BACKGROUND
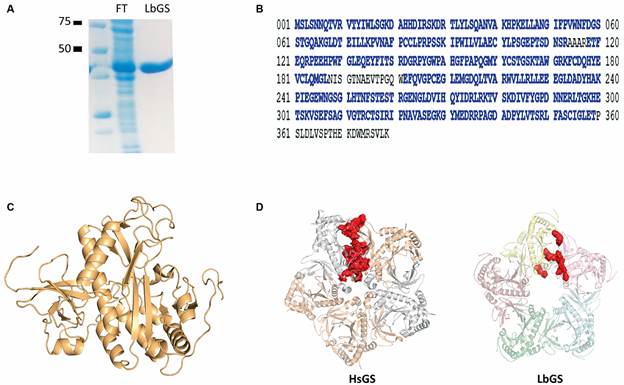
Leishmaniasis is a neglected tropical disease caused by the parasite Leishmania braziliensis, commonly found in Brazil and associated with cutaneous and visceral forms of this disease. Like other organisms, L. braziliensis has an enzyme called glutamine synthetase (LbGS) that acts on the synthesis of glutamine from glutamate. This enzyme plays an essential role in the metabolism of these parasites and can be a potential therapeutic target for treating this disease.
OBJECTIVES
Investigate LbGS structure and generate structural models of the protein.
METHODS
We use the method of crosslinking mass spectrometry (XLMS) and generate structural models in silico using I-TASSER.
FINDINGS
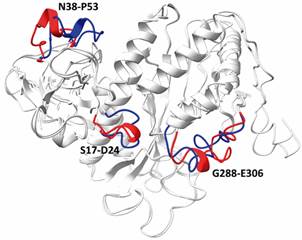
42 XLs peptides were identified, of which 37 are explained in a monomeric model with the other five indicating LbGS dimerization and pentamers interaction region. The comparison of 3D models generated in the presence and absence of XLMS restrictions probed the benefits of modeling with XLMS highlighting the inappropriate folding due to the absence of spatial restrictions.
MAIN CONCLUSIONS
In conclusion, we disclose the conservation of the active site and interface regions, but also unique features of LbGS showing the potential of XLMS to probe structural information and explore new drugs.
Key words:
structural proteomics; protein; glutamine synthetase; Leishmania braziliensis

 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail