Abstract
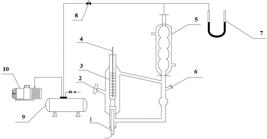
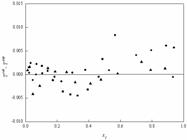
In this work, the vapor-liquid equilibrium experimental data for the systems of isopropyl chloroacetate + isopropanol, isopropyl chloroacetate + cyclohexane and isopropyl chloroacetate + benzene were measured by a modified Rose-type recirculating still under the pressure of 101.3 kPa. The thermodynamic consistency of the measured data was verified by the Herington and van Ness methods, respectively. The experimental data were correlated by the NRTL, Wilson, and UNIQUAC activity coefficient models, and the corresponding interaction parameters of the three models were obtained. The root-mean-square deviations between the experimental data and calculated results for the temperature and the mole fraction of the vapor phase were less than 0.58 K and 0.0066, respectively. In addition, the excess Gibbs energy was calculated for the three systems.
Keywords:
Vapor-liquid equilibrium; Isopropyl chloroacetate; Correlation; Thermodynamic model

 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail







