Abstract
We analyzed the reproductive phenology of Ditassa burchellii and the influence of abiotic factors on the species phenophases. The study was conducted on individuals of a natural population from a semi-deciduous forest (Viçosa municipality, Minas Gerais state, southeastern Brazil). We quantified the activity and intensity indices of the following phenophases: flower bud, flower, immature fruit, and dehiscent fruit. To test for seasonality of phenophases, we analyzed each of them using Rayleigh test. We evaluated whether there was any association between abiotic variables (mean temperature, rainfall, and day length) and phenophases, in the month of occurrence and in the three months prior to the occurrence of each phenophase. The analyzed phenophases occurred mainly at the end of the rainy season and during the dry season, with overlapping periods, but with sequential peaks. The periods of occurrence of reproductive phenophases were similar to the ones in other climbing species and were mainly related to the dispersal mode. All phenophases were seasonal and were associated with at least one abiotic variable, either in the month of their occurrence or in the previous months.
Key words:
anemochory; Atlantic Forest; flowering; fruiting; seasonality
Resumo
Analisamos a fenologia reprodutiva de Ditassa burchellii e a influência de fatores abióticos em suas fenofases. O estudo foi realizado em indivíduos de população natural em floresta estacional semidecídua (Viçosa, Minas Gerais, sudeste do Brasil). Quantificamos os índices de atividade e de intensidade das seguintes fenofases: botões florais, flores, frutos imaturos e frutos deiscentes. Para testar a sazonalidade dessas fenofases, analisamos cada uma usando o teste de Rayleigh. Avaliamos se houve associação entre as variáveis abióticas (temperatura média, precipitação e comprimento do dia) e as fenofases, no mês de ocorrência e também nos três meses anteriores à ocorrência de cada fenofase. As fenofases ocorreram principalmente no fim da estação chuvosa e durante a estação seca, com períodos sobrepostos, mas com picos sequenciais. Os períodos de ocorrência das fenofases reprodutivas foram similares aos de outras espécies de lianas e foram relacionados principalmente com o modo de dispersão. Todas as fenofases foram sazonais e foram associadas a pelo menos uma das variáveis abióticas, no mês de sua ocorrência ou nos meses anteriores.
Palavras-chave:
anemocoria; Floresta Atlântica; floração; frutificação; sazonalidade
Introduction
Plant phenology studies provide information on plant growth as well as on the effects of selective pressures imposed by the environment on flowering and fruiting (Fenner 1998Fenner M (1998) The phenology of growth and reproduction in plants. Perspectives in Plant Ecology, Evolution and Systematics 1: 78-91.). These studies are mainly performed on tree and treelet species (Morellato & Leitão-Filho 1996Morellato LPC & Leitão-Filho HF (1996) Reproductive phenology of climbers in a southeastern Brazilian forest. Biotropica 28: 180-191.). Climbing species (woody lianas and herbaceous vines sensuGentry 1991Gentry AH (1991) The distribution and evolution of climbing species. In: Putz FE & Mooney HA (eds.) The biology of vines. Cambridge University Press, Cambridge. Pp. 3-52.) usually have different phenological patterns from those of tree species, and they may also differ in their phenological responses according to the climber type (Putz & Windsor 1987Putz FE & Windsor DM (1987) Liana phenology on Barro Colorado Island, Panama. Biotropica 19: 334-341.; Morellato & Leitão-Filho 1996Morellato LPC & Leitão-Filho HF (1996) Reproductive phenology of climbers in a southeastern Brazilian forest. Biotropica 28: 180-191.).
In plant communities, reproductive phenophases (flowering and fruiting) are usually associated with the quantity and quality of resources available to pollinators and seed dispersers (Williams et al. 1999Williams RJ, Myers BA, Eamus D & Duff GA (1999) Reproductive phenology of woody species in a North Australian Tropical Savanna. Biotropica 31: 626-636.; Stevenson et al. 2008Stevenson PR, Castellanos MC, Cortés AI & Link A (2008) Flowering patterns in a seasonal tropical lowland Forest in western Amazonia. Biotropica 40: 559-567.; Rubim et al. 2010Rubim P, Nascimento HEM & Morellato LPC (2010) Variações interanuais na fenologia de uma comunidade arbórea de floresta semidecídua no sudeste do Brasil. Acta Botanica Brasilica 24: 756-764.; Fonseca et al. 2013Fonseca RS, Campos LAO & Vieira MF (2013) Melittophily and ornithochory in Tilesia baccata (L.f.) Pruski: an Asteraceae of the Atlantic Forest understory with fleshy fruits. Flora 208: 370-380.), besides providing information on the sexual reproductive mechanisms of plant species. In the case of climbing species, flowering can be unimodal (peaking in the dry season) or bimodal (peaking in both wet and dry seasons), while the fruiting peak is commonly associated with the type of fruit dispersal (Putz & Windsor 1987Putz FE & Windsor DM (1987) Liana phenology on Barro Colorado Island, Panama. Biotropica 19: 334-341.; Morellato & Leitão-Filho 1996Morellato LPC & Leitão-Filho HF (1996) Reproductive phenology of climbers in a southeastern Brazilian forest. Biotropica 28: 180-191.; Ramírez & Briceño 2011Ramírez N & Briceño H (2011) Reproductive phenology of 223 species from four herbaceous-shrubby communities in the Gran Sabana Plateau of Venezuela. AoB Plants 2011: 1-17.; Romaniuc Neto et al. 2012Romaniuc Neto S, Godoi JV, Villagra BLP, Almeida-Scabbia RJ & Melo MMRF (2012) Caracterização florística, fitossociológica e fenológica de trepadeiras de mata ciliar da Fazenda Campininha, Mogi Guaçu, SP, Brasil. Hoehnea 39: 145-155.; Ponnuchamy et al. 2013Ponnuchamy R, Pragasam A, Aravajy S, Patel P, Das L & Anupama K (2013) A floristic study on herbs and climbing plants at Puducherry, South India: an approach to biodiversity conservation and regeneration through eco-restoration. Check List 9: 555-600.). However, most of the studies addressing the reproductive phenophases of climbing species have focused on woody lianas and did not evaluate the influence of abiotic factors on the phenological responses of the species.
Asclepiadoideae, the largest subfamily in the Apocynaceae, commonly has herbaceous vine representatives in tropical forests (Morellato & Leitão-Filho 1996Morellato LPC & Leitão-Filho HF (1996) Reproductive phenology of climbers in a southeastern Brazilian forest. Biotropica 28: 180-191.; Ponnuchamy et al. 2013Ponnuchamy R, Pragasam A, Aravajy S, Patel P, Das L & Anupama K (2013) A floristic study on herbs and climbing plants at Puducherry, South India: an approach to biodiversity conservation and regeneration through eco-restoration. Check List 9: 555-600.), including Ditassa burchellii Hook. & Arn., the species addressed in the present study. This subfamily is represented by approximately 392 species distributed among 32 genera in Brazil (Rapini 2012Rapini A (2012) Taxonomy "under construction": advances in the systematics of Apocynaceae, with emphasis on the Brazilian Asclepiadoideae. Rodriguésia 63: 75-88.), where only the exotic shrub Calotropis procera (Aiton) W.T. Aiton has been the focus of studies involving reproductive phenophases (Sobrinho et al. 2013Sobrinho MS, Tabatinga GM, Machado IC & Lopes AV (2013) Reproductive phenological pattern of Calotropis procera (Apocynaceae), an invasive species in Brazil: annual in native areas; continuous in invaded areas of caatinga. Acta Botanica Brasilica 27: 456-459.).
There are no studies on the reproductive phenology of Ditassa representatives, despite the high endemic-species richness of the genus in Brazil (49 species; BFG 2018BFG - The Brazil Flora Group (2018) Brazilian flora 2020: innovation and collaboration to meet Target 1 of the Global Strategy for Plant Conservation (GSPC). Rodriguésia 69: 1513-1527.). Recent studies have shown that some Ditassa species interact with rare and specific groups of pollinators (Domingos-Melo et al. 2017Domingos-Melo A, Nadia TL & Machado IC (2017) Complex flowers and rare pollinators: Does ant pollination in Ditassa show stable system in Aclepiadoideae (Apocynaceae)? Arthropod-Plant Interactions 11: 339-349.; Fontes et al. 2018Fontes AL, Valentin-Silva A, Demuner AJ & Vieira MF (2018) Functional morphology and floral odor of Ditassa burchellii (Apocynaceae, Asclepiadoideae), a micromyiophilous species from southeastern Brazil. International Journal of Plant Sciences 179: 450-460.). These findings demonstrate the relevant role played by those plants in maintaining these poorly known mutualistic interactions. Thus, we aimed to describe the reproductive phenology of D. burchellii, a herbaceous vine, and determine whether abiotic variables are associated with the species phenological responses.
Material and Methods
Study area and species
We carried out the study from March 2013 to December 2014 in a forest fragment at the Mata do Paraíso Station for Research, Environmental Training and Education (hereafter referred to as Mata do Paraíso). Mata do Paraíso is located in Viçosa municipality (20º47'-48'S, 42º50'-52'W), Minas Gerais state, southeastern Brazil, covering an area of 194 ha, at 690-870 m above sea level. This forest fragment is the largest one in Viçosa (Silva et al. 2014Silva CA, Vieira MF, Okano RMC & Oliveira LO (2014) Reproductive success and genetic diversity of Psychotria hastisepala (Rubiaceae), in fragmented Atlantic forest, Southeastearn Brazil. Revista de Biología Tropical 62: 309-319.), and its vegetation is classified as seasonal semi-deciduous montane forest (Veloso et al. 1991Veloso HP, Rangel-Filho ALR & Lima JCA (1991) Classificação da vegetação brasileira adaptada a um sistema universal. IBGE, Rio de Janeiro. 124p.).
Climate in Viçosa is type Cwa (mesothermal with hot rainy summers and cold dry winters), according to Köppen's classification (Alvares et al. 2013Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM & Sparovek G (2013) Köppen's classification map for Brazil. Meteorologische Zeitschrift 22: 711-728.). Average annual rainfall is 1,221 mm and average annual temperature is 19.3 °C (averages of meteorological data from 1961-1990; DNMET 1992DNMET - Departamento Nacional de Meteorologia (1992) Normais climatológicas (1961-1990). SPI/EMBRAPA, Brasília. 84p.). The dry season extends from April to September (monthly rainfall < 60 mm; mean minimum and maximum monthly temperatures = 10 °C and 27 °C, respectively; mean photoperiod = 11.3 h) while the rainy season extends from October to March (monthly rainfall > 100 mm; mean minimum and maximum monthly temperatures = 16 °C and 30 °C, respectively; mean photoperiod = 12.9 h). Rainfall and temperature showed a seasonal distribution during the study period (Fig. 1).
Meteorological data on Viçosa municipality, Minas Gerais state, southeastern Brazil (dashed line = mean temperature, solid line = day length, bars = rainfall), during the study period. Black horizontal bar = dry season; white horizontal bar = rainy season.
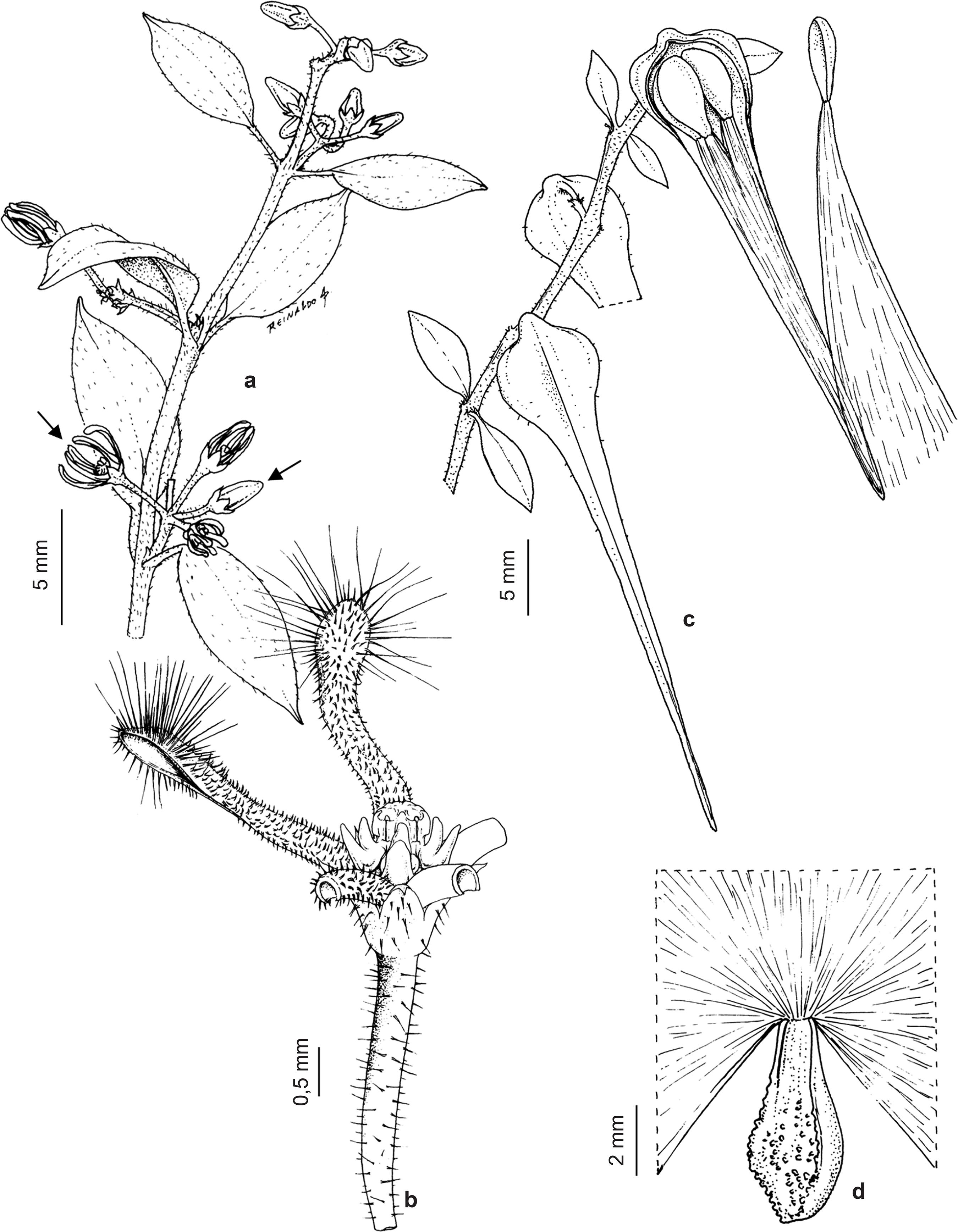
Ditassa burchellii inflorescences are umbelliform cymes (Fig. 2a) with tiny cream-colored, odoriferous flowers (ca. 3 mm; Fig. 2b); fruits are follicles, each of which contains four comose seeds (Fig. 2c-d). Each flower can produce one (single follicle; Fig. 2c) or two (twin follicles) fruits, as ovaries are apocarpous (Endress & Bruyns 2000Endress ME & Bruyns PV (2000) A revised classification of the Apocynaceae s.l. The Botanical Review 66: 1-56.). The species occurs commonly in "capoeira" (secondary) vegetation and at forest edges (Konno 2005Konno TUP (2005) Ditassa R. Br. no Brasil (Asclepiadoideae - Apocynaceae) e revisão taxonômica de Minaria T.U.P. Konno et Rapini. Tese de Doutorado. Universidade de São Paulo, São Paulo. 239p.). In the study site, plants are usually seen along trail edges in partially shaded locations, growing above the surrounding vegetation, which has a low (about 7 m) and discontinuous canopy. A voucher specimen was deposited in the Herbarium of Federal University of Viçosa (VIC Herbarium; no. 43,006).
Ditassa burchellii - a. branch with opposite leaves and one inflorescence per node; notice, on the first inflorescence (from bottom to top), flower bud (right arrow) and flower (left arrow); b. flower, with three petals having been removed; c. immature fruit (single follicle; left side) and dehiscent fruit with exposed comose seeds (right side); notice the occurrence of a single fruit per node; d. seed with the fully expanded coma being partially represented.
Reproductive phenology
We sampled seven adult plants, respecting a minimum distance of 10 m between each plant to avoid repeated evaluations in a same individual. We recorded the presence of flower buds (Fig. 2a), flowers (Fig. 2a-b), and fruits (immature and dehiscent; Fig. 2c) in two consecutive reproductive periods, with a total 22 months of observation. We performed evaluations on a fortnightly basis during the vegetative period and on a weekly basis in the reproductive period.
We analyzed reproductive phenophases by calculating the activity (percentage of plants at a given phenophase) and intensity indices (Bencke & Morellato 2002Bencke CSC & Morellato LPC (2002) Comparação de dois métodos de avaliação da fenologia em plantas, sua interpretação e representação. Revista Brasileira de Botânica 25: 269-275.). To calculate intensity index, each phenophase was scored on a semi-quantitative scale, as proposed by Fournier (1974)Fournier LA (1974) Un método cuantitativo para la medición de características fenológicas en árboles. Turrialba 24: 422-423.. To this end, we used the weekly values of each month for both indices. We classified the frequency of phenophases following Newstrom et al. (1994)Newstrom LE, Frankie GW & Baker HG (1994) A new classification for plant phenology based on flowering patterns in lowland tropical rain forest trees at La Selva, Costa Rica. Biotropica 26: 141-159., as continual, subannual, annual, or supra-annual. The annual phenophase was also classified according to its duration, as brief (< 1 month), intermediate (1-5 months), or extended (> 5 months).
In addition, we labeled 40 inflorescences (n = six individuals) having flower buds and monitored them on a daily basis from anthesis of the first flowers to fruiting. Thus, we recorded the duration of flowers and inflorescences, the number of flowers per inflorescence, and the number of open flowers per day per inflorescence. We monitored 40 other flowers (n = 40 inflorescences, six individuals) from ovary swelling to seed dispersal, to record the duration of fruit development stage. We determined the natural fruit set (open pollination) of 5655 flowers and made non-systematic observations on 650 inflorescences, throughout the two reproductive periods, to record the number of fruits per inflorescence and per flower.
Seasonality and association with abiotic variables
To test for seasonality of reproductive phenophases, we analyzed each phenophase using Rayleigh test (circular statistics; Zar 1999Zar JH (1999) Biostatistical analysis. Prentice-Hall, Upper Saddle River. 663p.; Morellato et al. 2010Morellato LPC, Alberti LF & Hudson IL (2010) Applications of circular statistics in plant phenology: a case studies approach. In: Keatley M & Hudson IL (eds.) Phenological research: methods for environmental and climate change analysis. Springer, New York. Pp. 339-359.). For that, we converted months to angles and calculated mean angle, mean date (for phenophases with significant mean angles), and vector length, considering the result significant when p < 0.01 and r > 0.5 (Morellato et al. 2010Morellato LPC, Alberti LF & Hudson IL (2010) Applications of circular statistics in plant phenology: a case studies approach. In: Keatley M & Hudson IL (eds.) Phenological research: methods for environmental and climate change analysis. Springer, New York. Pp. 339-359.). We carried out these analyses using BioEstat (Ayres et al. 2007Ayres M, Ayres Júnior M, Ayres DL & Santos AA (2007) BioEstat - aplicações estatísticas nas áreas das ciências biomédicas. Sociedade Civil Mamirauá, Belém. 364p.), based on the weekly values of number of individuals at each phenophase.
We evaluated whether there was any association between abiotic variables (mean temperature, rainfall, and day length) and phenophases, in the month of occurrence and also in the three months prior to the occurrence of each phenophase. To this end, we obtained meteorological data from the Meteorological Station of Federal University of Viçosa, which is 10 km away from the study area, and calculated the mean weekly values of mean temperature and day length and the sum of weekly rainfall values. We used generalized linear models with Poisson distribution and logarithmic link function, selecting the best model using the Akaike information criterion (AIC; Crawley 2007Crawley MJ (2007) The R book. Wiley, Chichester. 942p.). We carried out these analyses in R (R Core Team 2016R Core Team (2016) R: a language and environment for statistical computing. Available at <https://www.R-project.org/>. Access on 10 August 2016.
https://www.R-project.org/...
), considering the weekly values of number of individuals at each phenophase as the response variable and temperature, rainfall, and day length as explanatory variables.
Results
All individuals reproduced once a year during the study period. The reproductive phenophases occurred mainly at the end of the rainy season and during the dry season, with overlapping periods of occurrence, but with sequential peaks (Fig. 3a-d). Peaks of activity and intensity were similar for all phenophases (Fig. 3a-d).
a-d. Reproductive phenology of Ditassa burchellii at Mata do Paraíso, southeastern Brazi - a. activity (▲) and intensity (○) indices of phenophases flower buds; b. flowers; c. immature fruits; d. dehiscent fruits. Black horizontal bar = dry season; white horizontal bar = rainy season.
Flower buds were observed in March and April 2013 and 2014, during the transition from the rainy to the dry season, peaking in March (Fig. 3a). In both years, flowers were observed in the same period as flowers buds (about 35 days), but peaking in April (Fig. 3b). Both phenophases were annual with an intermediate duration.
Flowers opened early in the morning and lasted an average 6.4 days (with a range of 2-11 days). The mean number of flowers per inflorescence and mean inflorescence duration were, respectively, 8.7 ± 1.8 flowers and 21.1 ± 4.4 days. The mean number of open flowers per day per inflorescence was 4.1 ± 1.6, depending on the number of flowers per inflorescence and on the dynamics of flower opening, which occurred gradually.
In both years, immature fruits were mainly observed in the dry season (Fig. 3c), and such phenophase was annual with extended duration. Peaks occurred from May to September 2013 and in May 2014 for activity index and in May of both years for intensity index (Fig. 3c). The mean time of fruit development, from ovary swelling to seed dispersal, was 139.3 ± 24.2 days. Dehiscent fruits were observed from August to October 2013 and from July to September 2014, peaking in September 2013 and August 2014 (Fig. 3d), at the end of the dry season. This phenophase was annual with intermediate duration.
Natural fruit set was low (4.4%). Fruits were observed in 36.9% of the 650 inflorescences monitored over two reproductive episodes. There was a tendency of one flower per inflorescence to result in fruit (97.5%; Fig. 2c) and of one fruit to be produced per flower (single follicle, 96.8%; Fig. 2c).
All phenophases were seasonal, but the immature fruit phenophase was less concentrated and less synchronous around the mean data than the other ones (Tab. 1) due to the presence of immature fruits throughout the dry season. Phenophases were associated with at least one of the abiotic variables, either in the month of their occurrence or in the previous months (Tab. 2). The flower bud phenophase was associated with all abiotic variables, while flowering duration (the period with flowers at anthesis) was best explained by temperature and day length. Fruiting phenology was best explained by day length.
Results of circular statistical analysis on the occurrence of seasonality of reproductive phenophases of Ditassa burchellii at Mata do Paraíso, southeastern Brazil.
Models used to evaluate the association between abiotic variables and the reproductive phenophases of Ditassa burchellii. Numbers after each variable refer to the time lag used in the analyses. AIC = Akaike information criterion; temp = mean temperature; rain = rainfall; dayl = day length.
Occurrence of the flowering stage in the transition from the rainy to the dry season was also observed in herbaceous vines from other seasonal forests (Morellato & Leitão-Filho 1996Morellato LPC & Leitão-Filho HF (1996) Reproductive phenology of climbers in a southeastern Brazilian forest. Biotropica 28: 180-191.; Romaniuc Neto et al. 2012Romaniuc Neto S, Godoi JV, Villagra BLP, Almeida-Scabbia RJ & Melo MMRF (2012) Caracterização florística, fitossociológica e fenológica de trepadeiras de mata ciliar da Fazenda Campininha, Mogi Guaçu, SP, Brasil. Hoehnea 39: 145-155.). This period is the most favorable one for fruit ripening and diaspore dispersal by wind (Morellato & Leitão-Filho 1996Morellato LPC & Leitão-Filho HF (1996) Reproductive phenology of climbers in a southeastern Brazilian forest. Biotropica 28: 180-191.; Rossatto & Kolb 2011Rossatto DR & Kolb RM (2011) Comportamento fenológico de liana Pyrostegia venusta (Ker Gawl.) Miers (Bignoniaceae) em área de cerradão na Estação Ecológica de Assis, SP, Brasil. Revista Brasileira de Biociências 9: 289-296.). Flowering in some climbing species is stimulated by decrease in temperature and day length (Gentry 1974Gentry AH (1974) Flowering phenology and diversity in tropical Bignoniaceae. Biotropica 6: 64-68.; Rossatto & Kolb 2011Rossatto DR & Kolb RM (2011) Comportamento fenológico de liana Pyrostegia venusta (Ker Gawl.) Miers (Bignoniaceae) em área de cerradão na Estação Ecológica de Assis, SP, Brasil. Revista Brasileira de Biociências 9: 289-296.), similarly to the observed in D. burchellii.
Long-lived flowers such as those observed here are common in the Asclepiadoideae (Wyatt 1981Wyatt R (1981) The reproductive biology of Asclepias tuberosa: II. Factors determining fruit-set. New Phytologist 88: 375-385.; Skutch 1988Skutch AF (1988) Flowering and seed-production of Fischeria funebris (Asclepiadaceae). Brenesia 30: 13-17.; Lumer & Yost 1995Lumer C & Yost SE (1995) The reproductive biology of Vincetoxicum nigrum (L.) Moench (Asclepiadaceae), a Mediterranean weed in New York state. Bulletin of the Torrey Botanical Club 122: 15-23.; Vieira & Shepherd 1999Vieira MF & Shepherd GJ (1999) Sistema reprodutivo de Oxypetalum appendiculatum Mart. e Oxypetalum banksii Roem. & Schult. subsp. banksii (Asclepiadaceae). Acta Botanica Brasilica 13: 237-241.), similarly to the observed in Orchidaceae (Primack 1985Primack RB (1985) Longevity of individual flowers. Annual Review of Ecology and Systematics 16: 15-37.); pollen grains of plants from these two groups are clustered in pollinia and have complex pollination mechanisms in common (Endress 1994Endress PK (1994) Diversity and evolutionary biology of tropical flowers. Cambridge University Press, Cambridge. 511p., 2016Endress PK (2016) Development and evolution of extreme synorganization in angiosperm flowers and diversity: a comparison of Apocynaceae and Orchidaceae. Annals of Botany 117: 749-767.). Floral longevity may be associated with pollinator visitation rates (Schoen & Ashman 1995Schoen DJ & Ashman TL (1995) The evolution of floral longevity: Resource allocation to maintenance versus construction of repeated parts in modular organisms. Evolution 49: 131-139.). Thereby, the rates of removal and insertion of pollinia must be related to floral longevity, as unpollinated flowers tend to live longer (Devlin & Stephenson 1984Devlin B & Stephenson AG (1984) Factors that influence the duration of the staminate and pistillate phases of Lobelia cardinalis flowers. Botanical Gazette 145: 323-328.; Richardson & Stephenson 1989Richardson TE & Stephenson AG (1989) Pollen removal and pollen deposition affect the duration of the staminate and pistillate phases in Campanula rapunculoides. American Journal of Botany 76: 532-538.).
The occurrence of fruiting in D. burchellii during the dry season differed from what is common for herbaceous vines from seasonal semi-deciduous forests (with a slight peak at the end of the rainy season; Morellato & Leitão-Filho 1996Morellato LPC & Leitão-Filho HF (1996) Reproductive phenology of climbers in a southeastern Brazilian forest. Biotropica 28: 180-191.). This difference may also be related to the dispersal mode (Romaniuc Neto et al. 2012Romaniuc Neto S, Godoi JV, Villagra BLP, Almeida-Scabbia RJ & Melo MMRF (2012) Caracterização florística, fitossociológica e fenológica de trepadeiras de mata ciliar da Fazenda Campininha, Mogi Guaçu, SP, Brasil. Hoehnea 39: 145-155.). In fact, seed dispersal occurred at the end of the dry season, a period which might not only favor anemochorous dispersal (Morellato & Leitão-Filho 1996Morellato LPC & Leitão-Filho HF (1996) Reproductive phenology of climbers in a southeastern Brazilian forest. Biotropica 28: 180-191.; Rossatto & Kolb 2011Rossatto DR & Kolb RM (2011) Comportamento fenológico de liana Pyrostegia venusta (Ker Gawl.) Miers (Bignoniaceae) em área de cerradão na Estação Ecológica de Assis, SP, Brasil. Revista Brasileira de Biociências 9: 289-296.) of the species comose seeds but also be conducive to seed germination (Morellato et al. 1989Morellato LPC, Rodrigues RR, Leitão-Filho HF & Joly CA (1989) Estudo comparativo de espécies arbóreas de uma floresta de altitude e floresta mesófila semidecídua na Serra do Japi, Jundiaí, São Paulo. Revista Brasileira de Botânica 12: 85-98.; Rossatto & Kolb 2011Rossatto DR & Kolb RM (2011) Comportamento fenológico de liana Pyrostegia venusta (Ker Gawl.) Miers (Bignoniaceae) em área de cerradão na Estação Ecológica de Assis, SP, Brasil. Revista Brasileira de Biociências 9: 289-296.). Thus, new plants could use the entire rainy season to develop their root system (Fournier & Salas 1966Fournier LA & Salas S (1966) Algunas observaciones sobre la dinámica de la floración en el bosque tropical húmedo de Villa Cólon. Revista de Biología Tropical 14: 75-85.), which may have been additionally favored by the higher rates of decomposition and nutrient release from the litter accumulated in the dry season (Smythe 1970Smythe N (1970) Relationships between fruiting seasons and seed dispersal methods in a neotropical forest. The American Naturalist 104: 25-35.; Morellato 1992Morellato LPC (1992) Sazonalidade e dinâmica dos ecossistemas florestais da Serra do Japi. In: Morellato LPC (ed.) História natural da Serra do Japi: ecologia e preservação de uma área florestal no sudeste do Brasil. Ed. UNICAMP, Campinas. Pp. 98-110.). This strategy has also been described for other climbing species with anemochorous dispersal (Putz & Windsor 1987Putz FE & Windsor DM (1987) Liana phenology on Barro Colorado Island, Panama. Biotropica 19: 334-341.; Morellato & Leitão-Filho 1996Morellato LPC & Leitão-Filho HF (1996) Reproductive phenology of climbers in a southeastern Brazilian forest. Biotropica 28: 180-191.; Spina et al. 2001Spina AP, Ferreira WM & Leitão-Filho HF (2001) Floração, frutificação e síndromes de dispersão de uma comunidade de floresta de brejo na região de Campinas (SP). Acta Botanica Brasilica 15: 349-368.; Rossatto & Kolb 2011Rossatto DR & Kolb RM (2011) Comportamento fenológico de liana Pyrostegia venusta (Ker Gawl.) Miers (Bignoniaceae) em área de cerradão na Estação Ecológica de Assis, SP, Brasil. Revista Brasileira de Biociências 9: 289-296.; Romaniuc Neto et al. 2012Romaniuc Neto S, Godoi JV, Villagra BLP, Almeida-Scabbia RJ & Melo MMRF (2012) Caracterização florística, fitossociológica e fenológica de trepadeiras de mata ciliar da Fazenda Campininha, Mogi Guaçu, SP, Brasil. Hoehnea 39: 145-155.).
The association between fruiting and day length has also been observed in Pyrostegia venusta (Ker Gawl.) Miers (Bignoniaceae), a liana species (Rossatto & Kolb 2011Rossatto DR & Kolb RM (2011) Comportamento fenológico de liana Pyrostegia venusta (Ker Gawl.) Miers (Bignoniaceae) em área de cerradão na Estação Ecológica de Assis, SP, Brasil. Revista Brasileira de Biociências 9: 289-296.). Day length decreases along the dry season, when wind speed is more intense, which in turn favors anemochorous dispersal. Besides, fruiting of species from seasonal forests usually occurs on shorter days (lower temperature and precipitation; Stevenson et al. 2008Stevenson PR, Castellanos MC, Cortés AI & Link A (2008) Flowering patterns in a seasonal tropical lowland Forest in western Amazonia. Biotropica 40: 559-567.; Rubim et al. 2010Rubim P, Nascimento HEM & Morellato LPC (2010) Variações interanuais na fenologia de uma comunidade arbórea de floresta semidecídua no sudeste do Brasil. Acta Botanica Brasilica 24: 756-764.). We observed a similar pattern in this study, as fruiting peaked in the dry season.
The low fruit set observed was similar to values assessed in other Asclepiadoideae species (1-5%; Wyatt & Broyles 1994Wyatt R & Broyles SB (1994) Ecology and evolution of reproduction in milkweeds. Annual Review of Ecology and Systematics 25: 423-441.). This result was a consequence of the abortion of immature fruits, a common phenomenon in this plant group, which may be induced by extrinsic (pollinator-regulated) or intrinsic factors (plant-regulated) (Whyatt 1981; Queller 1985Queller DC (1985) Proximate and ultimate causes of low fruit production in Asclepias exaltata. Oikos 44: 373-381.; Wyatt & Broyles 1994Wyatt R & Broyles SB (1994) Ecology and evolution of reproduction in milkweeds. Annual Review of Ecology and Systematics 25: 423-441.). The tendency of one flower per inflorescence to result in fruit and of one fruit to be produced per flower is a characteristic commonly reported to Asclepiadoideae species (Liede & Whitehead 1991Liede S & Whitehead V (1991) Studies in the pollination biology of Sarcostemma viminale R. Br. sensu lato. South African Journal of Botany 57: 115-122.; Vieira & Shepherd 1999Vieira MF & Shepherd GJ (1999) Sistema reprodutivo de Oxypetalum appendiculatum Mart. e Oxypetalum banksii Roem. & Schult. subsp. banksii (Asclepiadaceae). Acta Botanica Brasilica 13: 237-241., 2002Vieira MF & Shepherd GJ (2002) Removal and insertion of pollinia in flowers of Oxypetalum (Asclepiadaceae) in southeastern Brazil. Revista de Biología Tropical 50: 37-43.), and it also contributed to the occurrence of a low fruit set.
This is the first study on the reproductive phenology of a native species of the Asclepiadoideae, a plant group with complex floral morphology and pollination mechanism. The periods of occurrence of reproductive phenophases in D. burchellii were similar to the ones in other climbing species and were mainly related to the species dispersal mode. Phenophases were adjusted to climatic seasonality, thus being influenced by abiotic factors, which is a remarkable feature of seasonal tropical forests.
Acknowledgements
We thank Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for the Master's Scholarship granted to the first author and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the Research Productivity Scholarship granted to the last author.
References
- Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM & Sparovek G (2013) Köppen's classification map for Brazil. Meteorologische Zeitschrift 22: 711-728.
- Ayres M, Ayres Júnior M, Ayres DL & Santos AA (2007) BioEstat - aplicações estatísticas nas áreas das ciências biomédicas. Sociedade Civil Mamirauá, Belém. 364p.
- Bencke CSC & Morellato LPC (2002) Comparação de dois métodos de avaliação da fenologia em plantas, sua interpretação e representação. Revista Brasileira de Botânica 25: 269-275.
- BFG - The Brazil Flora Group (2018) Brazilian flora 2020: innovation and collaboration to meet Target 1 of the Global Strategy for Plant Conservation (GSPC). Rodriguésia 69: 1513-1527.
- Crawley MJ (2007) The R book. Wiley, Chichester. 942p.
- Devlin B & Stephenson AG (1984) Factors that influence the duration of the staminate and pistillate phases of Lobelia cardinalis flowers. Botanical Gazette 145: 323-328.
- DNMET - Departamento Nacional de Meteorologia (1992) Normais climatológicas (1961-1990). SPI/EMBRAPA, Brasília. 84p.
- Domingos-Melo A, Nadia TL & Machado IC (2017) Complex flowers and rare pollinators: Does ant pollination in Ditassa show stable system in Aclepiadoideae (Apocynaceae)? Arthropod-Plant Interactions 11: 339-349.
- Endress PK (1994) Diversity and evolutionary biology of tropical flowers. Cambridge University Press, Cambridge. 511p.
- Endress ME & Bruyns PV (2000) A revised classification of the Apocynaceae s.l The Botanical Review 66: 1-56.
- Endress PK (2016) Development and evolution of extreme synorganization in angiosperm flowers and diversity: a comparison of Apocynaceae and Orchidaceae. Annals of Botany 117: 749-767.
- Fenner M (1998) The phenology of growth and reproduction in plants. Perspectives in Plant Ecology, Evolution and Systematics 1: 78-91.
- Fonseca RS, Campos LAO & Vieira MF (2013) Melittophily and ornithochory in Tilesia baccata (L.f.) Pruski: an Asteraceae of the Atlantic Forest understory with fleshy fruits. Flora 208: 370-380.
- Fontes AL, Valentin-Silva A, Demuner AJ & Vieira MF (2018) Functional morphology and floral odor of Ditassa burchellii (Apocynaceae, Asclepiadoideae), a micromyiophilous species from southeastern Brazil. International Journal of Plant Sciences 179: 450-460.
- Fournier LA & Salas S (1966) Algunas observaciones sobre la dinámica de la floración en el bosque tropical húmedo de Villa Cólon. Revista de Biología Tropical 14: 75-85.
- Fournier LA (1974) Un método cuantitativo para la medición de características fenológicas en árboles. Turrialba 24: 422-423.
- Gentry AH (1974) Flowering phenology and diversity in tropical Bignoniaceae. Biotropica 6: 64-68.
- Gentry AH (1991) The distribution and evolution of climbing species. In: Putz FE & Mooney HA (eds.) The biology of vines. Cambridge University Press, Cambridge. Pp. 3-52.
- Konno TUP (2005) Ditassa R. Br. no Brasil (Asclepiadoideae - Apocynaceae) e revisão taxonômica de Minaria T.U.P. Konno et Rapini. Tese de Doutorado. Universidade de São Paulo, São Paulo. 239p.
- Liede S & Whitehead V (1991) Studies in the pollination biology of Sarcostemma viminale R. Br. sensu lato South African Journal of Botany 57: 115-122.
- Lumer C & Yost SE (1995) The reproductive biology of Vincetoxicum nigrum (L.) Moench (Asclepiadaceae), a Mediterranean weed in New York state. Bulletin of the Torrey Botanical Club 122: 15-23.
- Morellato LPC, Rodrigues RR, Leitão-Filho HF & Joly CA (1989) Estudo comparativo de espécies arbóreas de uma floresta de altitude e floresta mesófila semidecídua na Serra do Japi, Jundiaí, São Paulo. Revista Brasileira de Botânica 12: 85-98.
- Morellato LPC (1992) Sazonalidade e dinâmica dos ecossistemas florestais da Serra do Japi. In: Morellato LPC (ed.) História natural da Serra do Japi: ecologia e preservação de uma área florestal no sudeste do Brasil. Ed. UNICAMP, Campinas. Pp. 98-110.
- Morellato LPC & Leitão-Filho HF (1996) Reproductive phenology of climbers in a southeastern Brazilian forest. Biotropica 28: 180-191.
- Morellato LPC, Alberti LF & Hudson IL (2010) Applications of circular statistics in plant phenology: a case studies approach. In: Keatley M & Hudson IL (eds.) Phenological research: methods for environmental and climate change analysis. Springer, New York. Pp. 339-359.
- Newstrom LE, Frankie GW & Baker HG (1994) A new classification for plant phenology based on flowering patterns in lowland tropical rain forest trees at La Selva, Costa Rica. Biotropica 26: 141-159.
- Ponnuchamy R, Pragasam A, Aravajy S, Patel P, Das L & Anupama K (2013) A floristic study on herbs and climbing plants at Puducherry, South India: an approach to biodiversity conservation and regeneration through eco-restoration. Check List 9: 555-600.
- Primack RB (1985) Longevity of individual flowers. Annual Review of Ecology and Systematics 16: 15-37.
- Putz FE & Windsor DM (1987) Liana phenology on Barro Colorado Island, Panama. Biotropica 19: 334-341.
- Queller DC (1985) Proximate and ultimate causes of low fruit production in Asclepias exaltata Oikos 44: 373-381.
- R Core Team (2016) R: a language and environment for statistical computing. Available at <https://www.R-project.org/>. Access on 10 August 2016.
» https://www.R-project.org/ - Ramírez N & Briceño H (2011) Reproductive phenology of 223 species from four herbaceous-shrubby communities in the Gran Sabana Plateau of Venezuela. AoB Plants 2011: 1-17.
- Rapini A (2012) Taxonomy "under construction": advances in the systematics of Apocynaceae, with emphasis on the Brazilian Asclepiadoideae. Rodriguésia 63: 75-88.
- Richardson TE & Stephenson AG (1989) Pollen removal and pollen deposition affect the duration of the staminate and pistillate phases in Campanula rapunculoides American Journal of Botany 76: 532-538.
- Romaniuc Neto S, Godoi JV, Villagra BLP, Almeida-Scabbia RJ & Melo MMRF (2012) Caracterização florística, fitossociológica e fenológica de trepadeiras de mata ciliar da Fazenda Campininha, Mogi Guaçu, SP, Brasil. Hoehnea 39: 145-155.
- Rossatto DR & Kolb RM (2011) Comportamento fenológico de liana Pyrostegia venusta (Ker Gawl.) Miers (Bignoniaceae) em área de cerradão na Estação Ecológica de Assis, SP, Brasil. Revista Brasileira de Biociências 9: 289-296.
- Rubim P, Nascimento HEM & Morellato LPC (2010) Variações interanuais na fenologia de uma comunidade arbórea de floresta semidecídua no sudeste do Brasil. Acta Botanica Brasilica 24: 756-764.
- Schoen DJ & Ashman TL (1995) The evolution of floral longevity: Resource allocation to maintenance versus construction of repeated parts in modular organisms. Evolution 49: 131-139.
- Silva CA, Vieira MF, Okano RMC & Oliveira LO (2014) Reproductive success and genetic diversity of Psychotria hastisepala (Rubiaceae), in fragmented Atlantic forest, Southeastearn Brazil. Revista de Biología Tropical 62: 309-319.
- Skutch AF (1988) Flowering and seed-production of Fischeria funebris (Asclepiadaceae). Brenesia 30: 13-17.
- Smythe N (1970) Relationships between fruiting seasons and seed dispersal methods in a neotropical forest. The American Naturalist 104: 25-35.
- Sobrinho MS, Tabatinga GM, Machado IC & Lopes AV (2013) Reproductive phenological pattern of Calotropis procera (Apocynaceae), an invasive species in Brazil: annual in native areas; continuous in invaded areas of caatinga. Acta Botanica Brasilica 27: 456-459.
- Spina AP, Ferreira WM & Leitão-Filho HF (2001) Floração, frutificação e síndromes de dispersão de uma comunidade de floresta de brejo na região de Campinas (SP). Acta Botanica Brasilica 15: 349-368.
- Stevenson PR, Castellanos MC, Cortés AI & Link A (2008) Flowering patterns in a seasonal tropical lowland Forest in western Amazonia. Biotropica 40: 559-567.
- Veloso HP, Rangel-Filho ALR & Lima JCA (1991) Classificação da vegetação brasileira adaptada a um sistema universal. IBGE, Rio de Janeiro. 124p.
- Vieira MF & Shepherd GJ (1999) Sistema reprodutivo de Oxypetalum appendiculatum Mart. e Oxypetalum banksii Roem. & Schult. subsp. banksii (Asclepiadaceae). Acta Botanica Brasilica 13: 237-241.
- Vieira MF & Shepherd GJ (2002) Removal and insertion of pollinia in flowers of Oxypetalum (Asclepiadaceae) in southeastern Brazil. Revista de Biología Tropical 50: 37-43.
- Williams RJ, Myers BA, Eamus D & Duff GA (1999) Reproductive phenology of woody species in a North Australian Tropical Savanna. Biotropica 31: 626-636.
- Wyatt R (1981) The reproductive biology of Asclepias tuberosa: II. Factors determining fruit-set. New Phytologist 88: 375-385.
- Wyatt R & Broyles SB (1994) Ecology and evolution of reproduction in milkweeds. Annual Review of Ecology and Systematics 25: 423-441.
- Zar JH (1999) Biostatistical analysis. Prentice-Hall, Upper Saddle River. 663p.
Edited by
Publication Dates
-
Publication in this collection
18 Mar 2019
History
-
Received
28 June 2017 -
Accepted
20 Dec 2017