Abstracts
Excess of heavy metals in agricultural soils is a matter of concern since it may decrease economic yield as a result of toxicity and lower product quality as a result of metal accumulation in edible plant parts. Among plant species and among cultivars within species a natural variation in uptake, translocation and distribution of trace elements occur. The transference of Cd and Zn, from soil to two lettuce (Lactuca sativa L.) cultivars grown in greenhouse, was evaluated in separate experiments for Cd and Zn.Plant dry and fresh matter yield and plant Cd and Zn concentrations were determined. Cultivar CRV showed greater potential for yield than CMM in both experiments. Cadmium and Zn translocation from roots to shoots increased with the increase of soil Cd or Zn, for both cultivars. There was Cd translocation from young to old leaves in CMM but not in CRV whereas for Zn it occurred in both cultivars, being higher in CRV. In both cultivars, old leaves had higher Cd and Zn concentrations (and lower dry matter yield) than young leaves. The CRV and CMM cultivars accumulate Cd differently in the leaves and the higher accumulation occurs in the former. Cultivar CRV also accumulates more Zn compared to CMM.
cadmium; food quality; lettuce; plant nutrition; soil; zinc
Excesso de metais pesados em solos agrícolas é motivo de preocupação uma vez que pode diminuir a produção econômica devido à toxidez e reduzir a qualidade do produto devido ao acúmulo de metais nas partes comestíveis. Entre espécies, e mesmo entre cultivares, há natural variação na absorção, translocação e distribuição dos elementos traço. Foi avaliada a transferência de Cd e Zn do solo para duas cultivares de alface (Lactuca sativa L.), cultivadas em casa de vegetação em experimentos individuais para Cd e Zn. Produção de matéria fresca e seca e concentrações de Cd e Zn foram determinados. A cultivar CRV mostrou maior potencial de crescimento comparado à CMM, nos dois experimentos. A translocação de Cd e Zn das raízes para a parte aérea aumentou com o aumento das doses de Cd ou Zn adicionadas ao solo, para ambas cultivares. Houve translocação de Cd das folhas jovens para as velhas na CMM, mas não na CRV, enquanto para Zn a translocação ocorreu para as duas cultivares, sendo maior na CRV. Nas duas cultivares, as concentrações de Cd e Zn foram maiores (e as produções de matéria seca, menores) nas folhas velhas em comparação com as novas. As cultivares CRV e CMM acumulam Cd nas folhas de forma distinta, ocorrendo maior acúmulo na CRV. Também para Zn, a CRV acumula mais o nutriente nas folhas do que a CMM.
cádmio; qualidade de alimentos; alface; nutrição de plantas; solo; zinco
INTRODUCTION
Cadmium and Zn uptake by plants depends on the soil properties, plant species and cultivar, fertilizers, agronomic management and properties of the metal source (Chaney 2010Chaney R. 2010. Cadmium and Zinc. In: Hooda PS (Ed), Trace Elements in Soils. Blackwell Publishing Ltd, UK, p. 410-439.). Heavy metal absorption from soil and translocation to edible plant parts is a potential risk for the food chain and has to be evaluated based on soil metal availability and plant efficiency for metal uptake and translocation (Alleoni et al. 2005Alleoni LRF, Borba RP and Camargo OA. 2005. Metais pesados: da cosmogênese aos solos brasileiros. In: Torrado PV, Alleoni LRF, Silva MAL and Cardoso EJ (Eds), Tópicos em ciência do solo. Sociedade Brasileira de Ciência do Solo, Viçosa, MG, Brasil, p. 1-42.).
Soil Zn2+ dynamics depends on pH, soil organic matter and soil clay contents. Zinc can be adsorbed to particles of Fe, Mn and Al oxides, clays and organic fraction (Agbenin and Olojo 2004Agbenin JO and Olojo LA. 2004. Competitive adsorption of copper and zinc by a Bt horizon of a savanna Alfisol as affected by pH and selective removal of hydrous oxides and organic matter. Geoderma 119: 85-95.). Cadmium has chemical characteristics similar to Zn, is absorbed by plants as Cd2+, and is retained to soil exchange sites similarly to cationic micronutrients (Fe2+, Mn2+, Zn2+ and Cu2+). Whereas Zn forms layered double hydroxides (LDH) compounds (Zn-Al) that become more stable over time (Roberts et al. 2003Roberts DR, Ford RG and Sparks DL. 2003. Kinetics and mechanisms of Zn complexation on metal oxides using EXAFS spectroscopy. J Coll Interfac Sci 263: 364-376.), LHD formation for Cd has not been found yet, and most Cd remains in the labile pool over time (Kukier et al. 2010Kukier U, Channey RL, Ryan JL, Daniels WL, Dowdy RH and Granato TC. 2010. Phytoavailability of Cd in longterm biosolid amended soils. J Environm Qual 39: 519-530.).
For plants in general, Cd concentrations in the mature leaf are considered normal (0.05-0.2 mg kg–1), excessive or toxic (5-30 mg kg–1) and tolerable (0.05-0.5 mg kg–1) (Kabata-Pendias 2010Kabata-Pendias A. 2010. Trace Elements in Soils and Plants, 4th ed. CRC Press, Boca Raton, Florida, 548 p.). In Brazil, the food legislation (ABIA 1991) allows a maximum of 1.0 mg kg–1 Cd in fresh food matter (Mesquita Filho et al. 2001). The proposed limit for Cd in lettuce, for consideration if it should be used as food, is 0.05 mg kg–1 in fresh weight or 4 mg kg–1 in dry weight (Simmons et al. 2005Simmons RW, Pongsakul P, Saiyasitpanich D and Klinphoklap S. 2005. Elevated levels of cadmium and zinc in paddy soils and elevated levels of cadmium in rice grain downstream of a zinc mineralized area in Thailand: implications for public health. Environ Geochem Health 27: 501-511.). For rice, the Codex Alimentarius Commission (2006)Codex Alimentarius Commission. 2006. Report of the 29th session of the Codex Alimentarius Commission. FAO/WHO. Geneve. Switzerland.established 0.4 mg kg–1 Cd as the background for the grain to be used as food. In the Netherlands, the background values for Cd in the fresh matter are 0.01 to 0.19 mg kg–1 for lettuce, 0.01 to 0.09 mg kg–1 for potatoes, and 0.01 to 0.26 mg kg–1for wheat (Staarink and Hakkenbrak 1991Staarink T and Hakkenbrak P. 1991. Book of Contaminants. Rijswijk, The Netherlands: Commodity Inspection Department.). These background values express limits for monitoring crop quality as related to undesirable Cd levels in agricultural products. For the fodder crops silage maize and ryegrass the ranges are 0.14 to 6.8 and 0.03 to 0.84 mg kg–1, respectively (Wiersma et al. 1981Wiersma D, Van Goor BJ and Van Der Veen NG. 1981. Cadmium, lead, mercury and arsenic concentrations in crops and corresponding soils in The Netherlands. J Agr Food Chem 34: 1067-1074.).
Cadmium provisional tolerable daily intake is 1 µg per kg of body weight (WHO 2006WHO. 2006. Evaluation of Certain Food Additives and Contaminants (Sixty-fourth Report of the Joint FAO/WHO Expert Committee on Food Additives). WHO-TRS 930-JECFA 64/26. World Health Organization, Geneva.). Expressed for a 70 kg adult this value corresponds to a tolerable daily intake of 70 µg Cd. Plant food is the highest source of dietary Cd (up to 75%) and cereals, particularly rice and potatoes may contribute above 50% of total intake (Louekari et al. 2000Louekari K, Mäkelä-Kurtto R, Virtanen JPV, Sippola J and Malm J. 2000. Cadmium in fertilizers. Risk to human health and the environment. Ministry Agric Forestry. Finland. Helsinki, 119 p.). Chen and Gao (1993)Chen J and Gao J. 1993. The Chinese total diet study in 1990. Part I. Chemical contaminants. JAOAC Int 76: 1193-1205. reported that cereals are responsible for 54.3 % of human daily intake of Cd, followed by vegetables (23.9 %).
Even being a component of enzymatic proteins required in plant physiological processes, Zn can be toxic if present at high concentrations (Clemens, 2001Clemens S. 2001. Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212: 475-486.). In contaminated soils, Zn excess has been a major environmental concern (Zarcinas et al. 2004Zarcinas BA, Pongsakul P, McLaughlin MJ and Cozens G. 2004. Heavy metals in soils and crops in Southeast Asia 2. Thailand Environ Geochem Health 26: 359-371.). Its build up on surface soil has been enhanced by Zn fertilizers, sewage sludge and other pollution sources (Kabata-Pendias 2010Kabata-Pendias A. 2010. Trace Elements in Soils and Plants, 4th ed. CRC Press, Boca Raton, Florida, 548 p.). There are agricultural soils with deficient and phytotoxic Zn levels, however, the major concern is with regards to deficiency (Chaney 2010Chaney R. 2010. Cadmium and Zinc. In: Hooda PS (Ed), Trace Elements in Soils. Blackwell Publishing Ltd, UK, p. 410-439.). Zinc deficiency is a fact in crops and humans (Hotz and Brown 2004Hotz C and Brown KH. 2004. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 25: 94-204., Welch and Graham 2004Welch RM and Graham RD. 2004. Breeding for micronutrients in staple food crops from a human nutrition perspective. J Exp Bot 55: 353-364.) and is currently listed as a major risk factor for human health and a cause of death globally (Cakmak 2008Cakmak I. 2008. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 302: 1-17.). For most crops, 10 to 100 µg g–1 Zn in leaf dry matter is considered adequate for growth (Pais and Jones Jr 2000). Sugarcane plants under excess Zn showed poor root growth due to changes in nutrient contents in different plant parts (Broadley et al. 2007Broadley MR, White PJ, Hammond JP, Zelko I and Lux A. 2007. Zinc in plants. New Phyt 173: 677-702.).
The Brazilian legislation for foods (ABIA 1991) states 50 mg kg–1 as the maximum Zn concentration in the fresh food matter (Mesquita Filho et al. 2001). In the Netherlands, the background values for Zn in the fresh matter are 1.2 to 4.3 mg kg–1 for lettuce, 1.9 to 11 mg kg–1 for potatoes, 1.9 to 10 mg kg–1for beans and 19 to 41 mg kg–1 for wheat (Staarink and Hakkenbrak 1991Staarink T and Hakkenbrak P. 1991. Book of Contaminants. Rijswijk, The Netherlands: Commodity Inspection Department.). The Zn DRI (Dietary Reference Intake) is 11 mg day–1 for males and 8 mg day–1 for females (WHO 1996WHO. 1996. Trace elements in human nutrition and health. WHO/FAO/IAEA. Geneva, 362 p.). Contrary to Cd, the ingestion of food with higher Zn contents is desirable, mainly in areas where Zn under-nutrition problems are found.
This work aimed to investigate the growth of two lettuce cultivars in soil amended with Cd or Zn, determining the concentrations of these metals in the shoot dry and fresh matter and the distribution of Cd and Zn in the shoots. The plant's metal concentrations were compared to critical levels for toxicity.
MATERIALS AND METHODS
Soil
Samples of a loam Red-Yellow Latossol (Oxisols) from the city of João Pinheiro, state of Minas Gerais, collected at the 0-20 cm depth and passed through 2 mm sieve for chemical and physical analysis (Table I) was used for plant growth. The soil analysis determined the following: pH in water(1:2.5 soil:water); organic carbon by Walkley-Black Method (Gaudette et al. 1974Gaudette HE, Flight WR, Toner L and Folger DW. 1974. An inexpensive titration method for the determination of organic carbon in recent sediments. J Sediment Petrol 44: 249-253.); P and K extracted by Mehlich-1 (dosage of P by molecular absorption spectrophotometry and K by atomic emission spectrophotometry); Al3+, Ca2+ and Mg2+extracted by KCl 1 mol L–1 (dosage by atomic absorption spectrophotometry); (H + Al) extracted by Ca(OAc)2 0,5 mol.L–1, pH 7,0 and dosage by acid-base titration; Cd2+, Fe2+, Zn2+, Cu2+, Mn2+ extracted by Mehlich-1 and dosage by atomic absorption spectrophotometry (Defelipo and Ribeiro 1981Defelipo BV and Ribeiro AC. 1981. Análise Química do Solo (Metodologia), (Boletim de Extensão, 29), Imprensa Universitária, Universidade Federal de Viçosa, Brasil, 17 p.); Soil texture by the Pipette Method (EMBRAPA 1997); Field capacity by Richards Method (EMBRAPA1997). Soil extracts concentrations of Al3+, Ca2+, Mg2+Cd2+, Fe2+, Zn2+, Cu2+ and Mn2+ were determined in a Varian (Model SpectrAA 200) atomic absorption spectrophotometer (detection limit 0.005 mg L–1 for Cd and Zn), with background correction, by direct aspiration of solution into air-acetylene flame (Jordão et al. 2006Jordão CP, Fialho LL, Cecon PR, Neves JCL, Mendonça E and Fontes RLF. 2006. Effects of Cu, Ni and Zn on lettuce grown in metal-enriched vermicompost amended soil. Water Air Soil Poll 172: 21-38.). Only analytical grade reagents were used and all glassware and materials were thoroughly cleaned.
In 3.0 dm3 polyethylene pots, 2.5 dm3 of soil was limed with analytical grade CaCO3 and MgCO3(ratio 4:1 mol) to reach pH 5.8 (CFSEMG 1999) and the material left incubated for 30 days with moisture at field capacity.
Doses of Cd (0.0; 0.4; 1.6; 3.2; 9.6 mg dm–3) (CdCl2.H2O) and Zn (ZnSO4.7H2O) at the recommended dose for horticultural crops in the state of Minas Gerais (Fontes 1999Fontes PCR. 1999. Sugestões para adubação para hortaliças em Minas Gerais. Alface. In: Ribeiro AC, Guimarães PTG and Alvarez VVH (Eds), Recomendações para o uso de corretivos e fertilizantes para Minas Gerais. 5a Aproximação. CFSEMG. Viçosa, Minas Gerais, Brasil, p. 171-177.) were applied to limed soil in pots. The material was incubated for 25 days, with moisture at field capacity.
Zinc doses (0.0; 2.0; 6.0; 18.0; 36.0 mg dm–3) (ZnSO4.7H2O) were applied to limed soil in pots and the material incubated for 25 days, with moisture at field capacity. The dose 2.0 mg dm–3 Zn was the control for adequate supply based on the Zn recommendation for horticultural crops in the state of Minas Gerais (Fontes 1999Fontes PCR. 1999. Sugestões para adubação para hortaliças em Minas Gerais. Alface. In: Ribeiro AC, Guimarães PTG and Alvarez VVH (Eds), Recomendações para o uso de corretivos e fertilizantes para Minas Gerais. 5a Aproximação. CFSEMG. Viçosa, Minas Gerais, Brasil, p. 171-177.).
In both experiments, after incubation, one day before seedling transplanting, the soil in the pot was fertilized with 60 mg/dm3N (NH4NO3); 88 mg/dm3 P (NH4H2PO4); 50 mg/dm3 K (K2SO4); 2,5 mg/dm3 S (FeSO4); 1.55 mg/kg Fe (FeCl3.6H2O); 3.5 mg/kg Mn (MnCl2.4H2O); 8.0 mg/dm3 Cu (CuSO4.5H2O); 4.0 mg/dm3 Ni (NiSO4); 1.0 mg/dm3 B (H3BO3); 0.14 mg/dm3 Mo [(NH4)6Mo7O24].
Plant
CMM and CRV seeds were sown in sand with moisture for seed germination provided by addition of distilled water. Twenty one days after sowing, three lettuce seedlings were transplanted into soil in polyethylene pots. After grown by ten days with moisture kept at field capacity, two seedlings were removed and one plant per pot was grown for thirty four days. Plants were cut near the soil surface and the roots separated from soil by washing them with deionized water. The eight basal leaves located at the lowest position in the plant (old leaves) were separated from the remaining leaves located closer to the plant top (young leaves). Shoot and fresh root weights were recorded, the material dried at 70 °C to constant weight, and dry weight recorded. Dry samples were grounded in a Willey mill, passed through a 16 mesh sieve, and analyzed for determination of Zn and Cd in the vegetal tissues.
A portion of 0.5 g dry matter of each sample was digested with nitric-perchloric solution (Zazoski and Burau 1977Zazoski RJ and Burau RG. 1977. A rapid nitric-perchloric acid digestion method for mult-element tissue analysis. Commun Soil Sci Plant 8: 425-436.). Plant extracts concentrations of Cd and Zn were determined in a Varian (Model SpectrAA 200) atomic absorption spectrophotometer (detection limit 0.005 mg L–1 for Cd and Zn), with background correction, by direct aspiration of solution into air-acetylene flame (Jordão et al. 2006Jordão CP, Fialho LL, Cecon PR, Neves JCL, Mendonça E and Fontes RLF. 2006. Effects of Cu, Ni and Zn on lettuce grown in metal-enriched vermicompost amended soil. Water Air Soil Poll 172: 21-38.). Between groups of samples, blanks were run to certify that there was no drift in the instrument. Only analytical grade reagents were used and all glassware and materials were thoroughly cleaned.
Cadmium and Zn contents were calculated for plant dry matter and concentrations of Cd and Zn were calculated for fresh shoot matter.
STATISTICAL ANALYSIS
The experimental design was a 2 x 5 factorial (2 lettuce cultivars and 5 treatments) arranged in a randomized block design with 4 replications. Blocks were set in individual greenhouse benches and pots rotated weekly among the benches. Experimental unit was one plant per 3.0 dm3 polyethylene pot.
Analysis of variance was performed to evaluate the effects of doses of Cd and Zn applied to soil. Linear, quadratic, cubic and exponential models were tested for adjustment of regression equations for plant dry matter weight, Cd and Zn plant concentrations, and Cd and Zn plant contents, as a function of treatments. Coefficients were tested based on the residue mean square of the analysis of variance, up to the 10% probability level. The software SAEG (Statistical Analysis System) (Ribeiro Jr 2001) was used.
RESULTS AND DISCUSSION
Soil Cd and Zn
Zinc in soil (0.26 mg dm–3) was very low Based on availability for plant absorption (Alvarezet al. 1999) and Cd was below the instrument detection limit (Table I). These low Cd and Zn availabilities found in the Red-Yellow Latosol are related to trace element characteristics, soil characteristics and the proportion of available chemical element which is very small in relation to total element concentration in soil. The soil nutrient concentration available for plant absorption is that concentration which correlates with plant growth (Cantarutti et al. 2007Cantarutti RB, Barros NF, Martinez HEP and Novais RF. 2007. Avaliação da fertilidade do solo e recomendação de fertilizantes. In: Novais RF, Alvarez VVH, Barros NF, Fontes RLF, Cantarutti RB and Neves JCL (Eds), Fertilidade do Solo. Viçosa, MG. Sociedade Brasileira de Ciencia do Solo. p. 645-736.). Most soil chemical elements are trapped in the soil structure, or retained in soil complex exchange in forms not available for root absorption. These forms, determined as soil total concentrations, are not useful in soil fertility evaluation. Plant nutrition and soil fertility studies require the quantification of soil metal fraction available for root absorption. Total forms should be analyzed to evaluate contamination of soil and water table due to application of inputs to improve agricultural production. Extraction of Zn2+ with Mehlich-1 (HCl 0,05 mol L–1+ H2SO4 0,0125 mol L–1) and DTPA (DTPA 0,005 mol L–1 + TEA 0,1 mol L–1 + CaCl2 0,01 mol L–1, pH 7,3) has been successfully used for soil fertility evaluation (Alvarez et al. 1999Alvarez VH, Novais FR, Barros NF, Cantarutti RB and Lopes AS. 1999. Interpretação dos resultados das análises se solos. In: Ribeiro AC, Guimarães PTG and Alvarez VVH (Eds), Recomendações para o uso de corretivos e fertilizantes para Minas Gerais. 5a Aproximação. CFSEMG. Viçosa, MG, Brasil, p. 25-32., Raij et al. 1996), however there is no method for soil Cd extraction based on fertility concepts. Cadmium extraction with Mehlich-1 and DTPA are options for determination of Cd availability to plants, based on its similarity with cationic micronutrients Fe, Mn, Zn, Cu and Ni.
Cadmium in Plants
Dry matter weight of CMM leaves decreased 47 % with the soil addition of 9.6 mg dm–3 Cd as compared to treatment without Cd; in CRV the decrease was 51 %. For roots, the correspondent reduction was 51% (CMM) and 37% (CRV) (Figure 1). Similar results, but with more severe effects (85% reduction in shoots and 80% in roots dry matter weight) was shown by Salviano et al. (2005)Salviano AM, Fontes RLF, Pereira JMN and Assis CP. 2005. Doses de cádmio no crescimento e na quantidade de clorofila em duas variedades de fumo cultivadas em solução nutritiva. Rev Caatinga 18(1): 6-12. in tobacco grown in 0.4 mg L–1 Cd nutrient solution. In corn, the dry matter and the height of the plants decreased significantly with 1.6 mg L–1 Cd in the nutrient solution (Nascimento and Fontes 2002Nascimento CWA and Fontes RLF. 2002. Absorção e distribuição de cádmio em duas cultivares de milho (Zea Mays L.) com diferentes graus de sensibilidade ao elemento. Rev Ceres 49(282): 169-179.).
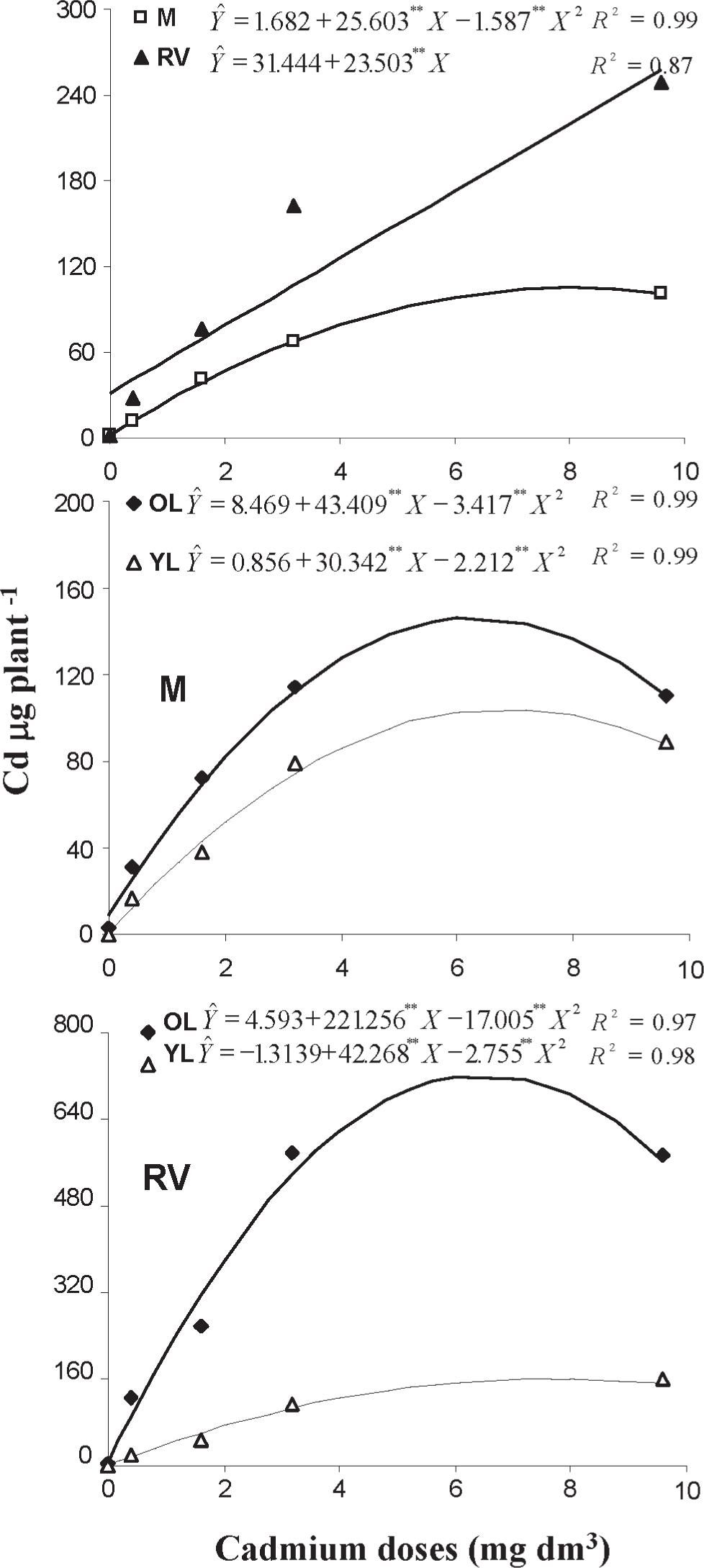
Dry matter of: (A) young leaves-YL and old leaves-OL of Mimosa-MM; (B) young leaves-YL and old leaves-OL of Regina Verao-RV; (C) roots; and (D) whole plant, in lettuce cultivars Mimosa and Regina Verao, as a result of Cd doses applied to soil (plants were grown for 44 days in the Cd amended soil).
The highest dry matter yield (8.52 g) was shown by young CRV leaves (zero Cd treatment), whereas the lowest (0.63 g) was shown by old leaves of CRV grown in soil amended with 9.6 mg dm3Cd (Figure 1). In CMM cultivar, the increase of Cd applied to soil decreased the dry matter in young leaves (exponentially) and old leaves (quadractically) whereas in CRV the decrease was exponential for young and old leaves (Figure 1).
For all Cd doses, in both cultivars, leaf dry matter weight was higher in young leaves compared to old leaves, reflecting the higher Cd concentrations observed in old leaves (Figure 2).
Cadmium concentrations in roots of Mimosa-M and Regina Verao-RV cultivars and in young leaves-YL and old leaves-OL of Mimosa-M and Regina Verao-RV, as a result of Cd doses applied to soil (plants were grown for 44 days in the Cd amended soil).
Simultaneously, in treatments with Cd addition (0.4, 1.6, 3.2 and 9.6 mg dm–3) young leaves showed higher Zn concentrations than old leaves (Table II). The higher Zn concentrations in young leaves attenuated the toxic effects of Cd in both cultivars as compared to old leaves. Some references for Zn-Cd interactions in plant uptake-transport processes mention antagonism and synergism between them (Kabata-Pendias 2010Kabata-Pendias A. 2010. Trace Elements in Soils and Plants, 4th ed. CRC Press, Boca Raton, Florida, 548 p.) and others point out antagonistic Cd-Zn interaction in some crops (Chaney 2010Chaney R. 2010. Cadmium and Zinc. In: Hooda PS (Ed), Trace Elements in Soils. Blackwell Publishing Ltd, UK, p. 410-439.). Here, Zn antagonized Cd helping the plant to cope with its toxicity. Additionally, the antagonistic effect of Zn over Cd depends on the Cd:Zn ratio of Cd input to soil, the lower the ratio the less likely the bioavailable Cd will accumulate in crops (Chaney 2010Chaney R. 2010. Cadmium and Zinc. In: Hooda PS (Ed), Trace Elements in Soils. Blackwell Publishing Ltd, UK, p. 410-439.).
The average CRV dry matter weight (calculated for treatments with Cd) was 1.6 times higher than for CMM cultivar (Figure 1), and Cd concentrations in CRV leaves for most Cd doses were about 2 to 3 times greater than in CMM leaves (Figure 2). Concomitantly, shoot Zn concentrations were higher in CRV than in CMM (Table II). This might have contributed towards CRV leaves coping with Cd detrimental effects in plant photosynthetic system. Additionally, Cd contents in leaves were higher in CRV cultivar (Figure 3). Compared to CMM, CRV cultivar had higher potential for growing when exposed to Cd. Considering the average of Cd concentrations in roots, the cultivars were similar (68.5 µg g–1 in CMM and 62.5 µg g–1 in CRV) (Figure 2), however, the contents in roots were higher in CRV (103 µg plant–1) than in CMM (44 µg plant–1) (Figure 3). This confirms the higher potential of CRV to grow when exposed to Cd. That is an advantage in terms of agricultural yield but a disadvantage considering product quality. In soils with Cd availability (Mehlich-1 extraction) above reasonable limits, higher Cd accumulation in CRV shoots will lower quality of produced lettuce. The concern with Cd is that in contaminated soil Cd2+ stays in the soil labile pool promptly available for plant uptake (Kukier et al. 2010Kukier U, Channey RL, Ryan JL, Daniels WL, Dowdy RH and Granato TC. 2010. Phytoavailability of Cd in longterm biosolid amended soils. J Environm Qual 39: 519-530.), posing a higher risk for Cd entry in the food-chain.
Cadmium contents in in roots of Mimosa-M and Regina Verao-RV cultivars and in young leaves-YL and old leaves-OL of Mimosa-M and Regina Verao-RV, as a result of Cd doses applied to soil (plants were grown for 44 days in the Cd amended soil).
It is known that the retention of Cd in a hyper-accumulator grass occurs mainly in the xylem roots and in the epidermis leaves (Solis-Dominguez et al. 2007Solis-Dominguez FA, Gonzalez-Chavez MC, Carrillo-Gonzalez R and Rodriguez-Vazquez R. 2007. Accumulation a nd localization of cadmium in Echinochloa polystachya grown within a hydroponic system. J Hazard Mater 141: 630-636.), and in the roots it occurs by adsorption, vacuolar sequestration, insoluble salt precipitation and phytochelatin complexation (DalCorso et al. 2008Dalcorso G, Farinat S, Maistri S and Furini A. 2008. How Plants Cope with Cadmium: Staking All on Metabolism and Gene Expression. J Integr Plant Biol 50(10): 1268-1280.). The retention also occurs by Cd bound to organic acids (COOH/OH) groups and/or cell wall components (Huguet et al. 2012Huguet S, Bert V, Laboudigue A, Barthes V, Isaure MP, Llorens I, Schat H and Sarret G. 2012. Cd speciation and localization in the hyperaccumulator Arabidopsis halleri. Environ Exp Bot 82: 54-65.) as well as to leaves and root cell walls of epidermal and bundle sheath vacuoles, playing a major role in the plant metal tolerance (Hu et al. 2009Hu PJ, Qiu RL, Senthilkumara P, Jiang D, Chen ZW, Tang YT and Liu FJ. 2009. Tolerance, accumulation and distribution of zinc and cadmium in hyperaccumulator Potentilla griffithii. Environ Exp Bot 66: 317-325.). More recently, Qiu et al. (2012)Qiu RL, Tang YT, Zeng XW, Thangavel PT, Tang L, Gan YY, Ying RR and Wang SZ. 2012. Mechanisms of Cd Hyperaccumulation and Detoxification in Heavy Metal Hyperaccumulators: How Plants Cope with Cd. In: Progress in Botany Vol. 73. Lüttge U, Beyschlag W, Büdel B and Francis D (Eds), Springer-Verlag, Berlin Heidelberg, p. 127-159., discussing experimental results, pointed out a clear indication that internal detoxification of Cd is achieved in the vacuoles. In the present work, even with CRV plants accumulating more Cd in their tissues (Figure 3) this cultivar produced more dry matter than CMM (Figure 1). It seems that a more efficient vacuolar structure for Cd sequestration/storage was active in CRV plants, which was responsible for its enhanced growth, in spite of higher accumulation of Cd in roots and shoots compared to CMM cultivar. Hence, the action of those mechanisms protecting the CRV photosynthetic apparatus against Cd assured the differential response of this cultivar to cope with the metal detrimental effects. The effective action of Cd inhibiting photosynthesis has been shown in corn (Ferretti et al. 1993Ferretti M, Ghisi R, Merlo L, Vecchia FD and Passera C. 1993. Effect of cadmium on photosynthesis and enzymes of photosynthetic sulphate and nitrate assimilation pathways in maize (Zea mays L.). Photosynthetica 29: 49-54.), pea, and sugar beets (Greger et al. 1994Greger M, Johansson M, Stihl A and Hamza K. 1994. Foliar uptake of Cd by pea (Pisum sativum) and sugar beet (Beta vulgaris). Physiol Plant 88: 563-570.).
The CMM Cd concentration varied in order: roots > old leaves > young leaves (Figure 2). In shoots of both cultivars, the percentage of Cd tended to decrease as a result of the elevation of the Cd dose applied to soil, with simultaneous metal increase in roots (Table III). Compared to the lower Cd doses, the highest (9.6 mg dm–3) resulted in an increase of Cd percentage in CRV roots (from 20 %, or less, to 25 %) and a decrease in shoots (from 80 %, or more, to 75 %).
Cadmium distribution between shoots and roots and between young and old leaves in lettuce cultivars CMM and CRV, 44 days after seedlings transplanting to soil treated with Cd.
Similarly, the correspondent variations in CMM were the increase from 29 (or less) to 34 % (roots) and decrease from 71 (or more) to 66 % (shoots) (Table III). This indicates that when the plants were exposed to high soil Cd its retention in roots increased notwithstanding the fact that Cd is a mobile element, easily absorbed by roots and transported to shoots. Although Cd mobility facilitates the uniform Cd distribution between roots and shoots, the plants tend to inhibit the transport of toxic elements from the roots to other parts, as a protection mechanism for the plant.
In CRV plants, 20 % Cd was in roots and 80% in shoots (average for all treatments) whereas in CMM the correspondent values were 27 (roots) and 73% (shoots), indicating a higher root to shoot Cd translocation in CRV cultivar. It should be noted that even with higher percentage of Cd in shoots (Table III), CRV cultivar produced more shoot dry matter than CMM (Figure 1), confirming the better growth of CRV under exposure to available Cd in the soil.
In the CMM cultivar, Cd percentage in young leaves decreased and in old leaves increased when higher doses of Cd were added to soil (Table III) suggesting that metal translocation from young to old leaves had been improved. Differently, in CRV, even with the increase of the CdCl2.H2O added to soil the Cd percentages in old and young leaves stayed relatively uniform (Table III), indicating no effect of Cd treatments in Cd translocation within shoots.
The Brazilian legislation for foods (ABIA 1991) establishes 1.0 mg kg–1 Cd as the maximum allowed concentration in fresh food matter (Mesquita Filho et al. 2001). In treatments with 0.0 and 0.4 mg dm–3 Cd, CMM fresh shoot matter showed Cdconcentrations below that maximum limit whereas in treatments with 1.6, 3.2 and 9.6 mg dm–3 Cd the limit was surpassed by, three and six times, respectively. In CRV cultivar, with no soil added Cd, shoot Cd concentrations were below the maximum limit whereas with 0.4, 1.6, 3.2 and 9.6 mg dm–3 soil added Cd, the metal in shoots reached twice, three, four, eleven and seventeen times that limit, respectively (Table IV).
Fresh matter weight of shoots (SFM), Cd concentrations in fresh shootmatter* (CdSFM) and amount of lettuce (Lettuce) to be consumed daily to reach the Cd provisional tolerable daily intake limit** for cultivars CMM and CRV, 44 days after seedlings transplanting to soil treated with Cd doses.
Based on the Cd provisional tolerable daily intake (57-71 µg) set for an adult with 70 kg weight (WHO 2006WHO. 2006. Evaluation of Certain Food Additives and Contaminants (Sixty-fourth Report of the Joint FAO/WHO Expert Committee on Food Additives). WHO-TRS 930-JECFA 64/26. World Health Organization, Geneva.), the limit range would be reached with a daily consumption of 70.3 g of CMM lettuce (produced in soil treated with 0.4 mg dm–3 Cd) (Table IV). For 1.6, 3.2 and 9.6 mg dm–3 Cd treatments, that limit would be reached with 28.6, 16.0 and 9.3 g of CMM lettuce, respectively (Table IV). For CRV, a daily consumption of 29.3 g of lettuce produced in soil amended with 0.4 mg dm–3 Cd would be enough to reach the limit (Table IV). In treatments with 1.6, 3.2 and 9.6 mg dm–3 Cd, the limit would be reached with 15.2, 5.3, and 3.4 g of CRV lettuce, respectively (Table IV). Although having more potential for growth, CRV cultivar represents a greater risk than CMM as related to the Cd transference from soil to vegetables in Cd contaminated soils. Cadmium is potentially harmful for plants posing a risk as environmental contaminant. It is one of the most toxic metals and exhibits adverse effects in humans, animals and plants, with great potential to adversely affect the environment and the quality of food (Kabata-Pendias 2010Kabata-Pendias A. 2010. Trace Elements in Soils and Plants, 4th ed. CRC Press, Boca Raton, Florida, 548 p.). Animal manure and fertilizers contribute most to Cd input in arable land in the Netherlands (Dach and Starmans 2005Dach J and Starmans D. 2005. Heavy metals balance inPolish and Dutch agronomy: actualstate and previsions for future. Agric Ecosystems Environ 107: 309-316.) and soil contamination with Cd is believed to be a serious health risk (Kabata-Pendias 2010Kabata-Pendias A. 2010. Trace Elements in Soils and Plants, 4th ed. CRC Press, Boca Raton, Florida, 548 p.). Measures to minimize its entry in the soil-plant system via agricultural inputs would contribute to prevent a long term basis Cd contamination.
Zinc in Plants
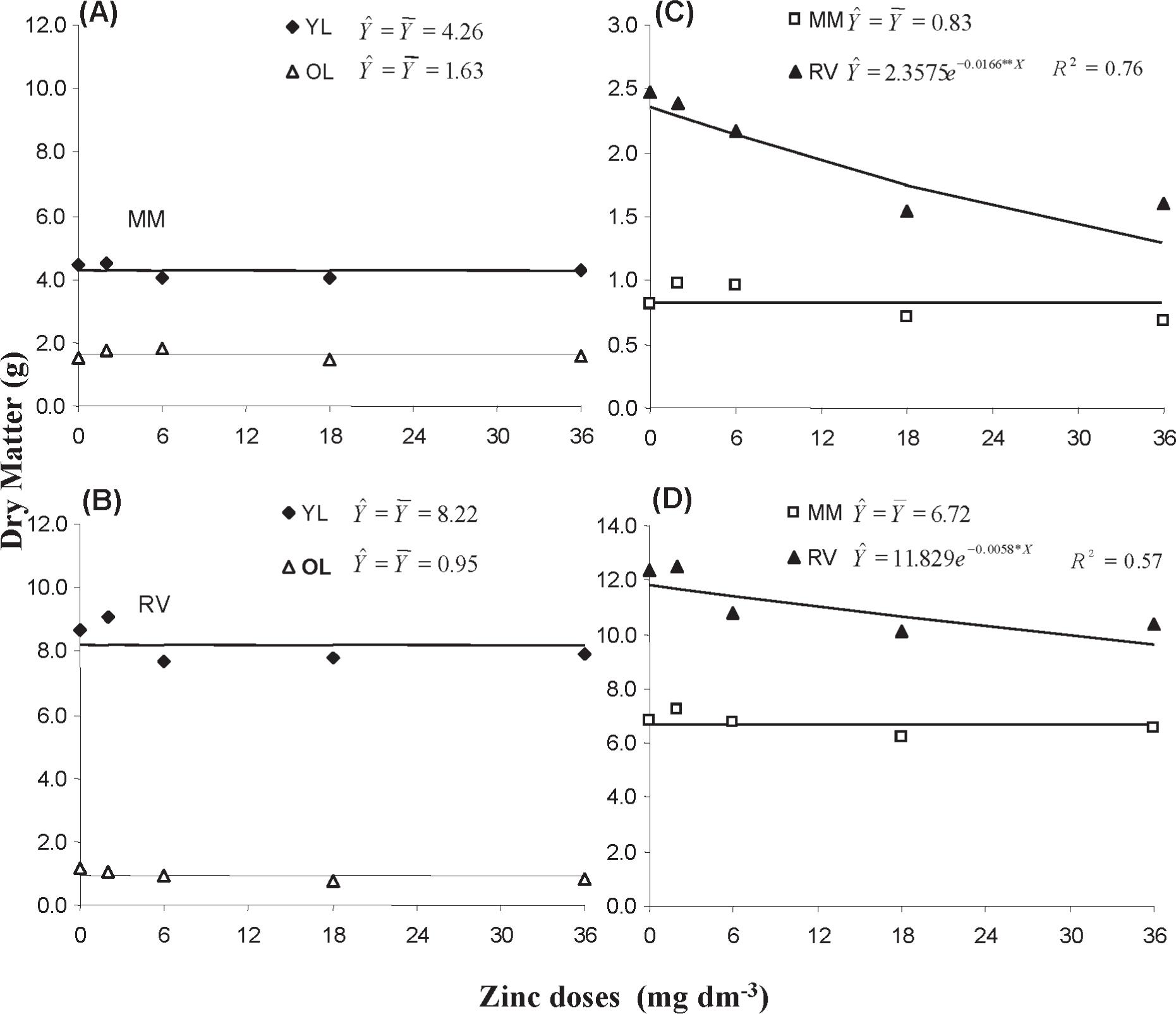
For both cultivars, there was no effect of soil applied Zn in shoot dry matter yield. The same was observed for root and total dry matter yield of CMM. On the other hand, the CRV root and total dry matter yield decreased exponentially as a function of soil added Zn (Figure 4). Zinc toxic effects were shown in sugarcane plants under excess Zn, expressed by poor root growth due to changes in nutrient contents in different plant parts (Broadley et al. 2007Broadley MR, White PJ, Hammond JP, Zelko I and Lux A. 2007. Zinc in plants. New Phyt 173: 677-702.). Comparing the two cultivars, CRV produced more dry matter than CMM (except in the old leaves). The average total dry matter weight (for Zn treatments) in CRV plants was 11.2 g whereas in CMM plants it was 6.7 g (Figure 4). This suggests CRV has higher potential for growth compared to CMM in a Zn amended soil.
Dry matter of: (A) young leaves-YL and old leaves-OL of Mimosa-MM; (B) young leaves-YL and old leaves-OL of Regina Verao-RV; (C)roots; and (D) whole plant, in lettuce cultivars Mimosa and Regina Verao, as a result of Zn doses applied to soil (plants were grown for 44 days in the Zn amended soil).
Plant species, in general, tolerate high concentrations of Zn in their tissues (Boardman and McGuire 1990Boardman R and McGuire D. 1990. The role of zinc in forestry. I. Zinc in forest environments, ecosystems and tree nutrition. Journal: Forest Ecol Manag 37 (1-3): 167-205.). Besides Zn vacuolar compartmentalization, cited as a potential mechanism for Zn detoxification (Hall 2002Hall JL. 2002. Cellular mechanism of heavy metal detoxification and tolerance. J Exp Bot 53(366): 1-11.), its retention by linking to the molecule of phytic acid in non vacuolated tissues may also play a role in Zn detoxification (Marschner 1995Marschner H. 1995. Mineral Nutrition of Higher Plants, Academic Press, New York, 889 p.). Addition of 36 mg dm–3 Zn (highest dose) to the soil resulted in Zn concentrations (old leaves and roots) above 1000 µg g–1, for both cultivars (Figure 5).
Zinc concentrations in roots of Mimosa-M and Regina Verao-RV cultivars and in young leaves-YL and old leaves-OL of Mimosa-M and Regina Verao-RV, as a result of Zn doses applied to soil (plants were grown for 44 days in the Zn amended soil).
Even though those concentrations were about ten times the adequate range for growth of most crops (10-100 µg g–1) (Pais and Jones Jr 2000), there was no remarkable dry matter weight decrease in comparison to treatments with lower Zn doses (Figure 4). These observations suggest that lettuce is among the plant species that tolerates high concentrations of Zn. Mechanisms that improve Zn sequestration for vacuolar compartmentalization and Zn retention by bounding to the phytic acid molecule may be active in the lettuce tissues.
In the present work, Zn concentrations in young leaves, at the highest Zn dose, were below 500 µg g–1 and about three times lower than in old leaves (Figure 5). Young leaves grew more than old leaves (Figure 4) and lettuce plants did not show any symptoms of Zn toxicity. As related to plant nutritional status, Zn in young leaves seem to better express the plant Zn nutritional status than Zn in old leaves.
The average of Zn concentrations in young and old leaves of CRV were about 1.8 times greater as compared to CMM cultivar and the correspondent values of Zn contents in shoots were about two times greater (Figures 5 and 6). Although there were no considerable differences in Zn root concentrations between cultivars, Zn contents in CRV roots were about 2.5 times higher than in CMM (Figure 6). Nevertheless, the dry matter produced by CRV was about 1.7 times greater than in CMM (Figure 4), suggesting that the CRV cultivar has higher potential for yielding than CMM.
Zinc contents in roots of Mimosa-M and Regina Verao-RV cultivars and in young leaves-YL and old leaves-OL of Mimosa-M and Regina Verao-RV, as a result of Zn doses applied to soil (plants were grown for 44 days in the Zn amended soil).
As Zn applied to soil became higher, the percentages of Zn diminished in roots and increased in shoots (Table V), showing that with more Zn its translocation from roots to shoots increased, in both cultivars. The average percentage of Zn in CRV was 30 % in roots and 70 % in shoots whereas for CMM the correspondent values were 25 % and 75 %, showing that translocation was higher in CRV.
Zinc distribution between shoots and roots and between young and old leaves in lettuce cultivars CMM and CRV, 44 days after seedlings transplanting to soil treated with Zn.
In shoots, comparing the zero dose to 36 mg dm–3Zn, there was an increase of Zn percentage in old leaves (three times in both cultivar) and a decrease in young leaves (1.2 times in CRV and twice in CMM) (Table V), showing that Zn translocation from young to old leaves occurred in both cultivars.
Comparing Zn distribution between old and young leaves, with 36 mg dm–3 of soil applied Zn, CMM and CRV old leaves had 57 % and 22 % (43 % and 78 % in young leaves), respectively. Taking the average from all treatments, the correspondent values were 37 % and 18 % for old leaves (63 % and 82 % for young) (Table V). The difference shows a higher translocation of Zn from old to young leaves in cultivar CRV.
The maximum Zn concentration allowed in fresh food matter by the Brazilian legislation is 50 mg kg–1 (ABIA 1991, Mesquita Filho et al. 2001). In all Zn treatments, fresh shoot matter Zn concentrations in CMM were below this reference level, whereas in CRV, the limit was surpassed in soil that received 18 and 36 mg dm–3 Zn (Table VI).
Fresh matter weight of shoots (SFM), Zn concentrations in fresh shoot matter* (ZnSFM) and amount of lettuce (Lettuce) to be consumed daily to reach the Zn provisional tolerable daily intake limit** for cultivars CMM and CRV, 44 days after seedlings transplanting to soil treated with Zn doses.
Based on the Zn provisional tolerable daily intake set for an adult (70 kg body weight) which is 21-70 mg Zn (WHO 2006WHO. 2006. Evaluation of Certain Food Additives and Contaminants (Sixty-fourth Report of the Joint FAO/WHO Expert Committee on Food Additives). WHO-TRS 930-JECFA 64/26. World Health Organization, Geneva.), this limit would be reached with a daily consumption of 3678, 1813, 888 and 480 g of CMM lettuce (grown in soil fertilized with 2, 6, 18 and 36 mg dm–3 Zn, respectively) (Table VI). For CRV cultivar, a smaller amount would be required since with a daily consumption of 1787, 1016, 401, and 290 g of lettuce (grown in soils amended with 2.0, 6, 18 and 36 mg dm–3 Zn, respectively) the limit would be reached (Table VI). This shows that both lettuce cultivars may accumulate considerable amounts of Zn in lettuce leaves, with CRV accumulating more than CMM. However, it is unlikely that lettuce daily consumption of both cultivars will cause Zn toxicity problems. In fact, a daily consumption of 290 g of CRV and 480 g of CMM (produced in soil amended with 36 mg dm–3 Zn) would be required to reach the lower limit (21 mg Zn).
The recommended USA eating plan for a daily supply of 2000 calories includes a consumption of 2 to 2.5 cups of vegetables (Dietary Guidelines for Americans 2005) (lettuce is a component of the vegetable group in the dietary guidelines). Considering 1 cup equivalent to 28.5 g, the weights for the cups would be 57 to 71 g.
Consumption of 60 g CRV lettuce would provide 0.70, 1.24, 3.14 and 4.33 mg Zn (for plants grown with 2, 6, 8 and 36 mg dm–3Zn, respectively). For CMM, it would be provided by 0.34, 0.69, 1.42, and 2.63 mg Zn (60 g lettuce grown with 2, 6, 8 and 36 mg dm–3 Zn, respectively), about half as compared to CRV. Taking only lettuce to fulfill the vegetable group recommendation (USA 2000 calories eating plan) and taking into account the Zn DRI (Dietary Reference Intake) for males (11 mg day–1) (WHO 1996WHO. 1996. Trace elements in human nutrition and health. WHO/FAO/IAEA. Geneva, 362 p.), 60 of CRV would provide 6.4, 11.3, 28.5 and 40.0 % of Zn DRI (with plants grown with 2, 6, 8 and 36 mg dm–3 Zn, respectively) and CMM would provide 3.0, 6.2, 12.9, and 23.9 % of Zn DRI. CRV would transfer about twice Zn to food-chain as compared to CMM, per unit of lettuce consumed.
It has to be noted that the recommended Zn dose for lettuce production in the state of Minas Gerais is 3 mg dm–3(Fontes 1999Fontes PCR. 1999. Sugestões para adubação para hortaliças em Minas Gerais. Alface. In: Ribeiro AC, Guimarães PTG and Alvarez VVH (Eds), Recomendações para o uso de corretivos e fertilizantes para Minas Gerais. 5a Aproximação. CFSEMG. Viçosa, Minas Gerais, Brasil, p. 171-177.) which corresponds to 6 kg ha–1 Zn, hence 36 mg dm–3 (72 kg ha–1) Zn is a very high dose compared to that.
Considering only lettuce to fulfill the vegetable group in the USA eating plan, 60 g CRV and 60 g CMM (produced with the highest Zn dose) would provide 40.0 % and 23.9 % Zn DRI, respectively. Considering Zn inputs for agricultural production, that amount (72 kg ha–1) might result in a long term basis soil Zn build up, which is a concern.
Zinc deficiency has been frequently associated to nutrition problems in humans, thus its excess in plants is not a matter of concern as it is for Cd. Even consuming appreciable amounts of high Zn leafy vegetables, the occurrence of Zn toxicity in humans is unlikely (Chaney 2010Chaney R. 2010. Cadmium and Zinc. In: Hooda PS (Ed), Trace Elements in Soils. Blackwell Publishing Ltd, UK, p. 410-439.). In fact, the research goal in numerous studies around the world has been to improve crop management for Zn increase in food. Recently, Hussain et al. (2012)Hussain S, Maqsood MA, Rengel Z and Aziz T. 2012. Biofortification and estimated human bioavailability of zinc in wheat grains as influenced by methods of zinc application. Plant Soil (27 March 2012), p. 1-12. doi:10.1007/s11104-012-1217-4.
https://doi.org/10.1007/s11104-012-1217-...
reported high soil application of Zn, increasing estimated Zn bioavailability to humans. Nutrient crop biofortification has been a matter of extensive research in recent years.
CONCLUSIONS
-
- The cultivar CRV showed greater potential for yield than CMM in both experiments.
-
- Cadmium and Zn translocation from roots to shoots increased with the increase of soil applied Cd or Zn, for both cultivars.
-
- Translocation from young to old leaves was observed for Cd in CMM but not in CRV shoots whereas for Zn it occurred in both cultivars, with a higher rate in CRV.
-
- The CMM and CRV cultivars showed a differential risk of Cd transference from soil to food-chain which was higher in CRV. In order to reach the provisional tolerable daily intake of Cd, the CMM lettuce should be consumed at least twice as much as the CRV.
-
- There is a differential risk of Zn transference from soil to food-chain between the cultivars and the CRV transferred more Zn than the CMM.
-
- Even with Zn soil addition at doses considerably higher than the usually recommended for lettuce, the risk of Zn toxicity by lettuce consumption is unlikely. However, high doses applied systematically in agricultural soils may lead to a soil Zn build up on a long term basis, which is a matter of concern.
RLFF thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the research fellowship and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for the research funding.
REFERENCES
- ABIA - Associação Brasileira das Indústrias da Alimentação. 1991. Compêndio da Legislação de Alimentos–Revisão. Vol 1, São Paulo, Brasil.
- Agbenin JO and Olojo LA. 2004. Competitive adsorption of copper and zinc by a Bt horizon of a savanna Alfisol as affected by pH and selective removal of hydrous oxides and organic matter. Geoderma 119: 85-95.
- Alleoni LRF, Borba RP and Camargo OA. 2005. Metais pesados: da cosmogênese aos solos brasileiros. In: Torrado PV, Alleoni LRF, Silva MAL and Cardoso EJ (Eds), Tópicos em ciência do solo. Sociedade Brasileira de Ciência do Solo, Viçosa, MG, Brasil, p. 1-42.
- Alvarez VH, Novais FR, Barros NF, Cantarutti RB and Lopes AS. 1999. Interpretação dos resultados das análises se solos. In: Ribeiro AC, Guimarães PTG and Alvarez VVH (Eds), Recomendações para o uso de corretivos e fertilizantes para Minas Gerais. 5a Aproximação. CFSEMG. Viçosa, MG, Brasil, p. 25-32.
- Boardman R and McGuire D. 1990. The role of zinc in forestry. I. Zinc in forest environments, ecosystems and tree nutrition. Journal: Forest Ecol Manag 37 (1-3): 167-205.
- Broadley MR, White PJ, Hammond JP, Zelko I and Lux A. 2007. Zinc in plants. New Phyt 173: 677-702.
- Cakmak I. 2008. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 302: 1-17.
- Cantarutti RB, Barros NF, Martinez HEP and Novais RF. 2007. Avaliação da fertilidade do solo e recomendação de fertilizantes. In: Novais RF, Alvarez VVH, Barros NF, Fontes RLF, Cantarutti RB and Neves JCL (Eds), Fertilidade do Solo. Viçosa, MG. Sociedade Brasileira de Ciencia do Solo. p. 645-736.
- CFSEMG - Comissão de Fertilidade do Solo do Estado de Minas Gerais. 1999. Recomendações para o Uso de Corretivos e Fertilizantes em Minas Gerais, 5a aproximação. Imprensa Universitária, Universidade Federal de Viçosa. Viçosa, MG, Brasil, 359 p.
- Chaney R. 2010. Cadmium and Zinc. In: Hooda PS (Ed), Trace Elements in Soils. Blackwell Publishing Ltd, UK, p. 410-439.
- Chen J and Gao J. 1993. The Chinese total diet study in 1990. Part I. Chemical contaminants. JAOAC Int 76: 1193-1205.
- Clemens S. 2001. Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212: 475-486.
- Codex Alimentarius Commission. 2006. Report of the 29th session of the Codex Alimentarius Commission. FAO/WHO. Geneve. Switzerland.
- Dach J and Starmans D. 2005. Heavy metals balance inPolish and Dutch agronomy: actualstate and previsions for future. Agric Ecosystems Environ 107: 309-316.
- Dalcorso G, Farinat S, Maistri S and Furini A. 2008. How Plants Cope with Cadmium: Staking All on Metabolism and Gene Expression. J Integr Plant Biol 50(10): 1268-1280.
- Defelipo BV and Ribeiro AC. 1981. Análise Química do Solo (Metodologia), (Boletim de Extensão, 29), Imprensa Universitária, Universidade Federal de Viçosa, Brasil, 17 p.
- Dietary Guidelines for Americans. 2005. US Department of Health and Human Services and USA. (www.health.gov/dietaryguidelines/dga2005/document).
» www.health.gov/dietaryguidelines/dga2005/document - EMBRAPA - Empresa Brasileira de Pesquisa Agropecuária. 1997. Manual de Métodos de Análise de Solos, Serviço Nacional de Levantamento e Conservação de Solos. Rio de Janeiro, Brasil, 212 p.
- Ferretti M, Ghisi R, Merlo L, Vecchia FD and Passera C. 1993. Effect of cadmium on photosynthesis and enzymes of photosynthetic sulphate and nitrate assimilation pathways in maize (Zea mays L.). Photosynthetica 29: 49-54.
- Fontes PCR. 1999. Sugestões para adubação para hortaliças em Minas Gerais. Alface. In: Ribeiro AC, Guimarães PTG and Alvarez VVH (Eds), Recomendações para o uso de corretivos e fertilizantes para Minas Gerais. 5a Aproximação. CFSEMG. Viçosa, Minas Gerais, Brasil, p. 171-177.
- Gaudette HE, Flight WR, Toner L and Folger DW. 1974. An inexpensive titration method for the determination of organic carbon in recent sediments. J Sediment Petrol 44: 249-253.
- Green CE, Chaney RL and Bouwkamp J. 2003. Interactions between cadmium uptake and phytotoxic levels of zinc in Hard Red Spring Wheat. J Plant Nut 26: 417-430.
- Greger M, Johansson M, Stihl A and Hamza K. 1994. Foliar uptake of Cd by pea (Pisum sativum) and sugar beet (Beta vulgaris). Physiol Plant 88: 563-570.
- Hall JL. 2002. Cellular mechanism of heavy metal detoxification and tolerance. J Exp Bot 53(366): 1-11.
- Hotz C and Brown KH. 2004. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 25: 94-204.
- Hu PJ, Qiu RL, Senthilkumara P, Jiang D, Chen ZW, Tang YT and Liu FJ. 2009. Tolerance, accumulation and distribution of zinc and cadmium in hyperaccumulator Potentilla griffithii. Environ Exp Bot 66: 317-325.
- Huguet S, Bert V, Laboudigue A, Barthes V, Isaure MP, Llorens I, Schat H and Sarret G. 2012. Cd speciation and localization in the hyperaccumulator Arabidopsis halleri. Environ Exp Bot 82: 54-65.
- Hussain S, Maqsood MA, Rengel Z and Aziz T. 2012. Biofortification and estimated human bioavailability of zinc in wheat grains as influenced by methods of zinc application. Plant Soil (27 March 2012), p. 1-12. doi:10.1007/s11104-012-1217-4.
» https://doi.org/10.1007/s11104-012-1217-4 - Jordão CP, Fialho LL, Cecon PR, Neves JCL, Mendonça E and Fontes RLF. 2006. Effects of Cu, Ni and Zn on lettuce grown in metal-enriched vermicompost amended soil. Water Air Soil Poll 172: 21-38.
- Kabata-Pendias A. 2010. Trace Elements in Soils and Plants, 4th ed. CRC Press, Boca Raton, Florida, 548 p.
- Kukier U, Channey RL, Ryan JL, Daniels WL, Dowdy RH and Granato TC. 2010. Phytoavailability of Cd in longterm biosolid amended soils. J Environm Qual 39: 519-530.
- Louekari K, Mäkelä-Kurtto R, Virtanen JPV, Sippola J and Malm J. 2000. Cadmium in fertilizers. Risk to human health and the environment. Ministry Agric Forestry. Finland. Helsinki, 119 p.
- Marschner H. 1995. Mineral Nutrition of Higher Plants, Academic Press, New York, 889 p.
- Mesquita Filho MV, Souza AF and Furlani PR. 2001. Hortaliças de bulbo, tubérculo, raiz e fruto. In: Ferreira ME, Cruz MCP, Raij B van and Abreu CA (Eds), Micronutrientes e elementos tóxicos na agricultura. Jaboticabal: CNPQ/FAPESP/POTAFOS, Cap. 20, p. 511-532.
- Nascimento CWA and Fontes RLF. 2002. Absorção e distribuição de cádmio em duas cultivares de milho (Zea Mays L.) com diferentes graus de sensibilidade ao elemento. Rev Ceres 49(282): 169-179.
- Pais I and Jones Jr JB. 2000. The handbook of trace elements. CRC Press, Boca Raton, Florida. 223 p.
- Qiu RL, Tang YT, Zeng XW, Thangavel PT, Tang L, Gan YY, Ying RR and Wang SZ. 2012. Mechanisms of Cd Hyperaccumulation and Detoxification in Heavy Metal Hyperaccumulators: How Plants Cope with Cd. In: Progress in Botany Vol. 73. Lüttge U, Beyschlag W, Büdel B and Francis D (Eds), Springer-Verlag, Berlin Heidelberg, p. 127-159.
- Raij B Van, Cantarella H, Quaggio JA and Furlani AMCF. 1996. Recomendação de adubação e calagem para o estado de São Paulo. 2a ed., Campinas, IAC, Boletim técnico 100, 285 p.
- Ribeiro Jr JI. 2001. Análise estatísticas no SAEG. Viçosa, Minas Gerais: Imprensa Universitária-UFV.
- Roberts DR, Ford RG and Sparks DL. 2003. Kinetics and mechanisms of Zn complexation on metal oxides using EXAFS spectroscopy. J Coll Interfac Sci 263: 364-376.
- Salviano AM, Fontes RLF, Pereira JMN and Assis CP. 2005. Doses de cádmio no crescimento e na quantidade de clorofila em duas variedades de fumo cultivadas em solução nutritiva. Rev Caatinga 18(1): 6-12.
- Simmons RW, Pongsakul P, Saiyasitpanich D and Klinphoklap S. 2005. Elevated levels of cadmium and zinc in paddy soils and elevated levels of cadmium in rice grain downstream of a zinc mineralized area in Thailand: implications for public health. Environ Geochem Health 27: 501-511.
- Solis-Dominguez FA, Gonzalez-Chavez MC, Carrillo-Gonzalez R and Rodriguez-Vazquez R. 2007. Accumulation a nd localization of cadmium in Echinochloa polystachya grown within a hydroponic system. J Hazard Mater 141: 630-636.
- Staarink T and Hakkenbrak P. 1991. Book of Contaminants. Rijswijk, The Netherlands: Commodity Inspection Department.
- Welch RM and Graham RD. 2004. Breeding for micronutrients in staple food crops from a human nutrition perspective. J Exp Bot 55: 353-364.
- WHO. 1996. Trace elements in human nutrition and health. WHO/FAO/IAEA. Geneva, 362 p.
- WHO. 2006. Evaluation of Certain Food Additives and Contaminants (Sixty-fourth Report of the Joint FAO/WHO Expert Committee on Food Additives). WHO-TRS 930-JECFA 64/26. World Health Organization, Geneva.
- Wiersma D, Van Goor BJ and Van Der Veen NG. 1981. Cadmium, lead, mercury and arsenic concentrations in crops and corresponding soils in The Netherlands. J Agr Food Chem 34: 1067-1074.
- Zarcinas BA, Pongsakul P, McLaughlin MJ and Cozens G. 2004. Heavy metals in soils and crops in Southeast Asia 2. Thailand Environ Geochem Health 26: 359-371.
- Zazoski RJ and Burau RG. 1977. A rapid nitric-perchloric acid digestion method for mult-element tissue analysis. Commun Soil Sci Plant 8: 425-436.
Publication Dates
-
Publication in this collection
June 2014
History
-
Received
14 Nov 2012 -
Accepted
13 May 2013