylon suture in the left internal carotid artery via external carotid artery, until it reach and occlude the ostium of the left middle cerebral artery.20 Following the ischemia, the obstructer was removed allowing reperfusion of the ischemic area. After reperfusion, the brain and samples of tissue for analysis were rapidly removed and the animals were sacrificed with a massive doses of halothane. The animals submitted to prolonged reperfusion were recovered from the anesthesia and sent back to their cages until the time for removal of the brain.
Evaluation of O2 consumption during mitochondrial respiration - The cerebral cortex was isolated and 4 samples of brain tissue were got from the ischemic/reperfused area (central left-CL), from the contralateral symmetric area (central right-CR), and two peripheral symmetric areas (right peripheral - RP, and left peripheral - LP) which represented the remainder cortex out of the middle cerebral artery territory. The samples of brain tissue were washed in saline solution at 0 to 4º C and the mitochondrias were isolated using differential centrifugation21,22. The consumption of O2 by the mitochondrias using the pyruvate/malate substrate was obtained using a Gilson's oxigraph (Medical Electronics - USA), attached to a Clark's electrode. The consumption of O2 in the states III and IV and the RRC (rate of respiratory control), were calculated using the graphic obtained in the oxygraph (Fig 1).6 The state IV were got adding 2 mg/ml of mitochondrial protein to 1,4 ml of the respiration substratum and the state III adding 200 moles of MgADP to the state IV. The values of states III and IV were obtained projecting two lines over the curves corresponding to these states and two other line parallels to the X and Y-axis were traced to form a right-angled triangle with the former (Fig 1). The side of this triangle parallel to X-axis should measure 1,5 cm, corresponding to a 1 minute in the graphic. The extension of the other side can be used as values expressed in nmoles of O2 consumed in one minute. As the amount of mitochondrias put into the O2 chamber was 2,0 mg, dividing the obtained value by 2, the mitochondrial respiration was expressed in nanoatoms of O2/minute/mg of mitochondria.
Statistical analysis - The comparisons among the parameters of the mitochondrial respiration (state 3, state 4 and RRC), were performed using the Friedman non-parametric test to gauged samples for comparing the 4 samples of the same animal. In the case of occurrence of any difference, the results were submitted to the multiple comparison test based in the additions of Friedman postulates. Comparison between the ischemic areas (central left area) and the non-ischemic areas in the different groups were performed using the Kruskal-Wallis non-parametric test. An a-error value not exceeding 5% was considered significant for the two-tailed tests.
RESULTS
Table 1 shows the mean of the consumption of O2 by the mitochondria in the stages III and IV and of the RRC in the different areas of the same group.
The O2 consumption by the mitochondrias in states III and IV in the different areas were similar in samples of animals of the groups 1 and 4. The RRC values were similar in different areas in samples of animals in each one of the groups.
There was a significant reduction in the O2 consumption in the central in relation to the peripheral areas in the state III (non parametric Friedman test, p = 0.0002) and there was a significant decrease in the consumption of O2 in the ischemic area in relation to the left peripheral area in the state IV (non parametric Friedman test, p = 0.0008) in the samples from the animals of group 2 (30 minutes of ischemia follwed by 10 minutes of reperfusion).
The O2 consumption was significantly reduced in the left central areas (ischemic) in relation to the peripheral areas in the state III after 60 minutes of ischemia follwed by 10 minutes of reperfusion (group 3) (non parametric Friedman test, p = 0.0001).There was significant decrease in the consumption of O2 in the central left area in relation to the peripheral area in the state III in the samples from the animals of group 5 (15 minutes of ischemia follwed by 19 hours of reperfusion) (non parametric Friedman test, p = 0.003). The central left areas presented a significant reduction in the consumption of O2 in relation to the left peripheral areas in state III in samples of animals of group 6 (30 minutes of ischemia follwed by 19 hours of reperfusion) (non parametric Friedman test, p = 0.01). It was observed a significant decrease in the consumptions in the central left area in relation to the right peripheral area in state III in the group 7 (60 minutes of ischemia follwed by 19 hours of reperfusion) (non parametric Friedman test, p = 0.01).
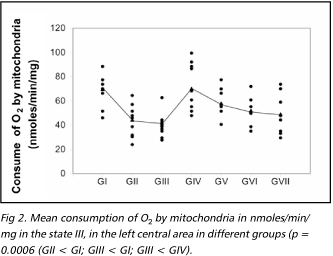
Figures 2 to 4 present the results of mitochondrial function evaluation through its respiration in the state III and IV, and the values of the RRC, obtained in the ischemic area in samples from animals of the different groups. There was a significant reduction in the consumption of O2 in the state III in the samples from animals of the group 2 in relation to the animals of group 1 and the consumption of O2 in the samples from animals of group III were significantly reduced in relation to animals of groups 1 and 4 (non parametric Kruskal Wallis test, p = 0.0006). Regarding the state IV, there was significant decrease in the consumption of O2 in samples of group 1 in relation to group 2 (non parametric Kruskal Wallis test, p = 0.04). The RRC values were similar among all the studied groups.
DISCUSSION
After the focal ischemic insult to the cerebral tissue some functional and histological changes occur and are identified in many ways of evaluation6,7,23. The restoration of the blood flow creates a possibility of stopping or reverse the ischemic process but it can lead to electrolytic and biochemistry alterations that become impossible the recuperation or can still aggravate the initial insult. This situation is known as "reperfusion injury"3. These modifications and its consequences are associated to the level of reduction of the blood flow and to the reduction or absence of blood flow2,24. Due to the difficulty of standardizing the clinical studies for evaluation of the ischemic/reperfusion phenomenon, experimental studies have been used, often using rats, although the transposition of these results to human beings has some limitations.6,12
In the present study halothane was used as anesthetic agent because it is considered safe and does not interfere on the mitochondrial respiration25 and there was no technical difficulties for performing the surgical procedures; the homeostatic parameter was also kept according to the similar studies for reducing interferences in mitochondrial respiration6. The analysis of mitochondrial respiration for functional evaluation of the effects of ischemia and reperfusion is justified because changes in this function are the first pathophysiological occurrence after the ischemia17-19, and because it's a biochemical method that can be precisely measured6.
Experimental studies showing the mitochondrial response to short period focal ischemia followed by reperfusion could not be found in the literature. Some experimental studies using longer periods of ischemia/reperfusion have showed partial or total functional recovery of the mitochondria after this phenomenon. Two hours of focal brain ischemia in rats, O2 consumption descreased to about 40% of control, improved to about 80% after 1 hour of reperfusion, but then decreased to about 60 and 50% of control after 2 and 4 hours of reperfusion9. Other authors observed that there was complete restoration of the mitochondrial function after 2 hours of ischemia followed by 2 hours of reperfusion and that this restoration persisted until 5 hours after the ischemia15. In a general way, the data from the literature show that short periods of ischemia are less aggressive to the mitochondria and also that the reperfusion leads to a partial or total mitochondrial recovery in its initial period and, after that, there is a decline in its function that is ischemia duration-related18,19. Other factors as high temperature, acidosis and increased glucose level can also contribute to aggravate the damage20. The results obtained in this study in the different cortical areas of the "sham" group (group 4), indicate a tendency to reduction in the consumption of O2 in the central areas than in the peripheral ones in the state III, but there was no statistic difference. In the state IV, the O2 consumption remained similar and the same occurred with the RRC values. These data were different from the data obtained by Carlotti Jr. et al.6 that found significant difference between the O2 consumption in the central and peripheral areas, in the state III.
The analysis of the different areas in each group of this study showed that to the "sham" group and to the group 1 (15-minutes of ischemia), followed by reperfusion and evaluated in a short period, the O2 consumption was similar. In the other groups, in the state III, the ischemic area was always different compared to at least one of the control areas of the animal. In the state IV, only in the group 2, the left central area presented less consumption related to at least one of the controls. The RRC was similar to all the areas in all the situations. These results demonstrate that short periods of ischemia followed by reperfusion do not affect the mitochondrial respiration. After longer periods of ischemia (30 and 60 minutes) followed by short period reperfusion there was a reduction in the mitochondrial respiration function during the coupling mechanism (state III). Only in the group 2 there was a significant compromising of the state IV (state of mitochondrial respiration rest). Sixty minutes of ischemia followed by 10 minutes reperfusion caused reduction in the mitochondrial respiration in the state IV, but it was not significant. Compromising of the state IV after short periods of ischemia followed or not by reperfusion, both in global and in focal ischemia, is not related in the literature. In the left central area (ischemic area), it was detected differences of O2 consumption in the states III and IV, between the groups 1 and 2, and in the state III between the groups 1 and 3 and groups 3 and 4. There was a tendency to lower consumption of O2 in the group 3 when compared with group 2 (not significant). Among the groups "sham", 1, 5, 6, and 7 there was not significant difference in the consumption. The evaluation of these results suggests that the focal ischemia after periods of 30 minutes or more causes alterations in the mitochondrial respiration. The reperfusion does not reverse the situation immediately (these results are similar to that obtained by Carlotti Jr. et al.,6 using ischemia without reperfusion). On the other hand, these results are different from the ones obtained by other authors that, studying the focal ischemia found alterations in the mitochondrial respiration only after longer ischemic periods.9,15 In the groups subjected to longer reperfusion (19 hours) the mitochondrial O2 consumption was similar to the control group (sham), and was independent of the duration of the ischemia. This suggests a recuperation of the mitochondrial respiratory function damaged during those periods (30 and 60 minutes). Another possibility would be the participation of mitochondria of inflammatory cells during longer periods of ischemia. The decline in the mitochondrial respiration after some hours of reperfusion in global ischemia of similar or longer-duration or focal ischemia with duration longer than 60 minutes has been observed by other authors8,10,26. These results were not observed in this study in which the reperfusion time was around 19 hours, suggesting that the effects of reperfusion were beneficial in this situation. On the other hand, it is possible that the respiration worsening had not been detected here because the period of ischemia was not long enough to produce electrolytic and metabolic changes in the mitochondria and start the mechanism that cause an irreversible process. When compared with morphological analysis of the ischemic cortical area14,27, the alterations in the mitochondrial respiration is detected much earlier and it is, therefore, a favorable method when trying to establish a therapeutic window or the safety time for interruption in the cerebral blood flow during neurosurgical vascular procedures. In this study, the evaluation of the mitochondrial respiration was not modified until 15 minutes of ischemia and seemed recovered after one-hour ischemia followed by 19 hours of reperfusion, suggesting a safety interval to the maintenance of the mitochondrial function and survival of the neurons. Similar evaluations in other species can contribute to approach the maximal objective, that is, to assure an "ischemic window" during which irreversible damages to the human cortex do not occur.
CONCLUSION
Short duration focal ischemia of cerebral cortex of rats (15 minutes) followed by short (10 minutes) or extended reperfusion (19 hours) did not change the mitochondrial respiration. Longer ischemic periods (30 to 60 minutes) followed by 10 minutes of reperfusion reduced the O2 consumption. Nevertheless, this was not detected after 19 hours of reperfusion. Short (10 minutes) or extended reperfusion (19 hours) did not increase the deleterious effects of the ischemic phenomenon.
Received 6 December 2002
Received in final formal 1 April 2003
Accepted 23 April 2003
Dr. Sinésio Grace Duarte - Rua Saldanha Marinho 2615 - 14400-000 Franca SP - Brasil E-mail: duarte.frc@netsite.com.br
- 1. Fisher M, Schaebitz W. An overview of acute stroke therapy: past, present, and future. Arch Intern Med 2000;160:3196-3206.
- 2. Hallenbeck JM, Dutka AJ. Background review and current concepts of reperfusion injury. Arch Neurol 1990;47:1245-1254.
- 3. Aronowski J, Strong R, Grotta JC. Reperfusion injury demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab 1997;17:1048-1056.
- 4. Taylor CL, Selman WR, Kiefer SP, Ratcheson RA. Temporary vessel occlusion during intracanial aneurysm repair. Neurosurgery 1996;39:893-905.
- 5. Goldman MS, Anderson RE, Meyer FB. Effects of intermittent reperfusion during temporal focal ischemia. J Neurosurg 1992;77:911-916.
- 6. Carlotti CG Jr., Colli BO, Kazuo JY. Avaliação da isquemia cerebral pela respiração mitocondrial: modelo experimental. Arq Neuropsiquiatr 2001;59:365-371.
- 7. Dias LAA, Colli BO, Coutinho J Neto, Lachat JJ. Avaliação da isquemia cerebral focal induzida pela oclusão da artéria cerebral média e a ação neuroprotetora do cetoprofeno em ratos. Arq Neuropsiquiatr 2000;58:1047-1054.
- 8. Phillis JW, O'regan MH, Estevez AY, Song D, Vanderheide SJ. Cerebral energy metabolism during severe ischemia of varying duration and following reperfusion. J Neurochem 1996;67:1525-1531.
- 9. Kuroda S, Katsura K, Hillered L, Bates TE, Siesjo BK. Delayed treatment with alpha-phenyl-N-tert-butyl nitrone (PBN) attenuates secondary mitochondrial dysfunction after transient focal cerebral ischemia in the rat. Neurobiol Dis 1996;3:149-157.
- 10. Li AL, Kristián T, He QP, Siesjö BK. Cyclosporin A enhances survival, ameliorates brain damage, and prevents secondary mitochondrial dysfunction after a 30-minutes period of transient cerebral ischemia. Exp Neurol 2000;165:153-163.
- 11. Memezawa H, Smith MJ, Siesjö BK. Penumbral tissues salvaged by reperfusion following middle cerebral artery occlusion in rats. Stroke 1992;23:552-559.
- 12. Siesjö BK. Pathophysiology and treatment of focal cerebral ischemia: Part I: Pathophysiology. J Neurosurg 1992;77:169-184.
- 13. Chen H, Chopp M, Schultz L, Bodzin G, Garcia JH. Sequential neuronal and astrocytic changes after transient middle cerebral artery occlusion in the rat. J Neurol Sci 1993;118:109-116.
- 14. Bartus R, Dean RL, Cavanaugh K, Eveleth D, Carriero DL, Lynch G. Time-related neuronal changes following middle cerebral artery occlusion: implications for therapeutic intervention and the role of calpain. J Cereb Blood Flow Metab 1995;15:969-979.
- 15. Anderson MF, Sims NR. Mitochondrial respiratory function and cell death in focal cerebral ischemia. J Neurochem 1999;73:1189-1199.
- 16. Folbergrová J, Kiyota Y, Pahlmark K, Memezawa H, Smith ML, Siesjö BK. Does ischemia with reperfusion lead to oxidative damage to proteins in the brain? J Cereb Blood Flow Metab 1993;13:145-152.
- 17. Fiskum G. Mitochondrial damage during cerebral ischemia. Ann Emerg Med 1985;14:810-815.
- 18. Siesjö BK, Siesjö P. Mechanisms of secondary brain injury. Eur J Anaesthesiol 1996;13:247-268.
- 19. White BC, Sullivan JM, Degracia DJ, et al. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci 2000;179:1-33.
- 20. Koiosumi J, Yoshida Y, Nakasawa T, Ooneda G. Experimental studies of ischemic brain edema: I: A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke 1986;8:1-8.
- 21. Lai JCK, Chark JB. Preparation of synaptic and nonsynaptic mitochondria from mammalian brain. Methods Enzimol 1974;60:51-64.
- 22. Curti C, Uyemura SA. Estudos da fosforilação oxidativa. In: Silva, OC Jr., Zucoloto S, Beer A Jr. (EDS) Modelos experimentais de pesquisa em cirurgia . São Paulo: Robe 1998:149-158.
- 23. Tegos TJ, Kalodiki E, Sabetai MM, Nicolaides AN. Stroke: pathogenesis, investigations, and prognosis: Part II of III. Angiology 2000;51:885-894.
- 24. Demchuk AM, Bucham AM. Predictor of stroke outcome. Neural Clinics 2000;19:455-473.
- 25. Kurup CKR, Kumaro KK, Dutk AAJ. Influence of cerebral ischemia and post-ischemic reperfusion on mitochondrial oxidative phosphorylation. J Bioenerg Biomembr 1990;22:61-80.
- 26. Sims NR, Willians VK, Zaidan E, Powell JA. The antioxidant defences of brain mitochondria during short-term forebrain ischemia and recicurlation in the rat. Brain Res Mol Brain Res 1998;60:141-149.
- 27. Kurokawa Y, Tranmer BI. Interrupted arterial occlusion reduces ischemic damage in a focal cerebral ischemia model of rats. Neurosurgery 1995;37:750-757.
Publication Dates
-
Publication in this collection
12 Nov 2003 -
Date of issue
Sept 2003
History
-
Reviewed
01 Apr 2003 -
Received
06 Dec 2002 -
Accepted
23 Apr 2003