Abstract
Rodent models in rats, mice, and guinea pigs have been extremely helpful to gain insight into pregnancy physiology and pathologies-related. Moreover, they have allowed understanding the mechanism that links an adverse intrauterine environment with the origin of adult disease. In this regard, the effects of diverse maternal conditions, such as undernutrition, obesity, hypoxia, and hyperandrogenism on placental function and its long-term consequences for the offspring, have been widely analyzed through rodents models involving dietary manipulations, modifications in environmental oxygen, surgical and pharmacological procedures that reduce uteroplacental blood flow and administrations of exogenous testosterone and dihydrotestosterone (DHT) mimicking maternal androgen excess. Both in human and in rodent models, these interventions induce modifications of placental morphology, transport of glucose, amino acid, and fatty acids, steroid synthesis, and signaling pathways control placental function. These changes are associated with the increase of pro-inflammatory and oxidative stress markers. For its part, offspring exhibit alterations in organs involved in metabolic control such as the hypothalamus, adipose tissue, liver, skeletal muscle, and pancreas altering the intake and preferences for certain foods, the metabolism of glucose and lipid, and hormonal function leading to fat accumulation, insulin resistance, fatty liver, dyslipidemia, and elevated glucose levels. Therefore, the present review discusses the evidence emerging from rodent models that relate maternal nutrition, hypoxia, and androgen exposure to the maternal mechanisms that lead to fetal programming and their metabolic consequences in postnatal life.
Keywords:
rodent models; maternal nutrition; hypoxia; and hyperandrogenism
Introduction
Fetal programming or the developmental origin of the health and disease (DOHaD) hypothesis postulate that adverse intrauterine environment induced by deleterious environments or the presence of pathologies during pregnancy trigger fetal adaptations and, in many cases, fetal growth restriction (FGR) and increased risk of developing chronic diseases as early as at adolescence or long-term during adulthood or aging (Perrone et al., 2016Perrone S, Santacroce A, Picardi A, Buonocore G. Fetal programming and early identification of newborns at high risk of free radical-mediated diseases. World J Clin Pediatr. 2016;5(2):172-81. http://dx.doi.org/10.5409/wjcp.v5.i2.172. PMid:27170927.
http://dx.doi.org/10.5409/wjcp.v5.i2.172...
). Among the factors that account for these phenomena are unhealthy maternal habits such as obesogenic diets, smoking, physical inactivity, and psychosocial stress. Complications related to pre-existing health problems or those that appear during pregnancy, like endocrine and metabolic diseases, also induce fetal programming (Perrone et al., 2016Perrone S, Santacroce A, Picardi A, Buonocore G. Fetal programming and early identification of newborns at high risk of free radical-mediated diseases. World J Clin Pediatr. 2016;5(2):172-81. http://dx.doi.org/10.5409/wjcp.v5.i2.172. PMid:27170927.
http://dx.doi.org/10.5409/wjcp.v5.i2.172...
; Marciniak et al., 2017Marciniak A, Patro-Małysza J, Kimber-Trojnar Ż, Marciniak B, Oleszczuk J, Leszczyńska-Gorzelak B. Fetal programming of the metabolic syndrome. Taiwan J Obstet Gynecol. 2017;56(2):133-8. http://dx.doi.org/10.1016/j.tjog.2017.01.001. PMid:28420495.
http://dx.doi.org/10.1016/j.tjog.2017.01...
). Interestingly, maternal obesity, polycystic ovary syndrome (PCOS), gestational diabetes, and preeclampsia are related to abnormal steroid synthesis that increases androgen levels affecting fetal growth and risk of metabolic, reproductive, and psychiatric diseases.
The placenta is the main organ of pregnancy that provides the growing fetus with nutrients, participates in gas exchanges, syntheses almost all peptides and steroid hormones, and removes fetal waste products. A substantial amount of evidence indicates the primary role of the placenta in the mechanism account of fetal programming. In this regard, the changes in the intrauterine environment influence oxygen and nutrient maternal-fetal transfer through alterations of metabolic and inflammatory pathways, modifications in steroid and cytokines synthesis, and lipid and protein oxidation (Sferruzzi-Perri and Camm, 2016Sferruzzi-Perri AN, Camm EJ. The programming power of the placenta. Front Physiol. 2016;7:33. http://dx.doi.org/10.3389/fphys.2016.00033. PMid:27014074.
http://dx.doi.org/10.3389/fphys.2016.000...
). Likewise, face to this environment, the fetus produces a redistribution of fetal blood flow to organs with higher metabolic demands like the heart and brain at the expense of others generating structural changes and regulating gene expression through epigenetic modifications of cell pathways that control the physiology of various organs causing fetal programming (Marciniak et al., 2017Marciniak A, Patro-Małysza J, Kimber-Trojnar Ż, Marciniak B, Oleszczuk J, Leszczyńska-Gorzelak B. Fetal programming of the metabolic syndrome. Taiwan J Obstet Gynecol. 2017;56(2):133-8. http://dx.doi.org/10.1016/j.tjog.2017.01.001. PMid:28420495.
http://dx.doi.org/10.1016/j.tjog.2017.01...
).
Animal models provide invaluable information for elucidating the mechanisms involved in fetal programming because they allow testing controlled the maternal exposure to diverse conditions that include nutritional and oxygen modifications or surgical and pharmacological paradigms that mimic multiple pregnancy pathologies (Warner and Ozanne, 2010Warner MJ, Ozanne SE. Mechanisms involved in the developmental programming of adulthood disease. Biochem J. 2010;427(3):333-47. http://dx.doi.org/10.1042/BJ20091861. PMid:20388123.
http://dx.doi.org/10.1042/BJ20091861...
). Because of their short gestation and lifespan, rodents are helpful and standard models for pregnancy physiology and the consequences for the offspring even more than one generation. Moreover, it is possible to obtain tissue samples from the mother, placenta, fetus, or offspring during any stages of the exposure allowing more insight into the short- and long-term relationships between mother and fetus in the development programming (Warner and Ozanne, 2010Warner MJ, Ozanne SE. Mechanisms involved in the developmental programming of adulthood disease. Biochem J. 2010;427(3):333-47. http://dx.doi.org/10.1042/BJ20091861. PMid:20388123.
http://dx.doi.org/10.1042/BJ20091861...
). In this regard, rats, mice, and guinea pigs are used widely for studies of nutritional interventions, maternal and fetal hypoxia, and prenatal androgen exposure. These rodent models share several similarities with the physiology of human pregnancy and placental function, giving essential information to understand the mechanism that affects fetal growth and development. However, they also show fundamental differences in the anatomy and physiology between them and in comparison to humans including uterine shape, the number of embryos, placental morphology, endocrine function, and metabolic requirements. Then, these differences should keep in mind when interpolating the findings in rodent models into human pregnancy.
Therefore, we will review and discuss the evidence emerging from rodent models that relate maternal nutrition, hypoxia, and androgen exposure to the maternal mechanisms that lead to fetal programming and their consequences in postnatal life.
Differences between rodent and human pregnancy
Pregnancy in rodents has significant differences with humans; the most evident is the litter size (Table 1). In rats, it ranges from 10 to 16 pups; in mice is 6 to 8, and in guinea pigs, usually, the number of pups is 2 to 4 (Andersen et al., 2018Andersen MD, Alstrup AKO, Duvald CS, Mikkelsen EFR, Vendelbo MH, Ovesen PG, Pedersen M. Animal models of fetal medicine and obstetrics. In: Ibeh B, editor. Experimental animal models of human diseases: an effective therapeutic strategy. London: InTech; 2018. http://dx.doi.org/10.5772/intechopen.74038.
http://dx.doi.org/10.5772/intechopen.740...
). In addition, the length of pregnancy varies between species and strains, being the delivery, in rats and mice between gestational day (GD) 19 to 21, whereas in guinea pigs is 60 to 70 days (Andersen et al., 2018Andersen MD, Alstrup AKO, Duvald CS, Mikkelsen EFR, Vendelbo MH, Ovesen PG, Pedersen M. Animal models of fetal medicine and obstetrics. In: Ibeh B, editor. Experimental animal models of human diseases: an effective therapeutic strategy. London: InTech; 2018. http://dx.doi.org/10.5772/intechopen.74038.
http://dx.doi.org/10.5772/intechopen.740...
). According to the degree of maturation of bone, muscle, and nervous system at birth, species can be classified as precocial, giving birth to pups with well-developed sensory and locomotor skills systems like in humans, guinea pigs, and spiny mice. On the other hand, altricial such as the rat, mouse, or hamster, birth underdeveloped, relatively immobile, lack of hair, and closed eyes. Then, the correct selection of the rodent species is critical to early postnatal studies, mainly in the neurodevelopmental field.
Placenta has a maternal portion, the decidua, and a fetal part, the chorion, and it consists of mesenchymal, immune, vascular, and trophoblast cells (cyto-and syncytiotrophoblast). Cytotrophoblasts fuse to form the syncytiotrophoblast, a specialized epithelium, carries out gas exchange and expresses nutrient transporters for glucose, amino acid, and fatty acid. Among the species, there are differences in placental shape, degree of the relationship between the chorion and uterine wall, number of layers of trophoblast, and maternal-fetal interdigitation (villous, trabecular, or labyrinthine)
According to the relationship between the chorion and uterine wall, the placenta is classified as epitheliochorial, in which fetal tissue is closely in contact with maternal uterine epithelium but does not invade it significantly. This type is found in horses, pigs, and ruminants. In contrast, those in which the trophoblast invades in different degrees the uterine wall are: the endotheliochorial that is present in eutherian mammals and carnivores, and it is characterized by a trophoblast invasion up to the level of the basal lamina of maternal endothelial cells. Humans and rodents possess a hemochorial placenta, in which cytotrophoblasts invade and replace endothelial and smooth muscle cells in spiral arteries remodeling from high-resistance/low-capacity to low-resistance/high-capacity vessels facilitating uterine blood flow and placental perfusion (Furukawa et al., 2014Furukawa S, Kuroda Y, Sugiyama A. A comparison of the histological structure of the placenta in experimental animals. J Toxicol Pathol. 2014;27(1):11-8. http://dx.doi.org/10.1293/tox.2013-0060. PMid:24791062.
http://dx.doi.org/10.1293/tox.2013-0060...
) (Figure 1). In guinea pigs and humans, there is only one layer of trophoblasts that separates the maternal blood space from the fetal capillaries; then, it is also classified as hemomonochorial (Figure1a and b). In contrast, in mice and rats, the placenta is hemotrichorial because there are three layers, one of cytotrophoblast and two of syncytiotrophoblasts, between maternal blood space and fetal capillaries (Figure 1c) (Andersen et al., 2018Andersen MD, Alstrup AKO, Duvald CS, Mikkelsen EFR, Vendelbo MH, Ovesen PG, Pedersen M. Animal models of fetal medicine and obstetrics. In: Ibeh B, editor. Experimental animal models of human diseases: an effective therapeutic strategy. London: InTech; 2018. http://dx.doi.org/10.5772/intechopen.74038.
http://dx.doi.org/10.5772/intechopen.740...
).
Schematic representation of placenta from human (a); guinea pig (b); and mouse or rat (c). The inset shows a magnification of the placental barrier.
The branching of the chorion forms the chorionic villi, which is the functional unit because it participates in maternal/ fetal exchange and hormone synthesis. Chorionic villi interdigitate into the intervillous space that receives maternal blood via the spiral arteries (Figure 1a). The placenta of guinea pigs is composed of labyrinthine lobes, where syncytiotrophoblast is embedded in maternal blood. The subplacenta connects the main placenta with the junctional zone and serves as the source of extravillous trophoblasts that invade the endometrium and have endocrine functions (Figure 1b) (Capellini et al., 2011Capellini I, Venditti C, Barton RA. Placentation and maternal investment in mammals. Am Nat. 2011;177(1):86-98. http://dx.doi.org/10.1086/657435. PMid:21087154.
http://dx.doi.org/10.1086/657435...
). In rats and mice, the placenta contains a fetal part that includes the labyrinthine zone, which meets the same exchange function of intervillous space in humans, the junctional zone that participates in hormone synthesis, and the yolk sac, which is not present in humans. The maternal part is formed of the decidua and metrial gland (Figure 1c).
Sex steroids play a central role in pregnancy, regulating immune tolerance, maternal food intake, glucose and lipid metabolism, uterine blood flow, placental angiogenesis and nutrient transport, and the timing of labor. In this regard, steroid receptors, including progesterone, estrogen, glucocorticoids, and androgen, are present in the placenta from mice, rats, and guinea pigs, indicating that steroid action regulates rodent placenta. However, there are more differences than similarities regarding steroid synthesis between humans and rodents (Table 1).
In humans, progesterone is produced at the beginning of pregnancy by corpora lutea under the control of chorionic gonadotropin secreted by the syncytiotrophoblast. From about eight weeks of gestation, progesterone is synthesized exclusively in the placental trophoblast. However, progesterone supporting pregnancy is produced mainly in mice and rats' ovaries (Warshaw et al., 1986Warshaw ML, Johnson DC, Khan I, Eckstein B, Gibori G. Placental secretion of androgens in the rat. Endocrinology. 1986;119(6):2642-8. http://dx.doi.org/10.1210/endo-119-6-2642. PMid:3490962.
http://dx.doi.org/10.1210/endo-119-6-264...
; Strauss et al., 1996Strauss JF 3rd, Martinez F, Kiriakidou M. Placental steroid hormone synthesis: unique features and unanswered questions. Biol Reprod. 1996;54(2):303-11. http://dx.doi.org/10.1095/biolreprod54.2.303. PMid:8788180.
http://dx.doi.org/10.1095/biolreprod54.2...
). On the other hand, guinea pigs, like humans, exhibit a luteo-placental shift in hormone production (Csapo et al., 1981Csapo AI, Puri CP, Tarró S. Relationship between timing of ovariectomy and maintenance of pregnancy in the guinea-pig. Prostaglandins. 1981;22(1):131-40. http://dx.doi.org/10.1016/0090-6980(81)90060-5. PMid:7291595.
http://dx.doi.org/10.1016/0090-6980(81)9...
). For its part, estrogens are produced from androgen precursors aromatized by P450 aromatase in the human placenta. On the contrary, the mouse and rat placenta expresses 17-hydroxylase activities, which is critical to synthesize androgens from progestogens, then can produce de novo androgens that are utilized as substrate by ovarian P450 aromatase to synthesize estrogens. Unlike humans, the placenta does not express aromatase activity in these species.
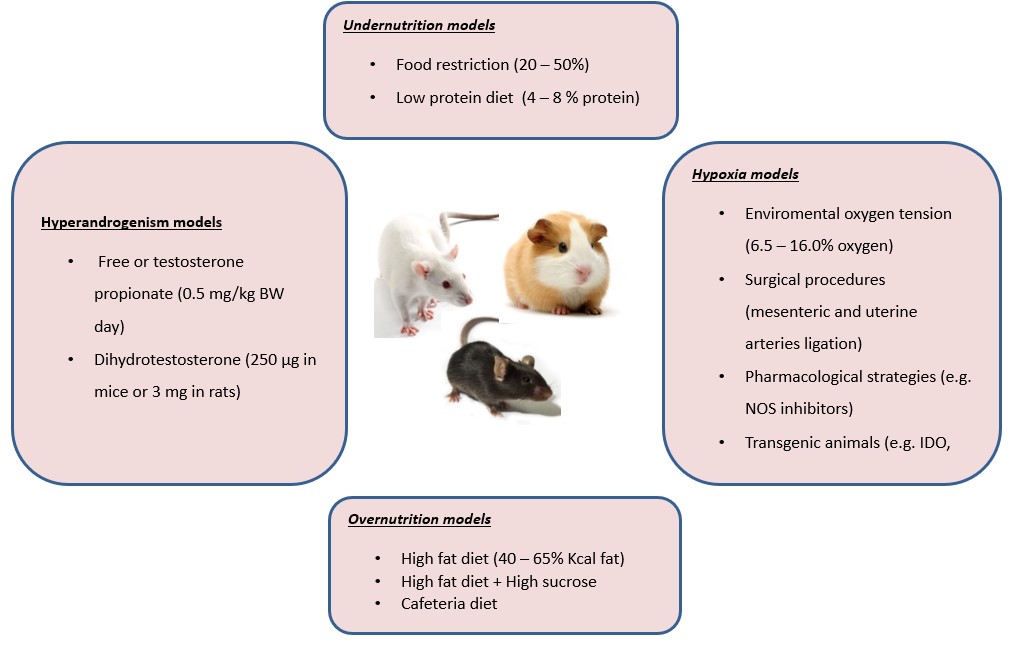
Rodent models of dietary interventions, hypoxia, and hyperandrogenism.
The interventions in rodent models that allow evaluating the effects of nutrition comprise under and over-nutrition with modifications in the energy content and nutrients components. In turn, hypoxia models can be reached through surgical techniques, environmental modifications, and genetic manipulations. On the other hand, gestational hyperandrogenism can be induced pharmacologically with hormones with androgens activity, mainly testosterone and dihydrotestosterone. These interventions are summarized in Figure 2 and will be described in the following sections.
Rodent models of caloric and nutrient manipulations have provided relevant insights into the effects of maternal undernutrition and obesity on placental nutrient transport, diet-associated mechanisms of fetal growth, and its long-term consequences for the offspring. The most extensively dietary rodent models of intrauterine growth restriction (IUGR) have applied a reduction of 20 to 50% of food intake or low protein diet (LPD) ranging from 4 to 8% of total calories instead of 19 to 22% in control chow (Warshaw et al., 1986Warshaw ML, Johnson DC, Khan I, Eckstein B, Gibori G. Placental secretion of androgens in the rat. Endocrinology. 1986;119(6):2642-8. http://dx.doi.org/10.1210/endo-119-6-2642. PMid:3490962.
http://dx.doi.org/10.1210/endo-119-6-264...
). On the other hand, to test nutrient excess, rodent models have involved the exposure, from before and during gestation, to high-fat (HFD) containing 18 to 65% of calories as fat with or without elevated levels of carbohydrates supplied as sucrose into the pellet diet or in drinking water (Williams et al., 2014Williams L, Seki Y, Vuguin PM, Charron MJ. Animal models of in utero exposure to a high fat diet: a review. Biochim Biophys Acta Mol Basis Dis. 2014;1842(3):507-19. http://dx.doi.org/10.1016/j.bbadis.2013.07.006. PMid:23872578.
http://dx.doi.org/10.1016/j.bbadis.2013....
). Another strategy that resembles human dietary habits is the cafeteria diet that includes human snack food items, i.e., cheesecake, chocolates, fried chips, and cookies, into the standard chow (Neri and Edlow, 2015Neri C, Edlow AG. Effects of maternal obesity on fetal programming: molecular approaches. Cold Spring Harb Perspect Med. 2015;6(2):a026591. http://dx.doi.org/10.1101/cshperspect.a026591. PMid:26337113.
http://dx.doi.org/10.1101/cshperspect.a0...
).
Oxygen is critical for mammals because it is a substrate for oxidative metabolism, and oxygen deprivation can damage several tissues. In physiological conditions, oxygen levels are nearly 20 mL O2/dL with slight differences in women due to minor hemoglobin levels, which mediates oxygen transport (Olive et al., 2018Olive EL, Xiao E, Natale DR, Fisher SA. Oxygen and lack of oxygen in fetal and placental development, feto–placental coupling, and congenital heart defects. Birth Defects Res. 2018;110(20):1517-30. http://dx.doi.org/10.1002/bdr2.1430. PMid:30576091.
http://dx.doi.org/10.1002/bdr2.1430...
). Prenatal hypoxia can originate from maternal, placental, and fetal conditions. Kingdom and Kaufmann classified hypoxia as pre-placental if the mother and fetus are affected due to external hypoxic environments (i.e., living at high altitudes, pulmonary hypertension, maternal anemia); uteroplacental due to an abnormal blood flow to these tissue product of occlusions or failed trophoblast invasion; and post-placental when the fetus is hypoxic due to fetal diseases (Kingdom and Kaufmann, 1997Kingdom JCP, Kaufmann P. Oxygen and placental villous development: origins of fetal hypoxia. Placenta. 1997;18(8):613-21. http://dx.doi.org/10.1016/S0143-4004(97)90000-X. PMid:9364596.
http://dx.doi.org/10.1016/S0143-4004(97)...
). It has been postulated that an abnormal trophoblast invasion leads to placental ischemia promotes chronic inflammation, endothelial dysfunction, and increased oxidative stress (Khalil and Granger, 2002Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol. 2002;283(1):R29-45. http://dx.doi.org/10.1152/ajpregu.00762.2001. PMid:12069928.
http://dx.doi.org/10.1152/ajpregu.00762....
; Redman, 2011Redman CWG. Preeclampsia: a multi-stress disorder. Rev Med Interne. 2011;32(Suppl. 1):S41-4. http://dx.doi.org/10.1016/j.revmed.2011.03.331. PMid:21530020.
http://dx.doi.org/10.1016/j.revmed.2011....
). Guinea pigs have been described as an excellent animal model to study the effects of hypoxia on placentation mechanisms because they present deep trophoblast invasion into the maternal decidua and a more extended gestational period than rats or mice species (Morrison et al., 2018Morrison JL, Botting KJ, Darby JRT, David AL, Dyson RM, Gatford KL, Gray C, Herrera EA, Hirst JJ, Kim B, Kind KL, Krause BJ, Matthews SG, Palliser HK, Regnault TRH, Richardson BS, Sasaki A, Thompson LP, Berry MJ. Guinea pig models for translation of the developmental origins of health and disease hypothesis into the clinic. J Physiol. 2018;596(23):5535-69. http://dx.doi.org/10.1113/JP274948. PMid:29633280.
http://dx.doi.org/10.1113/JP274948...
).
An abnormal uteroplacental blood flow has been implicated in preeclampsia's pathogenesis, which is pregnancy-related, defined as new-onset hypertension and proteinuria after 20 weeks of gestation. Preeclampsia is strongly associated with FGR and maternal morbidity because it can cause eclampsia, a severe and high-mortality pregnancy-pathology.
Preeclampsia can evolve in two stages: early-onset preeclampsia due to a defective placenta formation or a reduced uteroplacental blood flow, and late-onset preeclampsia, associated with placental pathologies (Roberts and Hubel, 2009Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30(Suppl. A):32-7. http://dx.doi.org/10.1016/j.placenta.2008.11.009. PMid:19070896.
http://dx.doi.org/10.1016/j.placenta.200...
). Animal models for the study of preeclampsia can be divided into four categories according to their mechanisms, as previously reported: spontaneous animal models of preeclampsia, surgical interventions, pharmacologically/substance-induced models, and transgenic animal (Erlandsson et al., 2016Erlandsson L, Nääv Å, Hennessy A, Vaiman D, Gram M, Åkerström B, Hansson SR. Inventory of novel animal models addressing etiology of preeclampsia in the development of new therapeutic/intervention opportunities. Am J Reprod Immunol. 2016;75(3):402-10. http://dx.doi.org/10.1111/aji.12460. PMid:26685057.
http://dx.doi.org/10.1111/aji.12460...
).
The BPH/5 mouse mimics both early and late-onset preeclampsia, showing elevated blood pressure, endothelial dysfunction, glomerular lesions, proteinuria, and fetoplacental defects (Davisson et al., 2002Davisson RL, Hoffmann DS, Butz GM, Aldape G, Schlager G, Merrill DC, Sethi S, Weiss RM, Bates JN. Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension. 2002;39(2):337-42. http://dx.doi.org/10.1161/hy02t2.102904. PMid:11882569.
http://dx.doi.org/10.1161/hy02t2.102904...
). On the other hand, surgical models involve mechanical occlusions in the uterine artery or the abdominal aorta that reduce uteroplacental perfusion (Li et al., 2012Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Heart Circ Physiol. 2012;303(1):H1-8. http://dx.doi.org/10.1152/ajpheart.00117.2012. PMid:22523250.
http://dx.doi.org/10.1152/ajpheart.00117...
). Pharmacologically induced models have shown that exposure to l-NAME, an inhibitor of nitric oxide synthase (NOS), or arginine vasopressin (AVP) can replicate classic maternal and fetal preeclampsia symptoms (Molnár et al., 1994; Santillan et al., 2014Santillan MK, Santillan DA, Scroggins SM, Min JY, Sandgren JA, Pearson NA, Leslie KK, Hunter SK, Zamba GKD, Gibson-Corley KN, Grobe JL. Vasopressin in preeclampsia: a novel very early human pregnancy biomarker and clinically relevant mouse model. Hypertension. 2014;64(4):852-9. http://dx.doi.org/10.1161/HYPERTENSIONAHA.114.03848. PMid:25001273.
http://dx.doi.org/10.1161/HYPERTENSIONAH...
). Finally, several genetically modified animal models simulate preeclampsia, including knockout (KO) mice for indoleamine 2,3-dioxygenase (IDO) that regulates endothelial-derived relaxing factors (Santillan et al., 2015Santillan MK, Pelham CJ, Ketsawatsomkron P, Santillan DA, Davis DR, Devor EJ, Gibson-Corley KN, Scroggins SM, Grobe JL, Yang B, Hunter SK, Sigmund CD. Pregnant mice lacking indoleamine 2,3-dioxygenase exhibit preeclampsia phenotypes. Physiol Rep. 2015;3(1):e12257. http://dx.doi.org/10.14814/phy2.12257. PMid:25602015.
http://dx.doi.org/10.14814/phy2.12257...
). Moreover, pregnant interleukin-4 (IL-4 -/-) exhibit mild preeclampsia-like symptoms, and interleukin-10 (IL-10 -/-) knockout mice exposed to hypoxia present placental injury, proteinuria, and hypertension.
In humans, increased circulating testosterone levels are consistently found in maternal malnutrition (Maliqueo et al., 2017Maliqueo M, Cruz G, Espina C, Contreras I, García M, Echiburú B, Crisosto N. Obesity during pregnancy affects sex steroid concentrations depending on fetal gender. Int J Obes. 2017;41(11):1636-45. http://dx.doi.org/10.1038/ijo.2017.159. PMid:28676682.
http://dx.doi.org/10.1038/ijo.2017.159...
; Barrett et al., 2019Barrett ES, Mbowe O, Thurston SW, Butts S, Wang C, Nguyen R, Bush N, Redmon JB, Sheshu S, Swan SH, Sathyanarayana S. Predictors of steroid hormone concentrations in early pregnancy: results from a multi-center cohort. Matern Child Health J. 2019;23(3):397-407. http://dx.doi.org/10.1007/s10995-018-02705-0. PMid:30659461.
http://dx.doi.org/10.1007/s10995-018-027...
), gestational diabetes (Villarroel et al., 2017Villarroel C, Salinas A, López P, Kohen P, Rencoret G, Devoto L, Codner E. Pregestational type 2 diabetes and gestational diabetes exhibit different sexual steroid profiles during pregnancy. Gynecol Endocrinol. 2017;33(3):212-7. http://dx.doi.org/10.1080/09513590.2016.1248933. PMid:27898283.
http://dx.doi.org/10.1080/09513590.2016....
), preeclampsia (Troisi et al., 2003Troisi R, Potischman N, Roberts J, Ness R, Crombleholme W, Lykins D, Siiteri P, Hoover RN. Maternal serum oestrogen and androgen concentrations in preeclamptic and uncomplicated pregnancies. Int J Epidemiol. 2003;32(3):455-60. http://dx.doi.org/10.1093/ije/dyg094. PMid:12777436.
http://dx.doi.org/10.1093/ije/dyg094...
), and polycystic ovary syndrome (PCOS) (Maliqueo et al., 2015Maliqueo M, Sundström Poromaa I, Vanky E, Fornes R, Benrick A, Åkerud H, Stridsklev S, Labrie F, Jansson T, Stener-Victorin E. Placental STAT3 signaling is activated in women with polycystic ovary syndrome. Hum Reprod. 2015;30(3):692-700. http://dx.doi.org/10.1093/humrep/deu351. PMid:25609240.
http://dx.doi.org/10.1093/humrep/deu351...
; Caanen et al., 2016Caanen MR, Kuijper EA, Hompes PG, Kushnir MM, Rockwood AL, Meikle WA, Homburg R, Lambalk CB. Mass spectrometry methods measured androgen and estrogen concentrations during pregnancy and in newborns of mothers with polycystic ovary syndrome. Eur J Endocrinol. 2016;174(1):25-32. http://dx.doi.org/10.1530/EJE-15-0699. PMid:26586837.
http://dx.doi.org/10.1530/EJE-15-0699...
). This latter is the most common endocrine-metabolic condition in women characterized by hyperandrogenism and oligo-anovulation, leading to reduced fertility and metabolic disorders like obesity, dyslipidemia, and type 2 diabetes (Boyle and Teede, 2016Boyle JA, Teede HJ. PCOS: refining diagnostic features in PCOS to optimize health outcomes. Nat Rev Endocrinol. 2016;12(11):630-1. http://dx.doi.org/10.1038/nrendo.2016.157. PMid:27636732.
http://dx.doi.org/10.1038/nrendo.2016.15...
). It is noteworthy that elevated maternal androgens are associated with placental dysfunction and FGR (Carlsen et al., 2006Carlsen SM, Jacobsen G, Romundstad P. Maternal testosterone levels during pregnancy are associated with offspring size at birth. Eur J Endocrinol. 2006;155(2):365-70. http://dx.doi.org/10.1530/eje.1.02200. PMid:16868152.
http://dx.doi.org/10.1530/eje.1.02200...
; Kumar et al., 2018Kumar S, Gordon GH, Abbott DH, Mishra JS. Androgens in maternal vascular and placental function: implications for preeclampsia pathogenesis. Reproduction. 2018;156(5):R155-67. http://dx.doi.org/10.1530/REP-18-0278. PMid:30325182.
http://dx.doi.org/10.1530/REP-18-0278...
). Rodent models in rats and mice involving subcutaneous injections of testosterone or dihydrotestosterone (DHT) at the last third of pregnancy have been commonly used to understand the role of androgens on placental function, fetal growth, and long-term consequences for offspring. Models in rats comprise the administration during five consecutive days of 0.5 mg/kg/day of testosterone (GD15 - GD19) or 3.0 mg/day of DHT during four days (GD16 - GD19) (Sathishkumar et al., 2011Sathishkumar K, Elkins R, Chinnathambi V, Gao H, Hankins GDV, Yallampalli C. Prenatal testosterone-induced fetal growth restriction is associated with down-regulation of rat placental amino acid transport. Reprod Biol Endocrinol. 2011;9(1):110. http://dx.doi.org/10.1186/1477-7827-9-110. PMid:21812961.
http://dx.doi.org/10.1186/1477-7827-9-11...
; Dean et al., 2012Dean A, Smith LB, Macpherson S, Sharpe RM. The effect of dihydrotestosterone exposure during or prior to the masculinization programming window on reproductive development in male and female rats. Int J Androl. 2012;35(3):330-9. http://dx.doi.org/10.1111/j.1365-2605.2011.01236.x. PMid:22248293.
http://dx.doi.org/10.1111/j.1365-2605.20...
; Sun et al., 2012Sun M, Maliqueo M, Benrick A, Johansson J, Shao R, Hou L, Jansson T, Wu X, Stener-Victorin E. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring. Am J Physiol Endocrinol Metab. 2012;303(11):E1373-85. http://dx.doi.org/10.1152/ajpendo.00421.2012. PMid:23047983.
http://dx.doi.org/10.1152/ajpendo.00421....
). For its part, mice models have used subcutaneous administration of 250 μg of DHT from GD16.5 - 18.5 (Fornes et al., 2017Fornes R, Maliqueo M, Hu M, Hadi L, Jimenez-Andrade JM, Ebefors K, Nyström J, Labrie F, Jansson T, Benrick A, Stener-Victorin E. The effect of androgen excess on maternal metabolism, placental function and fetal growth in obese dams. Sci Rep. 2017;7(1):8066. http://dx.doi.org/10.1038/s41598-017-08559-w. PMid:28808352.
http://dx.doi.org/10.1038/s41598-017-085...
). Moreover, this latter has been combined with the administration of high fat and high sucrose diet to understand the relationship between endocrine abnormalities and metabolic disorders (Risal et al., 2019Risal S, Pei Y, Lu H, Manti M, Fornes R, Pui HP, Zhao Z, Massart J, Ohlsson C, Lindgren E, Crisosto N, Maliqueo M, Echiburu B, Ladron de Guevara A, Sir-Petermann T, Larsson H, Rosenqvist MA, Cesta CE, Benrick A, Deng Q, Stener-Victorin E. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med. 2019;25(12):1894-904. http://dx.doi.org/10.1038/s41591-019-0666-1. PMid:31792459.
http://dx.doi.org/10.1038/s41591-019-066...
).
Placental adaptations in rodent models of dietary interventions, hypoxia, and hyperandrogenism
Food and chronic protein restrictions in rats and mice reduced maternal weight gain at the last third of pregnancy; and the placental and fetal weights (Jansson et al., 2006Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol. 2006;576(Pt 3):935-46. http://dx.doi.org/10.1113/jphysiol.2006.116509. PMid:16916910.
http://dx.doi.org/10.1113/jphysiol.2006....
; Gonzalez et al., 2016Gonzalez PN, Gasperowicz M, Barbeito-Andrés J, Klenin N, Cross JC, Hallgrímsson B. Chronic protein restriction in mice impacts placental function and maternal body weight before fetal growth. PLoS One. 2016;11(3):e0152227. http://dx.doi.org/10.1371/journal.pone.0152227. PMid:27018791.
http://dx.doi.org/10.1371/journal.pone.0...
; Connor et al., 2020Connor KL, Kibschull M, Matysiak-Zablocki E, Nguyen TT-TN, Matthews SG, Lye SJ, Bloise E. Maternal malnutrition impacts placental morphology and transporter expression: an origin for poor offspring growth. J Nutr Biochem. 2020;78:108329. http://dx.doi.org/10.1016/j.jnutbio.2019.108329. PMid:32004932.
http://dx.doi.org/10.1016/j.jnutbio.2019...
). Similarly, in C57BL/6J mice, it has been reported that four days of exposure to hypoxia (10.5% O2) at mid-pregnancy caused a decrease in the number of viable pups and a reduction of 36% in birth weight at gestational day 18.5 without changes in placental weight (Cuffe et al., 2014Cuffe JSM, Walton SL, Singh RR, Spiers JG, Bielefeldt-Ohmann H, Wilkinson L, Little MH, Moritz KM. Mid- to late term hypoxia in the mouse alters placental morphology, glucocorticoid regulatory pathways and nutrient transporters in a sex-specific manner. J Physiol. 2014;592(14):3127-41. http://dx.doi.org/10.1113/jphysiol.2014.272856. PMid:24801305.
http://dx.doi.org/10.1113/jphysiol.2014....
). In rat models, it has been reported that three days of hypoxia (12% O2) from GD18 until GD21 can induce a reduction of 10% in birth weight without changes in litter size (Thaete et al., 2004Thaete LG, Dewey ER, Neerhof MG. Endothelin and the regulation of uterine and placental perfusion in hypoxia-induced fetal growth restriction. J Soc Gynecol Investig. 2004;11(1):16-21. http://dx.doi.org/10.1016/j.jsgi.2003.07.001. PMid:14706678.
http://dx.doi.org/10.1016/j.jsgi.2003.07...
). Similarly, hypoxia levels for a longer period, starting from GD14 until GD20, can induce a reduction of 20% in weight (Jakoubek et al., 2008Jakoubek V, Bíbová J, Herget J, Hampl V. Chronic hypoxia increases fetoplacental vascular resistance and vasoconstrictor reactivity in the rat. Am J Physiol Heart Circ Physiol. 2008;294(4):H1638-44. http://dx.doi.org/10.1152/ajpheart.01120.2007. PMid:18310520.
http://dx.doi.org/10.1152/ajpheart.01120...
). Even more, hypoxia for 11 days causes a severe FGR with a decrease of 30% fetal-weight with smaller litter size and lower placental weight (Huang et al., 2004Huang S-TJ, Vo KCT, Lyell DJ, Faessen GH, Tulac S, Tibshirani R, Giaccia AJ, Giudice LC. Developmental response to hypoxia. FASEB J. 2004;18(12):1348-65. http://dx.doi.org/10.1096/fj.03-1377com. PMid:15333578.
http://dx.doi.org/10.1096/fj.03-1377com...
). In guinea pigs, hypoxia-induced by 10.5% O2 reduced fetal weight but increased placental weight (Thompson et al., 2016Thompson LP, Pence L, Pinkas G, Song H, Telugu BP. Placental hypoxia during early pregnancy causes maternal hypertension and placental insufficiency in the hypoxic guinea pig model. Biol Reprod. 2016;95(6):128. http://dx.doi.org/10.1095/biolreprod.116.142273. PMid:27806942.
http://dx.doi.org/10.1095/biolreprod.116...
). Meanwhile, in rats, maternal exposure to testosterone or DHT and in mice to DHT produce lower fetal weight and reduced placental size (Sun et al., 2012Sun M, Maliqueo M, Benrick A, Johansson J, Shao R, Hou L, Jansson T, Wu X, Stener-Victorin E. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring. Am J Physiol Endocrinol Metab. 2012;303(11):E1373-85. http://dx.doi.org/10.1152/ajpendo.00421.2012. PMid:23047983.
http://dx.doi.org/10.1152/ajpendo.00421....
; Risal et al., 2019Risal S, Pei Y, Lu H, Manti M, Fornes R, Pui HP, Zhao Z, Massart J, Ohlsson C, Lindgren E, Crisosto N, Maliqueo M, Echiburu B, Ladron de Guevara A, Sir-Petermann T, Larsson H, Rosenqvist MA, Cesta CE, Benrick A, Deng Q, Stener-Victorin E. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med. 2019;25(12):1894-904. http://dx.doi.org/10.1038/s41591-019-0666-1. PMid:31792459.
http://dx.doi.org/10.1038/s41591-019-066...
).
The effects on fetal growth observed by nutritional interventions, hypoxia, and hyperandrogenism are likely related to placental modifications due to reduced uteroplacental blood flow and nutrient transport. Thus, it is common to observe smaller placentas with alterations in specific placental zones that account for the modifications in nutrient transport and hormone synthesis. Both under and overnutrition-induced diets mainly affect the placental junctional zone, reducing the number of glycogen trophoblast cells, which meets a nutritional function. Moreover, rats fed with LPD increased density and number of spongiotrophoblast and giant cells producing prolactin-like, and lactogens hormones that maintain progesterone secretion from the corporea lutea and cytokines that regulate placental functions in the junctional zone were observed (Vomhof-DeKrey et al., 2016Vomhof-DeKrey E, Darland D, Ghribi O, Bundy A, Roemmich J, Claycombe K. Maternal low protein diet leads to placental angiogenic compensation via dysregulated M1/M2 macrophages and TNFα expression in Sprague-Dawley rats. J Reprod Immunol. 2016;118:9-17. http://dx.doi.org/10.1016/j.jri.2016.08.009. PMid:27596280.
http://dx.doi.org/10.1016/j.jri.2016.08....
). These data indicate that maternal undernutrition launches nutritional and endocrine compensatory mechanisms to pregnancy maintenance and avoid growth restriction; however, these seem to be insufficient considering that reduced fetal growth. LPD in rats could also affect steroid synthesis because it decreases the gene expression of 17-beta-hydroxysteroid dehydrogenase type 2 in the junctional zone in both female and male fetuses (Gao et al., 2012Gao H, Yallampalli U, Yallampalli C. Gestational protein restriction reduces expression of Hsd17b2 in rat placental labyrinth. Biol Reprod. 2012;87(3):68. http://dx.doi.org/10.1095/biolreprod.112.100479. PMid:22837477.
http://dx.doi.org/10.1095/biolreprod.112...
).
In guinea pigs, prenatal hypoxia (10.5% O2) during early gestation inhibits cytotrophoblast invasion of spiral arteries that affects placentation increasing maternal blood pressure that resembles changes observed in women with preeclampsia (Turan et al., 2017Turan S, Aberdeen GW, Thompson LP. Chronic hypoxia alters maternal uterine and fetal hemodynamics in the full-term pregnant Guinea pig. Am J Physiol Regul Integr Comp Physiol. 2017;313(4):R330-9. http://dx.doi.org/10.1152/ajpregu.00056.2017. PMid:28679680.
http://dx.doi.org/10.1152/ajpregu.00056....
). On the other hand, it was observed that hypoxia also induced a compensatory blood vessel expansion in the guinea pig labyrinth in response to inhibited upstream arterial remodeling (Thompson et al., 2016Thompson LP, Pence L, Pinkas G, Song H, Telugu BP. Placental hypoxia during early pregnancy causes maternal hypertension and placental insufficiency in the hypoxic guinea pig model. Biol Reprod. 2016;95(6):128. http://dx.doi.org/10.1095/biolreprod.116.142273. PMid:27806942.
http://dx.doi.org/10.1095/biolreprod.116...
). Interestingly, in CD1 mice, placentas of female fetuses responded differently to maternal hypoxia, showing reduced placental labyrinth blood spaces, which was not observed in male placenta (Cuffe et al., 2014Cuffe JSM, Walton SL, Singh RR, Spiers JG, Bielefeldt-Ohmann H, Wilkinson L, Little MH, Moritz KM. Mid- to late term hypoxia in the mouse alters placental morphology, glucocorticoid regulatory pathways and nutrient transporters in a sex-specific manner. J Physiol. 2014;592(14):3127-41. http://dx.doi.org/10.1113/jphysiol.2014.272856. PMid:24801305.
http://dx.doi.org/10.1113/jphysiol.2014....
). Overall, maternal exposure to hypoxia or surgical restriction of uterine blood flow induce placental hypoxia, evidenced by hypoxyprobe staining or protein expression of hypoxia-inducible factor 1α (HIF1α), increased apoptosis, reactive oxygen species (ROS), and mitochondrial or endoplasmic reticulum stress (Siragher and Sferruzzi-Perri, 2021Siragher E, Sferruzzi-Perri AN. Placental hypoxia: what have we learnt from small animal models? Placenta. 2021;113:29-47. http://dx.doi.org/10.1016/j.placenta.2021.03.018. PMid:34074553.
http://dx.doi.org/10.1016/j.placenta.202...
). Placental transcriptome analysis in mice exposed to 10.5% oxygen from GD 14.5 to 18.5 showed an altered expression of genes involved in vasculature development, hemostasis, adhesion, and extracellular matrix despite no changes in placental and fetal weights (Chu et al., 2019Chu A, Casero D, Thamotharan S, Wadehra M, Cosi A, Devaskar SU. The placental transcriptome in late gestational hypoxia resulting in murine intrauterine growth restriction parallels increased risk of adult cardiometabolic disease. Sci Rep. 2019;9(1):1243. http://dx.doi.org/10.1038/s41598-018-37627-y. PMid:30718791.
http://dx.doi.org/10.1038/s41598-018-376...
).
In this same line, maternal LPD decreased M1 macrophages and increased M2, producing TNFα, and invariant natural killer T (iNKT) cells, secreting significant amounts of cytokines in response to glycolipids, also suggesting a pro-inflammatory state. Similarly, HFD (45% kcal as fat) in pregnant mice significantly decreased placenta labyrinth thickness, where maternal-fetal exchange occurs, showing lower cell proliferation, increased macrophage activation, and elevated pro-inflammatory cytokine gene expression (Kim et al., 2014Kim DW, Young SL, Grattan DR, Jasoni CL. Obesity during pregnancy disrupts placental morphology, cell proliferation, and inflammation in a sex-specific manner across gestation in the mouse. Biol Reprod. 2014;90(6):130. http://dx.doi.org/10.1095/biolreprod.113.117259. PMid:24829026.
http://dx.doi.org/10.1095/biolreprod.113...
).
Along with the changes described above, LPD overexpressed placental angiogenic factors such as fibroblast growth factor 2 (FGF2), vascular endothelial growth factor receptor 1 (VEGFR1), and insulin-like growth factor 2 (IGF2) contributes to placental vascular defects because did not revert the FGR (Vomhof-DeKrey et al., 2016Vomhof-DeKrey E, Darland D, Ghribi O, Bundy A, Roemmich J, Claycombe K. Maternal low protein diet leads to placental angiogenic compensation via dysregulated M1/M2 macrophages and TNFα expression in Sprague-Dawley rats. J Reprod Immunol. 2016;118:9-17. http://dx.doi.org/10.1016/j.jri.2016.08.009. PMid:27596280.
http://dx.doi.org/10.1016/j.jri.2016.08....
). Similarly, maternal undernutrition downregulates VEGF and several antioxidant enzymatic systems that could compromise oxygen delivery and increase oxidative stress, mainly in the male fetus and moderately in females (Phuthong et al., 2020Phuthong S, Reyes-Hernández CG, Rodríguez-Rodríguez P, Ramiro-Cortijo D, Gil-Ortega M, González-Blázquez R, González MC, López de Pablo AL, Arribas SM. Sex differences in placental protein expression and efficiency in a rat model of fetal programming induced by maternal undernutrition. Int J Mol Sci. 2020;22(1):237. http://dx.doi.org/10.3390/ijms22010237. PMid:33379399.
http://dx.doi.org/10.3390/ijms22010237...
). On the other hand, hypoxia models increase placental expression of angiogenesis factors, including Flt1 and VEGF (Thompson et al., 2016Thompson LP, Pence L, Pinkas G, Song H, Telugu BP. Placental hypoxia during early pregnancy causes maternal hypertension and placental insufficiency in the hypoxic guinea pig model. Biol Reprod. 2016;95(6):128. http://dx.doi.org/10.1095/biolreprod.116.142273. PMid:27806942.
http://dx.doi.org/10.1095/biolreprod.116...
; Natale et al., 2018Natale BV, Mehta P, Vu P, Schweitzer C, Gustin K, Kotadia R, Natale DRC. Reduced Uteroplacental Perfusion Pressure (RUPP) causes altered trophoblast differentiation and pericyte reduction in the mouse placenta labyrinth. Sci Rep. 2018;8(1):17162. http://dx.doi.org/10.1038/s41598-018-35606-x. PMid:30464252.
http://dx.doi.org/10.1038/s41598-018-356...
). In the same way, mice fed with a highly palatable obesogenic diet supplemented with sweetened condensed produced an impaired labyrinth development associated with dysregulation of transcripts and pathway interactions related to placental vasculature and structure (Barros Mucci et al., 2020Barros Mucci D, Kusinski LC, Wilsmore P, Loche E, Pantaleão LC, Ashmore TJ, Blackmore HL, Fernandez-Twinn DS, Carmo MDGTD, Ozanne SE. Impact of maternal obesity on placental transcriptome and morphology associated with fetal growth restriction in mice. Int J Obes. 2020;44(5):1087-96. http://dx.doi.org/10.1038/s41366-020-0561-3. PMid:32203108.
http://dx.doi.org/10.1038/s41366-020-056...
). Interestingly, HFD also induced labyrinth placental endothelial damage in mice, which seems to be associated with oxidative stress because treatment with quercetin, an antioxidant, reverted this effect (Liang et al., 2010Liang C, DeCourcy K, Prater MR. High-saturated-fat diet induces gestational diabetes and placental vasculopathy in C57BL/6 mice. Metabolism. 2010;59(7):943-50. http://dx.doi.org/10.1016/j.metabol.2009.10.015. PMid:20022072.
http://dx.doi.org/10.1016/j.metabol.2009...
).
Pregnancy interventions have important implications in the pathways regulating placental nutrient transport, causing maternal-to-fetal nutrient transfer modifications. In this regard, rats fed with an isocaloric diet containing 4% of protein resulted in placental down-regulation of the system A and L amino acid transporters that uptake alanine, serine, proline, glycine, tryptophan, and neutral and branched-chain amino acids. However, glucose transport seems not to be affected despite the changes in gene expression of facilitated glucose transporter member 1 (Slc2a1) (Coan et al., 2010Coan PM, Vaughan OR, Sekita Y, Finn SL, Burton GJ, Constancia M, Fowden AL. Adaptations in placental phenotype support fetal growth during undernutrition of pregnant mice. J Physiol. 2010;588(3):527-38. http://dx.doi.org/10.1113/jphysiol.2009.181214. PMid:19948659.
http://dx.doi.org/10.1113/jphysiol.2009....
). Regarding the placental fatty acid transporters, in food-restricted mice, this was dependent on fetal sex, showing that protein expression of fatty acid transporter protein (FATP) 4 and gene expression of the fatty acid-binding protein plasma membrane (fabppm) were higher, and fatty acid translocase (Fat/Cd36) lower in male placentas. In contrast, endothelial lipase (El) was higher in females than in male placentas (Connor et al., 2020Connor KL, Kibschull M, Matysiak-Zablocki E, Nguyen TT-TN, Matthews SG, Lye SJ, Bloise E. Maternal malnutrition impacts placental morphology and transporter expression: an origin for poor offspring growth. J Nutr Biochem. 2020;78:108329. http://dx.doi.org/10.1016/j.jnutbio.2019.108329. PMid:32004932.
http://dx.doi.org/10.1016/j.jnutbio.2019...
).
Similarly, Sprague-Dawley rats fed with 40% calories as fat show placental lipid accumulation associated with lower mRNA levels of crucial lipid transport and storage genes (Cd36, Fabp3, Pparg, Plin2, and Irs1) (Louwagie et al., 2018Louwagie EJ, Larsen TD, Wachal AL, Baack ML. Placental lipid processing in response to a maternal high-fat diet and diabetes in rats. Pediatr Res. 2018;83(3):712-22. http://dx.doi.org/10.1038/pr.2017.288. PMid:29166372.
http://dx.doi.org/10.1038/pr.2017.288...
). These changes were concomitant with the inhibition of placental signaling for key metabolic pathways, including insulin, mammalian target of rapamycin (mTOR), and signal transducer and activator of transcription 3 (STAT3) (Rosario et al., 2011Rosario FJ, Jansson N, Kanai Y, Prasad PD, Powell TL, Jansson T. Maternal protein restriction in the rat inhibits placental insulin, mTOR, and STAT3 signaling and down-regulates placental amino acid transporters. Endocrinology. 2011;152(3):1119-29. http://dx.doi.org/10.1210/en.2010-1153. PMid:21285325.
http://dx.doi.org/10.1210/en.2010-1153...
). On the other hand, maternal obesity-induced by HFD (41 kcal% fat) supplemented with 20% of sucrose showed increased activity and protein expression of the system A and L amino acid transport in the placental barrier, in addition to higher expression of glucose transporters, which was associated with fetal overgrowth (Rosario et al., 2015Rosario FJ, Kanai Y, Powell TL, Jansson T. Increased placental nutrient transport in a novel mouse model of maternal obesity with fetal overgrowth. Obesity. 2015;23(8):1663-70. http://dx.doi.org/10.1002/oby.21165. PMid:26193061.
http://dx.doi.org/10.1002/oby.21165...
). Interestingly, signaling pathways are associated with energy metabolism, such as mTOR, insulin, growth factors, and leptin was overactivated, explaining the stimulation of placental nutrient transporters (Rosario et al., 2016Rosario FJ, Powell TL, Jansson T. Activation of placental insulin and mTOR signaling in a mouse model of maternal obesity associated with fetal overgrowth. Am J Physiol Regul Integr Comp Physiol. 2016;310(1):R87-93. http://dx.doi.org/10.1152/ajpregu.00356.2015. PMid:26491103.
http://dx.doi.org/10.1152/ajpregu.00356....
).
Meanwhile, 13% oxygen during GD14 to GD19 in C57BL/6 mice increased placental glucose transfer without affecting the amino acid transfer. On the other hand, 10% oxygen reduced amino acid transport without modifications in glucose transport (Cuffe et al., 2014Cuffe JSM, Walton SL, Singh RR, Spiers JG, Bielefeldt-Ohmann H, Wilkinson L, Little MH, Moritz KM. Mid- to late term hypoxia in the mouse alters placental morphology, glucocorticoid regulatory pathways and nutrient transporters in a sex-specific manner. J Physiol. 2014;592(14):3127-41. http://dx.doi.org/10.1113/jphysiol.2014.272856. PMid:24801305.
http://dx.doi.org/10.1113/jphysiol.2014....
). In addition, in placentas from CD1 mice exposed to hypoxia, mRNA expression of Glut1, Igf2 and Igf1r was reduced only in female placentas (Cuffe et al., 2014Cuffe JSM, Walton SL, Singh RR, Spiers JG, Bielefeldt-Ohmann H, Wilkinson L, Little MH, Moritz KM. Mid- to late term hypoxia in the mouse alters placental morphology, glucocorticoid regulatory pathways and nutrient transporters in a sex-specific manner. J Physiol. 2014;592(14):3127-41. http://dx.doi.org/10.1113/jphysiol.2014.272856. PMid:24801305.
http://dx.doi.org/10.1113/jphysiol.2014....
). These changes were associated with alterations in the insulin-IGF signaling pathway in the labyrinth zone. Interestingly, the lowest oxygen levels produced a higher FGR (Higgins et al., 2016Higgins JS, Vaughan OR, Fernandez de Liger E, Fowden AL, Sferruzzi-Perri AN. Placental phenotype and resource allocation to fetal growth are modified by the timing and degree of hypoxia during mouse pregnancy. J Physiol. 2016;594(5):1341-56. http://dx.doi.org/10.1113/JP271057. PMid:26377136.
http://dx.doi.org/10.1113/JP271057...
). Moreover, increased Akt-mTOR signaling in the hypoxic placentas consistent with heavier placenta was also observed (Matheson et al., 2016Matheson H, Veerbeek JHW, Charnock-Jones DS, Burton GJ, Yung HW. Morphological and molecular changes in the murine placenta exposed to normobaric hypoxia throughout pregnancy. J Physiol. 2016;594(5):1371-88. http://dx.doi.org/10.1113/JP271073. PMid:26278110.
http://dx.doi.org/10.1113/JP271073...
). In addition, another study that analyzed placental transcriptome analysis confirms these observations, including also, MAPK and genes associated with inflammatory responses (Chu et al., 2019Chu A, Casero D, Thamotharan S, Wadehra M, Cosi A, Devaskar SU. The placental transcriptome in late gestational hypoxia resulting in murine intrauterine growth restriction parallels increased risk of adult cardiometabolic disease. Sci Rep. 2019;9(1):1243. http://dx.doi.org/10.1038/s41598-018-37627-y. PMid:30718791.
http://dx.doi.org/10.1038/s41598-018-376...
). In the same way, AMPK was activated in the uterine artery and the labyrinth zone in response to 10% hypoxia (Skeffington et al., 2016Skeffington KL, Higgins JS, Mahmoud AD, Evans AM, Sferruzzi-Perri AN, Fowden AL, Yung HW, Burton GJ, Giussani DA, Moore LG. Hypoxia, AMPK activation and uterine artery vasoreactivity. J Physiol. 2016;594(5):1357-69. http://dx.doi.org/10.1113/JP270995. PMid:26110512.
http://dx.doi.org/10.1113/JP270995...
)
Testosterone during gestation leads to reduced placental system A amino acid transport activity, which seems to be associated with the downregulating of amino acid transporter slc38a2/Snat2. On the other hand, testosterone exposure did not modify glucose transport capacity (Sathishkumar et al., 2011Sathishkumar K, Elkins R, Chinnathambi V, Gao H, Hankins GDV, Yallampalli C. Prenatal testosterone-induced fetal growth restriction is associated with down-regulation of rat placental amino acid transport. Reprod Biol Endocrinol. 2011;9(1):110. http://dx.doi.org/10.1186/1477-7827-9-110. PMid:21812961.
http://dx.doi.org/10.1186/1477-7827-9-11...
). Although alterations in nutrient transport were not related to mTOR signaling, as in the case of dietary modifications or hypoxia, overactivation of the STAT3 pathway was observed (Hu et al., 2015Hu M, Richard JE, Maliqueo M, Kokosar M, Fornes R, Benrick A, Jansson T, Ohlsson C, Wu X, Skibicka KP, Stener-Victorin E. Maternal testosterone exposure increases anxiety-like behavior and impacts the limbic system in the offspring. Proc Natl Acad Sci USA. 2015;112(46):14348-53. http://dx.doi.org/10.1073/pnas.1507514112. PMid:26578781.
http://dx.doi.org/10.1073/pnas.150751411...
). Interestingly, testosterone exposure also leads to alterations in the expression of estrogen and androgen receptors and 17-beta-hydroxysteroid dehydrogenase type 2 (17β-HSD2) that suggest an altered placental steroid signaling and steroidogenesis (Sun et al., 2012Sun M, Maliqueo M, Benrick A, Johansson J, Shao R, Hou L, Jansson T, Wu X, Stener-Victorin E. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring. Am J Physiol Endocrinol Metab. 2012;303(11):E1373-85. http://dx.doi.org/10.1152/ajpendo.00421.2012. PMid:23047983.
http://dx.doi.org/10.1152/ajpendo.00421....
). However, of these models is not possible to dissect the role of estrogen because testosterone, under the action of P450 aromatase, is aromatized to estradiol. To address this dilemma, non-aromatizable androgens such as dihydrotestosterone (DHT) or blocking androgen or estrogen action like flutamide or tamoxifen, respectively, have been used (Walters, 2016Walters KA. Androgens in polycystic ovary syndrome: lessons from experimental models. Curr Opin Endocrinol Diabetes Obes. 2016;23(3):257-63. http://dx.doi.org/10.1097/MED.0000000000000245. PMid:26866639.
http://dx.doi.org/10.1097/MED.0000000000...
). In this regard, it has been observed that in mice, the administration of 250 µg of DHT at late pregnancy reduced placental and fetal weight affecting androgen and estrogen receptor expression similarly to testosterone administration (Fornes et al., 2017Fornes R, Maliqueo M, Hu M, Hadi L, Jimenez-Andrade JM, Ebefors K, Nyström J, Labrie F, Jansson T, Benrick A, Stener-Victorin E. The effect of androgen excess on maternal metabolism, placental function and fetal growth in obese dams. Sci Rep. 2017;7(1):8066. http://dx.doi.org/10.1038/s41598-017-08559-w. PMid:28808352.
http://dx.doi.org/10.1038/s41598-017-085...
; Risal et al., 2019Risal S, Pei Y, Lu H, Manti M, Fornes R, Pui HP, Zhao Z, Massart J, Ohlsson C, Lindgren E, Crisosto N, Maliqueo M, Echiburu B, Ladron de Guevara A, Sir-Petermann T, Larsson H, Rosenqvist MA, Cesta CE, Benrick A, Deng Q, Stener-Victorin E. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med. 2019;25(12):1894-904. http://dx.doi.org/10.1038/s41591-019-0666-1. PMid:31792459.
http://dx.doi.org/10.1038/s41591-019-066...
).
Fetal programming in rodent models of dietary interventions, hypoxia, and hyperandrogenism
The placental changes originated from maternal dietary interventions, hypoxia, and elevated androgen levels lead to adaptations that redistribute fetal blood flow to ensure oxygen and nutrient supplies to organs with higher metabolic demands like the heart and brain at the expense of others. However, these changes can induce modifications and damage in specific organs that generate, among others, metabolic and cardiovascular disorders (Peeters et al., 1979Peeters LLH, Sheldon RE, Jones MD Jr, Makowski EL, Meschia G. Blood flow to fetal organs as a function of arterial oxygen content. Am J Obstet Gynecol. 1979;135(5):637-46. http://dx.doi.org/10.1016/S0002-9378(16)32989-1. PMid:507116.
http://dx.doi.org/10.1016/S0002-9378(16)...
; Fajersztajn and Veras, 2017Fajersztajn L, Veras MM. Hypoxia: from placental development to fetal programming. Birth Defects Res. 2017;109(17):1377-85. http://dx.doi.org/10.1002/bdr2.1142. PMid:29105382.
http://dx.doi.org/10.1002/bdr2.1142...
).
Rodent models of maternal undernutrition have demonstrated that offspring showing reduced pancreatic β cells, insulin resistance, alterations in the regulatory mechanisms that favor energy storage after birth, despite adequate nutrients availability, resulting in obesity, diabetes, and various metabolic disorders (Hales and Barker, 2001Hales CN, Barker DJP. The thrifty phenotype hypothesis. Br Med Bull. 2001;60(1):5-20. http://dx.doi.org/10.1093/bmb/60.1.5. PMid:11809615.
http://dx.doi.org/10.1093/bmb/60.1.5...
; Marciniak et al., 2017Marciniak A, Patro-Małysza J, Kimber-Trojnar Ż, Marciniak B, Oleszczuk J, Leszczyńska-Gorzelak B. Fetal programming of the metabolic syndrome. Taiwan J Obstet Gynecol. 2017;56(2):133-8. http://dx.doi.org/10.1016/j.tjog.2017.01.001. PMid:28420495.
http://dx.doi.org/10.1016/j.tjog.2017.01...
). Obesity in offspring born to food-restricted mothers results from hyperphagia and adipocyte dysfunction. Maternal undernutrition induces higher hypothalamic expression of the orexigenic peptides agouti-related protein (AgRP) and neuropeptide Y (NPY) and lowers gene expression for the anorexigenic peptide proopiomelanocortin (Pomc) (Vickers et al., 2000Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279(1):E83-7. http://dx.doi.org/10.1152/ajpendo.2000.279.1.E83. PMid:10893326.
http://dx.doi.org/10.1152/ajpendo.2000.2...
; Fukami et al., 2012Fukami T, Sun X, Li T, Desai M, Ross M. Mechanism of programmed obesity in intrauterine fetal growth restricted offspring: paradoxically enhanced appetite stimulation in fed and fasting states. Reprod Sci. 2012;19(4):423-30. http://dx.doi.org/10.1177/1933719111424448. PMid:22344733.
http://dx.doi.org/10.1177/19337191114244...
). Interestingly, LPD throughout pregnancy enhances the preference for fatty foods in the offspring, which is associated with increased motivation for food reward and an altered expression pattern of opioid receptors and other reward-related genes in the nucleus accumbens and other structures of the brain (Bellinger et al., 2004Bellinger L, Lilley C, Langley-Evans SC. Prenatal exposure to a maternal low-protein diet programmes a preference for high-fat foods in the young adult rat. Br J Nutr. 2004;92(3):513-20. http://dx.doi.org/10.1079/BJN20041224. PMid:15469656.
http://dx.doi.org/10.1079/BJN20041224...
; Vucetic et al., 2010Vucetic Z, Totoki K, Schoch H, Whitaker KW, Hill-Smith T, Lucki I, Reyes TM. Early life protein restriction alters dopamine circuitry. Neuroscience. 2010;168(2):359-70. http://dx.doi.org/10.1016/j.neuroscience.2010.04.010. PMid:20394806.
http://dx.doi.org/10.1016/j.neuroscience...
; Alves et al., 2015Alves MB, Dalle Molle R, Desai M, Ross MG, Silveira PP. Increased palatable food intake and response to food cues in intrauterine growth-restricted rats are related to tyrosine hydroxylase content in the orbitofrontal cortex and nucleus accumbens. Behav Brain Res. 2015;287:73-81. http://dx.doi.org/10.1016/j.bbr.2015.03.019. PMid:25796489.
http://dx.doi.org/10.1016/j.bbr.2015.03....
).
Maternal LPD during gestation and lactation affects adipose tissue by reducing the adipocyte size of rat offspring. In contrast, rat offspring from food-restricted dams showed hypertrophic adipocytes. Moreover, increased catecholamine levels and adrenoreceptors and upregulation of CCAAT/Enhancer-binding protein α (Cebpa) and peroxisome proliferator-activated receptor-gamma (Pparg), regulators of fatty acid oxidation, gene expressions were observed in adipocytes, indicating an altered lipogenesis (Petry et al., 2000Petry CJ, Dorling MW, Wang CL, Pawlak DB, Ozanne SE. Catecholamine levels and receptor expression in low protein rat offspring. Diabet Med. 2000;17(12):848-53. http://dx.doi.org/10.1046/j.1464-5491.2000.00392.x. PMid:11168327.
http://dx.doi.org/10.1046/j.1464-5491.20...
; Lecoutre and Breton, 2015Lecoutre S, Breton C. Maternal nutritional manipulations program adipose tissue dysfunction in offspring. Front Physiol. 2015;6:158. http://dx.doi.org/10.3389/fphys.2015.00158. PMid:26029119.
http://dx.doi.org/10.3389/fphys.2015.001...
). In the liver of growth-restricted male offspring, elevated levels of triglycerides along with increased protein expression levels of lipoprotein lipase (LPL) were explained by an increase in the hepatic expression levels of liver X receptors (LXRs)-α expression that binds to the putative response elements in the LPL promoter regions (Zhu et al., 2016Zhu W, Zhu J, Liang L, Shen Z, Wang Y. Maternal undernutrition leads to elevated hepatic triglycerides in male rat offspring due to increased expression of lipoprotein lipase. Mol Med Rep. 2016;13(5):4487-93. http://dx.doi.org/10.3892/mmr.2016.5040. PMid:27035287.
http://dx.doi.org/10.3892/mmr.2016.5040...
). In the same way, Wistar rats born to dams fed with 50% restricted diet showed induction of cholesterol biosynthesis with higher concentrations of very-low-density lipoprotein (VLDL) and low-density lipoprotein (LDL), lipoproteins, and triglycerides (Sarli et al., 2021Sarli PM, Manousopoulou A, Efthymiou E, Zouridis A, Potiris A, Pervanidou P, Panoulis K, Vlahos N, Deligeoroglou E, Garbis SD, Eleftheriades M. Liver proteome profile of growth restricted and appropriately grown newborn Wistar rats associated with maternal undernutrition. Front Endocrinol. 2021;12:684220. http://dx.doi.org/10.3389/fendo.2021.684220. PMid:34127923.
http://dx.doi.org/10.3389/fendo.2021.684...
). Therefore, abnormalities in adipose tissue and liver lead to dyslipidemia with metabolic consequences. In the same way, hyperinsulinemia and reduced glucose uptake insulin-stimulated and expression of total and phosphorylated specific insulin-signaling proteins in skeletal muscle have also been demonstrated in male and female offspring at 15 and 21 months old, respectively, indicating an insulin-resistance state induced by maternal LPD during pregnancy (Fernandez-Twinn et al., 2005Fernandez-Twinn DS, Wayman A, Ekizoglou S, Martin MS, Hales CN, Ozanne SE. Maternal protein restriction leads to hyperinsulinemia and reduced insulin-signaling protein expression in 21-mo-old female rat offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R368-73. http://dx.doi.org/10.1152/ajpregu.00206.2004. PMid:15514105.
http://dx.doi.org/10.1152/ajpregu.00206....
).
In rodent models of maternal overnutrition, offspring present weight gain, hyperphagia (Ozanne et al., 2004Ozanne SE, Lewis R, Jennings BJ, Hales CN. Early programming of weight gain in mice prevents the induction of obesity by a highly palatable diet. Clin Sci. 2004;106(2):141-5. http://dx.doi.org/10.1042/CS20030278. PMid:14507258.
http://dx.doi.org/10.1042/CS20030278...
), leptin resistance (Samuelsson et al., 2008Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EHJM, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51(2):383-92. http://dx.doi.org/10.1161/HYPERTENSIONAHA.107.101477. PMid:18086952.
http://dx.doi.org/10.1161/HYPERTENSIONAH...
; Morris and Chen, 2009Morris MJ, Chen H. Established maternal obesity in the rat reprograms hypothalamic appetite regulators and leptin signaling at birth. Int J Obes. 2009;33(1):115-22. http://dx.doi.org/10.1038/ijo.2008.213. PMid:18982008.
http://dx.doi.org/10.1038/ijo.2008.213...
) and hyperleptinemia (McMillen et al., 2006McMillen IC, Edwards LJ, Duffield J, Muhlhausler BS. Regulation of leptin synthesis and secretion before birth: implications for the early programming of adult obesity. Reproduction. 2006;131(3):415-27. http://dx.doi.org/10.1530/rep.1.00303. PMid:16514185.
http://dx.doi.org/10.1530/rep.1.00303...
), increased adiposity (Li et al., 2013Li M, Reynolds CM, Sloboda DM, Gray C, Vickers MH. Effects of taurine supplementation on hepatic markers of inflammation and lipid metabolism in mothers and offspring in the setting of maternal obesity. PLoS One. 2013;8(10):e76961. http://dx.doi.org/10.1371/journal.pone.0076961. PMid:24146946.
http://dx.doi.org/10.1371/journal.pone.0...
), arterial hypertension (Samuelsson et al., 2008Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EHJM, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51(2):383-92. http://dx.doi.org/10.1161/HYPERTENSIONAHA.107.101477. PMid:18086952.
http://dx.doi.org/10.1161/HYPERTENSIONAH...
; Elahi et al., 2009Elahi MM, Cagampang FR, Mukhtar D, Anthony FW, Ohri SK, Hanson MA. Long-term maternal high-fat feeding from weaning through pregnancy and lactation predisposes offspring to hypertension, raised plasma lipids and fatty liver in mice. Br J Nutr. 2009;102(4):514-9. http://dx.doi.org/10.1017/S000711450820749X. PMid:19203419.
http://dx.doi.org/10.1017/S0007114508207...
), insulin resistance, higher hepatic gluconeogenesis (Strakovsky et al., 2011Strakovsky RS, Zhang X, Zhou D, Pan YX. Gestational high fat diet programs hepatic phosphoenolpyruvate carboxykinase gene expression and histone modification in neonatal offspring rats. J Physiol. 2011;589(Pt 11):2707-17. http://dx.doi.org/10.1113/jphysiol.2010.203950. PMid:21486814.
http://dx.doi.org/10.1113/jphysiol.2010....
; Taylor et al., 2014Taylor PD, Samuelsson AM, Poston L. Maternal obesity and the developmental programming of hypertension: a role for leptin. Acta Physiol (Oxf). 2014;210(3):508-23. http://dx.doi.org/10.1111/apha.12223. PMid:24433239.
http://dx.doi.org/10.1111/apha.12223...
), lipid profile abnormalities (Elahi et al., 2009Elahi MM, Cagampang FR, Mukhtar D, Anthony FW, Ohri SK, Hanson MA. Long-term maternal high-fat feeding from weaning through pregnancy and lactation predisposes offspring to hypertension, raised plasma lipids and fatty liver in mice. Br J Nutr. 2009;102(4):514-9. http://dx.doi.org/10.1017/S000711450820749X. PMid:19203419.
http://dx.doi.org/10.1017/S0007114508207...
), in addition to increasing pro-inflammatory mediators and vascular endothelial dysfunction, which are key factors in the development of metabolic syndrome, fatty liver, hepatic inflammation and steatosis (Li et al., 2013Li M, Reynolds CM, Sloboda DM, Gray C, Vickers MH. Effects of taurine supplementation on hepatic markers of inflammation and lipid metabolism in mothers and offspring in the setting of maternal obesity. PLoS One. 2013;8(10):e76961. http://dx.doi.org/10.1371/journal.pone.0076961. PMid:24146946.
http://dx.doi.org/10.1371/journal.pone.0...
) and cardiovascular disease (Vickers et al., 2000Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279(1):E83-7. http://dx.doi.org/10.1152/ajpendo.2000.279.1.E83. PMid:10893326.
http://dx.doi.org/10.1152/ajpendo.2000.2...
; Ghosh et al., 2001Ghosh P, Bitsanis D, Ghebremeskel K, Crawford MA, Poston L. Abnormal aortic fatty acid composition and small artery function in offspring of rats fed a high fat diet in pregnancy. J Physiol. 2001;533(Pt 3):815-22. http://dx.doi.org/10.1111/j.1469-7793.2001.00815.x. PMid:11410637.
http://dx.doi.org/10.1111/j.1469-7793.20...
; Khan et al., 2005Khan IY, Dekou V, Douglas G, Jensen R, Hanson MA, Poston L, Taylor PD. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R127-33. http://dx.doi.org/10.1152/ajpregu.00354.2004. PMid:15308487.
http://dx.doi.org/10.1152/ajpregu.00354....
; Chen et al., 2008Chen H, Simar D, Lambert K, Mercier J, Morris MJ. Maternal and postnatal overnutrition differentially impact appetite regulators and fuel metabolism. Endocrinology. 2008;149(11):5348-56. http://dx.doi.org/10.1210/en.2008-0582. PMid:18635655.
http://dx.doi.org/10.1210/en.2008-0582...
; Ashino et al., 2012Ashino NG, Saito KN, Souza FD, Nakutz FS, Roman EA, Velloso LA, Torsoni AS, Torsoni MA. Maternal high-fat feeding through pregnancy and lactation predisposes mouse offspring to molecular insulin resistance and fatty liver. J Nutr Biochem. 2012;23(4):341-8. http://dx.doi.org/10.1016/j.jnutbio.2010.12.011. PMid:21543214.
http://dx.doi.org/10.1016/j.jnutbio.2010...
). Metabolic alterations in offspring born to mothers fed with high-fat diet consumption appear early during postnatal life. In Sprague–Dawley rats, maternal HFD induced intrauterine inflammation, showing enhanced inflammatory cytokines (IL6, IL-1β, and TNF-α) in umbilical cord blood and the placenta that contribute with metabolic disorders in neonates characterized by alterations in hepatic genes both lipid synthesis- and β-oxidation that promote hepatic lipid accumulation (Cao et al., 2020Cao B, Liu C, Zhang Q, Dong Y. Maternal high-fat diet leads to non-alcoholic fatty liver disease through upregulating hepatic SCD1 expression in neonate rats. Front Nutr. 2020;7:581723. http://dx.doi.org/10.3389/fnut.2020.581723. PMid:33282902.
http://dx.doi.org/10.3389/fnut.2020.5817...
). On postnatal day 10, they exhibit, in a sex-specific manner, changes in dopamine-related gene expression (Th and Slc6a3, also known as Dat1) in the hypothalamus that could contribute to hyperphagia and preference for fatty, sugary, and salty foods at the expense of protein-rich foods (Bayol et al., 2007Bayol SA, Farrington SJ, Stickland NC. A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. Br J Nutr. 2007;98(4):843-51.. http://dx.doi.org/10.1017/S0007114507812037. PMid:17697422.
http://dx.doi.org/10.1017/S0007114507812...
; Barrand et al., 2017Barrand S, Crowley TM, Wood-Bradley RJ, De Jong KA, Armitage JA. Impact of maternal high fat diet on hypothalamic transcriptome in neonatal Sprague Dawley rats. PLoS One. 2017;12(12):e0189492. http://dx.doi.org/10.1371/journal.pone.0189492. PMid:29240779.
http://dx.doi.org/10.1371/journal.pone.0...
). Both male and female offsprings show increased adiposity, which is more pronounced in females than males. In the same way, elevated expression of genes involved in insulin signaling (Igf1 and Irs1), angiogenesis (Vegfa), lipogenesis (Pparg and Lpl), adipocyte function (Lep and Adipoq), and glucose uptake (Slc2A1 and Slc2A3) in females fed cafeteria diet compared with females did not have access to this diet (Bayol et al., 2008Bayol SA, Simbi BH, Bertrand JA, Stickland NC. Offspring from mothers fed a ‘junk food’ diet in pregnancy and lactation exhibit exacerbated adiposity that is more pronounced in females. J Physiol. 2008;586(13):3219-30. http://dx.doi.org/10.1113/jphysiol.2008.153817. PMid:18467362.
http://dx.doi.org/10.1113/jphysiol.2008....
).
Therefore, rodent models of maternal overnutrition in mice and rats show similar alterations in the hypothalamus, adipose tissue, muscle, and liver than those observed in models of undernutrition, although these appear earlier than offspring born to overnourished than undernourished mothers.
Like those observed in nutritional interventions, hypoxia can lead to cardiovascular and metabolic alterations in the offspring. In adulthood, the hearts of offspring express abnormal phenotypes, including diastolic dysfunction, increases in myocardial contractility, and responsiveness to β-adrenoreceptor stimulation (Giussani et al., 2012Giussani DA, Camm EJ, Niu Y, Richter HG, Blanco CE, Gottschalk R, Blake EZ, Horder KA, Thakor AS, Hansell JA, Kane AD, Wooding FBP, Cross CM, Herrera EA. Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS One. 2012;7(2):e31017. http://dx.doi.org/10.1371/journal.pone.0031017. PMid:22348036.
http://dx.doi.org/10.1371/journal.pone.0...
; Niu et al., 2018Niu Y, Kane AD, Lusby CM, Allison BJ, Chua YY, Kaandorp JJ, Nevin-Dolan R, Ashmore TJ, Blackmore HL, Derks JB, Ozanne SE, Giussani DA. Maternal allopurinol prevents cardiac dysfunction in adult male offspring programmed by chronic hypoxia during pregnancy. Hypertension. 2018;72(4):971-8. http://dx.doi.org/10.1161/HYPERTENSIONAHA.118.11363. PMid:30354714.
http://dx.doi.org/10.1161/HYPERTENSIONAH...
). Although these alterations are developed over time during postnatal life because fetal heart function measured in vivo is not disrupted by hypoxia, despite FGR, suggesting a functional capacity to adapt to prolonged hypoxic stress (Thompson et al., 2016Thompson LP, Pence L, Pinkas G, Song H, Telugu BP. Placental hypoxia during early pregnancy causes maternal hypertension and placental insufficiency in the hypoxic guinea pig model. Biol Reprod. 2016;95(6):128. http://dx.doi.org/10.1095/biolreprod.116.142273. PMid:27806942.
http://dx.doi.org/10.1095/biolreprod.116...
). Interestingly, in deer mice (Peromyscus maniculatus), a native rodent to high altitude (4350 m.a.s.l), hypoxia exposure during early life likely contributes to the ability to cope with hypoxia, increasing their plasticity on metabolism, oxygen consumption rate, and body temperature regulation (Ivy and Scott, 2021Ivy CM, Scott GR. Life-long exposure to hypoxia affects metabolism and respiratory physiology across life stages in high-altitude deer mice (Peromyscus maniculatus). J Exp Biol. 2021;224(1). http://dx.doi.org/10.1242/jeb.237024. PMid:33268530.
http://dx.doi.org/10.1242/jeb.237024...
). Likewise, FGR induced by bilateral uterine ligation alters nephrogenesis, leading to increased serum corticosterone levels, decreased nephron number, and cause adult-onset hypertension (Baserga et al., 2007Baserga M, Hale MA, Wang ZM, Yu X, Callaway CW, McKnight RA, Lane RH. Uteroplacental insufficiency alters nephrogenesis and downregulates cyclooxygenase-2 expression in a model of IUGR with adult-onset hypertension. Am J Physiol Regul Integr Comp Physiol. 2007;292(5):R1943-55. http://dx.doi.org/10.1152/ajpregu.00558.2006. PMid:17272666.
http://dx.doi.org/10.1152/ajpregu.00558....
).
Rodent models of hypoxia exposure during gestation show gene and protein expression changes in different organs involved in nutrient metabolism. Maternal hypoxia increased body weight and food consumption, reduced daily energy expenditure, increased adiposity index and insulin resistance, systemic elevations of lipid levels, and altered macrophage populations in adult male, but not female, mice offspring (Khalyfa et al., 2017Khalyfa A, Cortese R, Qiao Z, Ye H, Bao R, Andrade J, Gozal D. Late gestational intermittent hypoxia induces metabolic and epigenetic changes in male adult offspring mice. J Physiol. 2017;595(8):2551-68. http://dx.doi.org/10.1113/JP273570. PMid:28090638.
http://dx.doi.org/10.1113/JP273570...
). Interestingly, these effects can be attributed to altered leptin action in hypothalamic arcuate nuclei in male offspring in the Sprague-Dawley rat (Vargas et al., 2017Vargas VE, Gurung S, Grant B, Hyatt K, Singleton K, Myers SM, Saunders D, Njoku C, Towner R, Myers DA. Gestational hypoxia disrupts the neonatal leptin surge and programs hyperphagia and obesity in male offspring in the Sprague-Dawley rat. PLoS One. 2017;12(9):e0185272. http://dx.doi.org/10.1371/journal.pone.0185272. PMid:28957383.
http://dx.doi.org/10.1371/journal.pone.0...
). Moreover, reduced protein phosphorylation related to insulin signaling and lipid accumulation in the liver, along with lower expression of GLUT4 in skeletal muscle, were also observed (Camm et al., 2011Camm EJ, Martin-Gronert MS, Wright NL, Hansell JA, Ozanne SE, Giussani DA. Prenatal hypoxia independent of undernutrition promotes molecular markers of insulin resistance in adult offspring. FASEB J. 2011;25(1):420-7. http://dx.doi.org/10.1096/fj.10-158188. PMid:20923964.
http://dx.doi.org/10.1096/fj.10-158188...
; Cao et al., 2012Cao L, Mao C, Li S, Zhang Y, Lv J, Jiang S, Xu Z. Hepatic insulin signaling changes: possible mechanism in prenatal hypoxia-increased susceptibility of fatty liver in adulthood. Endocrinology. 2012;153(10):4955-65. http://dx.doi.org/10.1210/en.2012-1349. PMid:22903613.
http://dx.doi.org/10.1210/en.2012-1349...
). It is interesting to note that gestational hypoxia leads to abnormalities in maternal lipid and carbohydrate metabolism, suggesting that described effects in the offspring can result from alterations in maternal-fetal nutrient transport (Määttä et al., 2018Määttä J, Sissala N, Dimova EY, Serpi R, Moore LG, Koivunen P. Hypoxia causes reductions in birth weight by altering maternal glucose and lipid metabolism. Sci Rep. 2018;8(1):13583. http://dx.doi.org/10.1038/s41598-018-31908-2. PMid:30206264.
http://dx.doi.org/10.1038/s41598-018-319...
).
In females, exposure to chronic maternal hypoxia during fetal development leads to accelerated ovarian aging of somatic cells and reduced ovarian reserve at pubertal age, associated with gene pathways regulating folliculogenesis and steroidogenesis (Aiken et al., 2019Aiken CE, Tarry-Adkins JL, Spiroski A-M, Nuzzo AM, Ashmore TJ, Rolfo A, Sutherland MJ, Camm EJ, Giussani DA, Ozanne SE. Chronic gestational hypoxia accelerates ovarian aging and lowers ovarian reserve in next-generation adult rats. FASEB J. 2019;33(6):7758-66. http://dx.doi.org/10.1096/fj.201802772R. PMid:30888848.
http://dx.doi.org/10.1096/fj.201802772R...
; Pampanini et al., 2019Pampanini V, Jahnukainen K, Sahlin L, Germani D, Puglianiello A, Cianfarani S, Söder O. Impact of uteroplacental insufficiency on ovarian follicular pool in the rat. Reprod Biol Endocrinol. 2019;17(1):10. http://dx.doi.org/10.1186/s12958-019-0453-3. PMid:30630482.
http://dx.doi.org/10.1186/s12958-019-045...
). In turn, male offspring exhibited abnormalities in the proliferation and differentiation of spermatic cells and alterations in angiogenesis and connective tissue growth. Thus, together with metabolic and cardiovascular alterations, maternal hypoxia also results in reproductive dysfunctions.
Differences in the timing of exposure to androgen excess lead to variations in the appearance of PCOS features observed in animal models, implying that developmental stages are likely to be a key determinant in PCOS pathogenesis (Caldwell et al., 2014Caldwell AS, Middleton LJ, Jimenez M, Desai R, McMahon AC, Allan CM, Handelsman DJ, Walters KA. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology. 2014;155(8):3146-59. http://dx.doi.org/10.1210/en.2014-1196. PMid:24877633.
http://dx.doi.org/10.1210/en.2014-1196...
). Rats and mouse models of prenatal exposure to testosterone or DHT excess have been reported to induce most of the relevant reproductive, endocrine, and metabolic features of PCOS (Walters, 2015Walters KA. Role of androgens in normal and pathological ovarian function. Reproduction. 2015;149(4):R193-218. http://dx.doi.org/10.1530/REP-14-0517. PMid:25516989.
http://dx.doi.org/10.1530/REP-14-0517...
). Longer anogenital distance and smaller weight are frequent features observed in female pups born to androgenized rats and mice (Sun et al., 2012Sun M, Maliqueo M, Benrick A, Johansson J, Shao R, Hou L, Jansson T, Wu X, Stener-Victorin E. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring. Am J Physiol Endocrinol Metab. 2012;303(11):E1373-85. http://dx.doi.org/10.1152/ajpendo.00421.2012. PMid:23047983.
http://dx.doi.org/10.1152/ajpendo.00421....
; Risal et al., 2019Risal S, Pei Y, Lu H, Manti M, Fornes R, Pui HP, Zhao Z, Massart J, Ohlsson C, Lindgren E, Crisosto N, Maliqueo M, Echiburu B, Ladron de Guevara A, Sir-Petermann T, Larsson H, Rosenqvist MA, Cesta CE, Benrick A, Deng Q, Stener-Victorin E. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med. 2019;25(12):1894-904. http://dx.doi.org/10.1038/s41591-019-0666-1. PMid:31792459.
http://dx.doi.org/10.1038/s41591-019-066...
). Moreover, they had irregular cycles, polycystic ovary morphology, lower androgen levels being DHT lower in rat models, and testosterone and androstenedione in mice models (Hu et al., 2015Hu M, Richard JE, Maliqueo M, Kokosar M, Fornes R, Benrick A, Jansson T, Ohlsson C, Wu X, Skibicka KP, Stener-Victorin E. Maternal testosterone exposure increases anxiety-like behavior and impacts the limbic system in the offspring. Proc Natl Acad Sci USA. 2015;112(46):14348-53. http://dx.doi.org/10.1073/pnas.1507514112. PMid:26578781.
http://dx.doi.org/10.1073/pnas.150751411...
). Along with reproductive phenotype, metabolic and cardiovascular abnormalities have been described, including clear signs of non-alcoholic fatty liver (Sun et al., 2012Sun M, Maliqueo M, Benrick A, Johansson J, Shao R, Hou L, Jansson T, Wu X, Stener-Victorin E. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring. Am J Physiol Endocrinol Metab. 2012;303(11):E1373-85. http://dx.doi.org/10.1152/ajpendo.00421.2012. PMid:23047983.
http://dx.doi.org/10.1152/ajpendo.00421....
), which seems to be established during fetal life such that the expression of the transcription factor Pparg was decreased in fetal livers exposed to DHT (Fornes et al., 2017Fornes R, Maliqueo M, Hu M, Hadi L, Jimenez-Andrade JM, Ebefors K, Nyström J, Labrie F, Jansson T, Benrick A, Stener-Victorin E. The effect of androgen excess on maternal metabolism, placental function and fetal growth in obese dams. Sci Rep. 2017;7(1):8066. http://dx.doi.org/10.1038/s41598-017-08559-w. PMid:28808352.
http://dx.doi.org/10.1038/s41598-017-085...
), in addition, insulin resistance, alterations in the adipocyte functions and adipogenesis, and dyslipidemia have also been described (Sun et al., 2012Sun M, Maliqueo M, Benrick A, Johansson J, Shao R, Hou L, Jansson T, Wu X, Stener-Victorin E. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring. Am J Physiol Endocrinol Metab. 2012;303(11):E1373-85. http://dx.doi.org/10.1152/ajpendo.00421.2012. PMid:23047983.
http://dx.doi.org/10.1152/ajpendo.00421....
; Risal et al., 2019Risal S, Pei Y, Lu H, Manti M, Fornes R, Pui HP, Zhao Z, Massart J, Ohlsson C, Lindgren E, Crisosto N, Maliqueo M, Echiburu B, Ladron de Guevara A, Sir-Petermann T, Larsson H, Rosenqvist MA, Cesta CE, Benrick A, Deng Q, Stener-Victorin E. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med. 2019;25(12):1894-904. http://dx.doi.org/10.1038/s41591-019-0666-1. PMid:31792459.
http://dx.doi.org/10.1038/s41591-019-066...
). Elevated blood pressure has been demonstrated in rat models showing different regulatory mechanisms being NO-related in females and endothelium-derived hyperpolarizing factor (EDHF) -related in males. Sex steroids, in general, are central in the regulation of neurodevelopment processes; therefore, it is expected that both testosterone and DHT affect the behavior, in this regard, anxiety-like behavior in females, which is associated with decreased gene expression of androgen receptor (Ar) and increased expression of GABAergic and serotoninergic receptors, and genes involved in calcium signaling, among others, causing an anxiety-like behavior increased anxiety-like behavior (Hu et al., 2015Hu M, Richard JE, Maliqueo M, Kokosar M, Fornes R, Benrick A, Jansson T, Ohlsson C, Wu X, Skibicka KP, Stener-Victorin E. Maternal testosterone exposure increases anxiety-like behavior and impacts the limbic system in the offspring. Proc Natl Acad Sci USA. 2015;112(46):14348-53. http://dx.doi.org/10.1073/pnas.1507514112. PMid:26578781.
http://dx.doi.org/10.1073/pnas.150751411...
; Risal et al., 2021Risal S, Manti M, Lu H, Fornes R, Larsson H, Benrick A, Deng Q, Cesta CE, Rosenqvist MA, Stener-Victorin E. Prenatal androgen exposure causes a sexually dimorphic transgenerational increase in offspring susceptibility to anxiety disorders. Transl Psychiatry. 2021;11(1):45. http://dx.doi.org/10.1038/s41398-020-01183-9. PMid:33441551.
http://dx.doi.org/10.1038/s41398-020-011...
). Interestingly, these alterations can be transgenerationally transmitted by maternal and paternal lineage according to DHT-mice modes have demonstrated (Risal et al., 2019Risal S, Pei Y, Lu H, Manti M, Fornes R, Pui HP, Zhao Z, Massart J, Ohlsson C, Lindgren E, Crisosto N, Maliqueo M, Echiburu B, Ladron de Guevara A, Sir-Petermann T, Larsson H, Rosenqvist MA, Cesta CE, Benrick A, Deng Q, Stener-Victorin E. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med. 2019;25(12):1894-904. http://dx.doi.org/10.1038/s41591-019-0666-1. PMid:31792459.
http://dx.doi.org/10.1038/s41591-019-066...
, 2021Risal S, Manti M, Lu H, Fornes R, Larsson H, Benrick A, Deng Q, Cesta CE, Rosenqvist MA, Stener-Victorin E. Prenatal androgen exposure causes a sexually dimorphic transgenerational increase in offspring susceptibility to anxiety disorders. Transl Psychiatry. 2021;11(1):45. http://dx.doi.org/10.1038/s41398-020-01183-9. PMid:33441551.
http://dx.doi.org/10.1038/s41398-020-011...
)
In rodents, early postnatal exposure to dihydrotestosterone (DHT) produces the closest PCOS-like phenotype because the differentiation of reproductive tissues occurs during neonatal life contrary to humans (Caldwell et al., 2014Caldwell AS, Middleton LJ, Jimenez M, Desai R, McMahon AC, Allan CM, Handelsman DJ, Walters KA. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology. 2014;155(8):3146-59. http://dx.doi.org/10.1210/en.2014-1196. PMid:24877633.
http://dx.doi.org/10.1210/en.2014-1196...
; Kauffman et al., 2015Kauffman AS, Thackray VG, Ryan GE, Tolson KP, Glidewell-Kenney CA, Semaan SJ, Poling MC, Iwata N, Breen KM, Duleba AJ, Stener-Victorin E, Shimasaki S, Webster NJ, Mellon PL. A novel letrozole model recapitulates both the reproductive and metabolic phenotypes of polycystic ovary syndrome in female mice. Biol Reprod. 2015;93(3):69. http://dx.doi.org/10.1095/biolreprod.115.131631. PMid:26203175.
http://dx.doi.org/10.1095/biolreprod.115...
; Walters et al., 2018Walters KA, Bertoldo MJ, Handelsman DJ. Evidence from animal models on the pathogenesis of PCOS. Best Pract Res Clin Endocrinol Metab. 2018;32(3):271-81. http://dx.doi.org/10.1016/j.beem.2018.03.008. PMid:29779581.
http://dx.doi.org/10.1016/j.beem.2018.03...
). The perinatal androgenization induces irregular cycles, oligo-anovulation, PCO morphology, increased preantral/antral follicles, hyperandrogenism, LH hypersecretion, and disturbances in fat metabolism without insulin resistance. One study that evaluated pre and postnatal androgen-induced-PCOS mouse models found that a model using DHT treatment in early postnatal provided the highest concordance with clinical features of PCOS (Caldwell et al., 2014Caldwell AS, Middleton LJ, Jimenez M, Desai R, McMahon AC, Allan CM, Handelsman DJ, Walters KA. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology. 2014;155(8):3146-59. http://dx.doi.org/10.1210/en.2014-1196. PMid:24877633.
http://dx.doi.org/10.1210/en.2014-1196...
). In summary, analysis of androgenized rodent PCOS animal models has demonstrated closely mimic human PCOS as androgen excess consistently induces a wide breadth of characteristics of these disorders, which may vary depending on the type of androgen used and the timing of the androgenization.
Conclusions
Rodent models of dietary interventions, hypoxia, and hyperandrogenism have shown that the mechanisms of fetal programming are mediated by placental dysfunctions (Figure 3). These alterations in insulin, mammalian target of rapamycin (mTOR), and signal transducer and activator of transcription 3 (STAT3) pathways are associated with modifications in uteroplacental blood flow, amino acids, and fatty acid transport, increased in pro-inflammatory cytokines and ROS lead mainly to FGR.
Schematic representation of the effects of dietary interventions, hypoxia, and hyperandrogenism based on observations obtained by rodent models in rats, mice, and guinea pigs. These models have shown that the mechanisms of fetal programming are mediated by placental dysfunctions involving nutrient transport, steroid action (androgens), reduced oxygen supply (modifications in uteroplacental blood flow), increased in pro-inflammatory cytokines and reactive oxygen species (ROS), leading mainly to fetal growth restriction (FGR). These modifications in the prenatal environment affect various fetal organs resulting in metabolic, cardiovascular, and behavioral disorders in postnatal life mediated by epigenetics modifications, sex dimorphism, and early postnatal life.
Modifications in the prenatal environment affect fetal organs such as the brain, adipose tissue, liver, heart, and endothelium, programming its function at postnatal life inducing hyperphagia, obesity, hepatic fat accumulation, blunted insulin signaling in muscle resulting in insulin resistance and cardiovascular disorders. In these alterations, sex dimorphism plays a central role such that females and males exhibit differential phenotypes.
Acknowledgements
This work was supported by Fondo Nacional de Desarrollo Científico y Tecnológico (National Fund for Scientific and Technological Research) FONDECYT Grant 1181798.
-
Financial support: MM and BE received funding for this research from Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) (grant numbers #1181798 and 1201483, respectively).
-
How to cite: Aguilera N, Salas F, Ortíz M, Álvarez D, Echiburú B, Maliqueo M. Rodent models in placental research. Implications for fetal origins of adult disease. Anim Reprod. 2022;19(1):e20210134. https://doi.org/10.1590/1984-3143-AR2021-0134
References
- Aiken CE, Tarry-Adkins JL, Spiroski A-M, Nuzzo AM, Ashmore TJ, Rolfo A, Sutherland MJ, Camm EJ, Giussani DA, Ozanne SE. Chronic gestational hypoxia accelerates ovarian aging and lowers ovarian reserve in next-generation adult rats. FASEB J. 2019;33(6):7758-66. http://dx.doi.org/10.1096/fj.201802772R PMid:30888848.
» http://dx.doi.org/10.1096/fj.201802772R - Alves MB, Dalle Molle R, Desai M, Ross MG, Silveira PP. Increased palatable food intake and response to food cues in intrauterine growth-restricted rats are related to tyrosine hydroxylase content in the orbitofrontal cortex and nucleus accumbens. Behav Brain Res. 2015;287:73-81. http://dx.doi.org/10.1016/j.bbr.2015.03.019 PMid:25796489.
» http://dx.doi.org/10.1016/j.bbr.2015.03.019 - Andersen MD, Alstrup AKO, Duvald CS, Mikkelsen EFR, Vendelbo MH, Ovesen PG, Pedersen M. Animal models of fetal medicine and obstetrics. In: Ibeh B, editor. Experimental animal models of human diseases: an effective therapeutic strategy. London: InTech; 2018. http://dx.doi.org/10.5772/intechopen.74038
» http://dx.doi.org/10.5772/intechopen.74038 - Ashino NG, Saito KN, Souza FD, Nakutz FS, Roman EA, Velloso LA, Torsoni AS, Torsoni MA. Maternal high-fat feeding through pregnancy and lactation predisposes mouse offspring to molecular insulin resistance and fatty liver. J Nutr Biochem. 2012;23(4):341-8. http://dx.doi.org/10.1016/j.jnutbio.2010.12.011 PMid:21543214.
» http://dx.doi.org/10.1016/j.jnutbio.2010.12.011 - Barrand S, Crowley TM, Wood-Bradley RJ, De Jong KA, Armitage JA. Impact of maternal high fat diet on hypothalamic transcriptome in neonatal Sprague Dawley rats. PLoS One. 2017;12(12):e0189492. http://dx.doi.org/10.1371/journal.pone.0189492 PMid:29240779.
» http://dx.doi.org/10.1371/journal.pone.0189492 - Barrett ES, Mbowe O, Thurston SW, Butts S, Wang C, Nguyen R, Bush N, Redmon JB, Sheshu S, Swan SH, Sathyanarayana S. Predictors of steroid hormone concentrations in early pregnancy: results from a multi-center cohort. Matern Child Health J. 2019;23(3):397-407. http://dx.doi.org/10.1007/s10995-018-02705-0 PMid:30659461.
» http://dx.doi.org/10.1007/s10995-018-02705-0 - Barros Mucci D, Kusinski LC, Wilsmore P, Loche E, Pantaleão LC, Ashmore TJ, Blackmore HL, Fernandez-Twinn DS, Carmo MDGTD, Ozanne SE. Impact of maternal obesity on placental transcriptome and morphology associated with fetal growth restriction in mice. Int J Obes. 2020;44(5):1087-96. http://dx.doi.org/10.1038/s41366-020-0561-3 PMid:32203108.
» http://dx.doi.org/10.1038/s41366-020-0561-3 - Baserga M, Hale MA, Wang ZM, Yu X, Callaway CW, McKnight RA, Lane RH. Uteroplacental insufficiency alters nephrogenesis and downregulates cyclooxygenase-2 expression in a model of IUGR with adult-onset hypertension. Am J Physiol Regul Integr Comp Physiol. 2007;292(5):R1943-55. http://dx.doi.org/10.1152/ajpregu.00558.2006 PMid:17272666.
» http://dx.doi.org/10.1152/ajpregu.00558.2006 - Bayol SA, Farrington SJ, Stickland NC. A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. Br J Nutr. 2007;98(4):843-51.. http://dx.doi.org/10.1017/S0007114507812037 PMid:17697422.
» http://dx.doi.org/10.1017/S0007114507812037 - Bayol SA, Simbi BH, Bertrand JA, Stickland NC. Offspring from mothers fed a ‘junk food’ diet in pregnancy and lactation exhibit exacerbated adiposity that is more pronounced in females. J Physiol. 2008;586(13):3219-30. http://dx.doi.org/10.1113/jphysiol.2008.153817 PMid:18467362.
» http://dx.doi.org/10.1113/jphysiol.2008.153817 - Bellinger L, Lilley C, Langley-Evans SC. Prenatal exposure to a maternal low-protein diet programmes a preference for high-fat foods in the young adult rat. Br J Nutr. 2004;92(3):513-20. http://dx.doi.org/10.1079/BJN20041224 PMid:15469656.
» http://dx.doi.org/10.1079/BJN20041224 - Boyle JA, Teede HJ. PCOS: refining diagnostic features in PCOS to optimize health outcomes. Nat Rev Endocrinol. 2016;12(11):630-1. http://dx.doi.org/10.1038/nrendo.2016.157 PMid:27636732.
» http://dx.doi.org/10.1038/nrendo.2016.157 - Caanen MR, Kuijper EA, Hompes PG, Kushnir MM, Rockwood AL, Meikle WA, Homburg R, Lambalk CB. Mass spectrometry methods measured androgen and estrogen concentrations during pregnancy and in newborns of mothers with polycystic ovary syndrome. Eur J Endocrinol. 2016;174(1):25-32. http://dx.doi.org/10.1530/EJE-15-0699 PMid:26586837.
» http://dx.doi.org/10.1530/EJE-15-0699 - Caldwell AS, Middleton LJ, Jimenez M, Desai R, McMahon AC, Allan CM, Handelsman DJ, Walters KA. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology. 2014;155(8):3146-59. http://dx.doi.org/10.1210/en.2014-1196 PMid:24877633.
» http://dx.doi.org/10.1210/en.2014-1196 - Camm EJ, Martin-Gronert MS, Wright NL, Hansell JA, Ozanne SE, Giussani DA. Prenatal hypoxia independent of undernutrition promotes molecular markers of insulin resistance in adult offspring. FASEB J. 2011;25(1):420-7. http://dx.doi.org/10.1096/fj.10-158188 PMid:20923964.
» http://dx.doi.org/10.1096/fj.10-158188 - Cao B, Liu C, Zhang Q, Dong Y. Maternal high-fat diet leads to non-alcoholic fatty liver disease through upregulating hepatic SCD1 expression in neonate rats. Front Nutr. 2020;7:581723. http://dx.doi.org/10.3389/fnut.2020.581723 PMid:33282902.
» http://dx.doi.org/10.3389/fnut.2020.581723 - Cao L, Mao C, Li S, Zhang Y, Lv J, Jiang S, Xu Z. Hepatic insulin signaling changes: possible mechanism in prenatal hypoxia-increased susceptibility of fatty liver in adulthood. Endocrinology. 2012;153(10):4955-65. http://dx.doi.org/10.1210/en.2012-1349 PMid:22903613.
» http://dx.doi.org/10.1210/en.2012-1349 - Capellini I, Venditti C, Barton RA. Placentation and maternal investment in mammals. Am Nat. 2011;177(1):86-98. http://dx.doi.org/10.1086/657435 PMid:21087154.
» http://dx.doi.org/10.1086/657435 - Carlsen SM, Jacobsen G, Romundstad P. Maternal testosterone levels during pregnancy are associated with offspring size at birth. Eur J Endocrinol. 2006;155(2):365-70. http://dx.doi.org/10.1530/eje.1.02200 PMid:16868152.
» http://dx.doi.org/10.1530/eje.1.02200 - Chen H, Simar D, Lambert K, Mercier J, Morris MJ. Maternal and postnatal overnutrition differentially impact appetite regulators and fuel metabolism. Endocrinology. 2008;149(11):5348-56. http://dx.doi.org/10.1210/en.2008-0582 PMid:18635655.
» http://dx.doi.org/10.1210/en.2008-0582 - Chu A, Casero D, Thamotharan S, Wadehra M, Cosi A, Devaskar SU. The placental transcriptome in late gestational hypoxia resulting in murine intrauterine growth restriction parallels increased risk of adult cardiometabolic disease. Sci Rep. 2019;9(1):1243. http://dx.doi.org/10.1038/s41598-018-37627-y PMid:30718791.
» http://dx.doi.org/10.1038/s41598-018-37627-y - Coan PM, Vaughan OR, Sekita Y, Finn SL, Burton GJ, Constancia M, Fowden AL. Adaptations in placental phenotype support fetal growth during undernutrition of pregnant mice. J Physiol. 2010;588(3):527-38. http://dx.doi.org/10.1113/jphysiol.2009.181214 PMid:19948659.
» http://dx.doi.org/10.1113/jphysiol.2009.181214 - Connor KL, Kibschull M, Matysiak-Zablocki E, Nguyen TT-TN, Matthews SG, Lye SJ, Bloise E. Maternal malnutrition impacts placental morphology and transporter expression: an origin for poor offspring growth. J Nutr Biochem. 2020;78:108329. http://dx.doi.org/10.1016/j.jnutbio.2019.108329 PMid:32004932.
» http://dx.doi.org/10.1016/j.jnutbio.2019.108329 - Csapo AI, Puri CP, Tarró S. Relationship between timing of ovariectomy and maintenance of pregnancy in the guinea-pig. Prostaglandins. 1981;22(1):131-40. http://dx.doi.org/10.1016/0090-6980(81)90060-5 PMid:7291595.
» http://dx.doi.org/10.1016/0090-6980(81)90060-5 - Cuffe JSM, Walton SL, Singh RR, Spiers JG, Bielefeldt-Ohmann H, Wilkinson L, Little MH, Moritz KM. Mid- to late term hypoxia in the mouse alters placental morphology, glucocorticoid regulatory pathways and nutrient transporters in a sex-specific manner. J Physiol. 2014;592(14):3127-41. http://dx.doi.org/10.1113/jphysiol.2014.272856 PMid:24801305.
» http://dx.doi.org/10.1113/jphysiol.2014.272856 - Davisson RL, Hoffmann DS, Butz GM, Aldape G, Schlager G, Merrill DC, Sethi S, Weiss RM, Bates JN. Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension. 2002;39(2):337-42. http://dx.doi.org/10.1161/hy02t2.102904 PMid:11882569.
» http://dx.doi.org/10.1161/hy02t2.102904 - Dean A, Smith LB, Macpherson S, Sharpe RM. The effect of dihydrotestosterone exposure during or prior to the masculinization programming window on reproductive development in male and female rats. Int J Androl. 2012;35(3):330-9. http://dx.doi.org/10.1111/j.1365-2605.2011.01236.x PMid:22248293.
» http://dx.doi.org/10.1111/j.1365-2605.2011.01236.x - Elahi MM, Cagampang FR, Mukhtar D, Anthony FW, Ohri SK, Hanson MA. Long-term maternal high-fat feeding from weaning through pregnancy and lactation predisposes offspring to hypertension, raised plasma lipids and fatty liver in mice. Br J Nutr. 2009;102(4):514-9. http://dx.doi.org/10.1017/S000711450820749X PMid:19203419.
» http://dx.doi.org/10.1017/S000711450820749X - Erlandsson L, Nääv Å, Hennessy A, Vaiman D, Gram M, Åkerström B, Hansson SR. Inventory of novel animal models addressing etiology of preeclampsia in the development of new therapeutic/intervention opportunities. Am J Reprod Immunol. 2016;75(3):402-10. http://dx.doi.org/10.1111/aji.12460 PMid:26685057.
» http://dx.doi.org/10.1111/aji.12460 - Fajersztajn L, Veras MM. Hypoxia: from placental development to fetal programming. Birth Defects Res. 2017;109(17):1377-85. http://dx.doi.org/10.1002/bdr2.1142 PMid:29105382.
» http://dx.doi.org/10.1002/bdr2.1142 - Fernandez-Twinn DS, Wayman A, Ekizoglou S, Martin MS, Hales CN, Ozanne SE. Maternal protein restriction leads to hyperinsulinemia and reduced insulin-signaling protein expression in 21-mo-old female rat offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R368-73. http://dx.doi.org/10.1152/ajpregu.00206.2004 PMid:15514105.
» http://dx.doi.org/10.1152/ajpregu.00206.2004 - Fornes R, Maliqueo M, Hu M, Hadi L, Jimenez-Andrade JM, Ebefors K, Nyström J, Labrie F, Jansson T, Benrick A, Stener-Victorin E. The effect of androgen excess on maternal metabolism, placental function and fetal growth in obese dams. Sci Rep. 2017;7(1):8066. http://dx.doi.org/10.1038/s41598-017-08559-w PMid:28808352.
» http://dx.doi.org/10.1038/s41598-017-08559-w - Fukami T, Sun X, Li T, Desai M, Ross M. Mechanism of programmed obesity in intrauterine fetal growth restricted offspring: paradoxically enhanced appetite stimulation in fed and fasting states. Reprod Sci. 2012;19(4):423-30. http://dx.doi.org/10.1177/1933719111424448 PMid:22344733.
» http://dx.doi.org/10.1177/1933719111424448 - Furukawa S, Kuroda Y, Sugiyama A. A comparison of the histological structure of the placenta in experimental animals. J Toxicol Pathol. 2014;27(1):11-8. http://dx.doi.org/10.1293/tox.2013-0060 PMid:24791062.
» http://dx.doi.org/10.1293/tox.2013-0060 - Gao H, Yallampalli U, Yallampalli C. Gestational protein restriction reduces expression of Hsd17b2 in rat placental labyrinth. Biol Reprod. 2012;87(3):68. http://dx.doi.org/10.1095/biolreprod.112.100479 PMid:22837477.
» http://dx.doi.org/10.1095/biolreprod.112.100479 - Ghosh P, Bitsanis D, Ghebremeskel K, Crawford MA, Poston L. Abnormal aortic fatty acid composition and small artery function in offspring of rats fed a high fat diet in pregnancy. J Physiol. 2001;533(Pt 3):815-22. http://dx.doi.org/10.1111/j.1469-7793.2001.00815.x PMid:11410637.
» http://dx.doi.org/10.1111/j.1469-7793.2001.00815.x - Giussani DA, Camm EJ, Niu Y, Richter HG, Blanco CE, Gottschalk R, Blake EZ, Horder KA, Thakor AS, Hansell JA, Kane AD, Wooding FBP, Cross CM, Herrera EA. Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS One. 2012;7(2):e31017. http://dx.doi.org/10.1371/journal.pone.0031017 PMid:22348036.
» http://dx.doi.org/10.1371/journal.pone.0031017 - Gonzalez PN, Gasperowicz M, Barbeito-Andrés J, Klenin N, Cross JC, Hallgrímsson B. Chronic protein restriction in mice impacts placental function and maternal body weight before fetal growth. PLoS One. 2016;11(3):e0152227. http://dx.doi.org/10.1371/journal.pone.0152227 PMid:27018791.
» http://dx.doi.org/10.1371/journal.pone.0152227 - Hales CN, Barker DJP. The thrifty phenotype hypothesis. Br Med Bull. 2001;60(1):5-20. http://dx.doi.org/10.1093/bmb/60.1.5 PMid:11809615.
» http://dx.doi.org/10.1093/bmb/60.1.5 - Higgins JS, Vaughan OR, Fernandez de Liger E, Fowden AL, Sferruzzi-Perri AN. Placental phenotype and resource allocation to fetal growth are modified by the timing and degree of hypoxia during mouse pregnancy. J Physiol. 2016;594(5):1341-56. http://dx.doi.org/10.1113/JP271057 PMid:26377136.
» http://dx.doi.org/10.1113/JP271057 - Hu M, Richard JE, Maliqueo M, Kokosar M, Fornes R, Benrick A, Jansson T, Ohlsson C, Wu X, Skibicka KP, Stener-Victorin E. Maternal testosterone exposure increases anxiety-like behavior and impacts the limbic system in the offspring. Proc Natl Acad Sci USA. 2015;112(46):14348-53. http://dx.doi.org/10.1073/pnas.1507514112 PMid:26578781.
» http://dx.doi.org/10.1073/pnas.1507514112 - Huang S-TJ, Vo KCT, Lyell DJ, Faessen GH, Tulac S, Tibshirani R, Giaccia AJ, Giudice LC. Developmental response to hypoxia. FASEB J. 2004;18(12):1348-65. http://dx.doi.org/10.1096/fj.03-1377com PMid:15333578.
» http://dx.doi.org/10.1096/fj.03-1377com - Ivy CM, Scott GR. Life-long exposure to hypoxia affects metabolism and respiratory physiology across life stages in high-altitude deer mice (Peromyscus maniculatus). J Exp Biol. 2021;224(1). http://dx.doi.org/10.1242/jeb.237024 PMid:33268530.
» http://dx.doi.org/10.1242/jeb.237024 - Jakoubek V, Bíbová J, Herget J, Hampl V. Chronic hypoxia increases fetoplacental vascular resistance and vasoconstrictor reactivity in the rat. Am J Physiol Heart Circ Physiol. 2008;294(4):H1638-44. http://dx.doi.org/10.1152/ajpheart.01120.2007 PMid:18310520.
» http://dx.doi.org/10.1152/ajpheart.01120.2007 - Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol. 2006;576(Pt 3):935-46. http://dx.doi.org/10.1113/jphysiol.2006.116509 PMid:16916910.
» http://dx.doi.org/10.1113/jphysiol.2006.116509 - Kauffman AS, Thackray VG, Ryan GE, Tolson KP, Glidewell-Kenney CA, Semaan SJ, Poling MC, Iwata N, Breen KM, Duleba AJ, Stener-Victorin E, Shimasaki S, Webster NJ, Mellon PL. A novel letrozole model recapitulates both the reproductive and metabolic phenotypes of polycystic ovary syndrome in female mice. Biol Reprod. 2015;93(3):69. http://dx.doi.org/10.1095/biolreprod.115.131631 PMid:26203175.
» http://dx.doi.org/10.1095/biolreprod.115.131631 - Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol. 2002;283(1):R29-45. http://dx.doi.org/10.1152/ajpregu.00762.2001 PMid:12069928.
» http://dx.doi.org/10.1152/ajpregu.00762.2001 - Khalyfa A, Cortese R, Qiao Z, Ye H, Bao R, Andrade J, Gozal D. Late gestational intermittent hypoxia induces metabolic and epigenetic changes in male adult offspring mice. J Physiol. 2017;595(8):2551-68. http://dx.doi.org/10.1113/JP273570 PMid:28090638.
» http://dx.doi.org/10.1113/JP273570 - Khan IY, Dekou V, Douglas G, Jensen R, Hanson MA, Poston L, Taylor PD. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R127-33. http://dx.doi.org/10.1152/ajpregu.00354.2004 PMid:15308487.
» http://dx.doi.org/10.1152/ajpregu.00354.2004 - Kim DW, Young SL, Grattan DR, Jasoni CL. Obesity during pregnancy disrupts placental morphology, cell proliferation, and inflammation in a sex-specific manner across gestation in the mouse. Biol Reprod. 2014;90(6):130. http://dx.doi.org/10.1095/biolreprod.113.117259 PMid:24829026.
» http://dx.doi.org/10.1095/biolreprod.113.117259 - Kingdom JCP, Kaufmann P. Oxygen and placental villous development: origins of fetal hypoxia. Placenta. 1997;18(8):613-21. http://dx.doi.org/10.1016/S0143-4004(97)90000-X PMid:9364596.
» http://dx.doi.org/10.1016/S0143-4004(97)90000-X - Kumar S, Gordon GH, Abbott DH, Mishra JS. Androgens in maternal vascular and placental function: implications for preeclampsia pathogenesis. Reproduction. 2018;156(5):R155-67. http://dx.doi.org/10.1530/REP-18-0278 PMid:30325182.
» http://dx.doi.org/10.1530/REP-18-0278 - Lecoutre S, Breton C. Maternal nutritional manipulations program adipose tissue dysfunction in offspring. Front Physiol. 2015;6:158. http://dx.doi.org/10.3389/fphys.2015.00158 PMid:26029119.
» http://dx.doi.org/10.3389/fphys.2015.00158 - Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Heart Circ Physiol. 2012;303(1):H1-8. http://dx.doi.org/10.1152/ajpheart.00117.2012 PMid:22523250.
» http://dx.doi.org/10.1152/ajpheart.00117.2012 - Li M, Reynolds CM, Sloboda DM, Gray C, Vickers MH. Effects of taurine supplementation on hepatic markers of inflammation and lipid metabolism in mothers and offspring in the setting of maternal obesity. PLoS One. 2013;8(10):e76961. http://dx.doi.org/10.1371/journal.pone.0076961 PMid:24146946.
» http://dx.doi.org/10.1371/journal.pone.0076961 - Liang C, DeCourcy K, Prater MR. High-saturated-fat diet induces gestational diabetes and placental vasculopathy in C57BL/6 mice. Metabolism. 2010;59(7):943-50. http://dx.doi.org/10.1016/j.metabol.2009.10.015 PMid:20022072.
» http://dx.doi.org/10.1016/j.metabol.2009.10.015 - Louwagie EJ, Larsen TD, Wachal AL, Baack ML. Placental lipid processing in response to a maternal high-fat diet and diabetes in rats. Pediatr Res. 2018;83(3):712-22. http://dx.doi.org/10.1038/pr.2017.288 PMid:29166372.
» http://dx.doi.org/10.1038/pr.2017.288 - Määttä J, Sissala N, Dimova EY, Serpi R, Moore LG, Koivunen P. Hypoxia causes reductions in birth weight by altering maternal glucose and lipid metabolism. Sci Rep. 2018;8(1):13583. http://dx.doi.org/10.1038/s41598-018-31908-2 PMid:30206264.
» http://dx.doi.org/10.1038/s41598-018-31908-2 - Maliqueo M, Cruz G, Espina C, Contreras I, García M, Echiburú B, Crisosto N. Obesity during pregnancy affects sex steroid concentrations depending on fetal gender. Int J Obes. 2017;41(11):1636-45. http://dx.doi.org/10.1038/ijo.2017.159 PMid:28676682.
» http://dx.doi.org/10.1038/ijo.2017.159 - Maliqueo M, Sundström Poromaa I, Vanky E, Fornes R, Benrick A, Åkerud H, Stridsklev S, Labrie F, Jansson T, Stener-Victorin E. Placental STAT3 signaling is activated in women with polycystic ovary syndrome. Hum Reprod. 2015;30(3):692-700. http://dx.doi.org/10.1093/humrep/deu351 PMid:25609240.
» http://dx.doi.org/10.1093/humrep/deu351 - Marciniak A, Patro-Małysza J, Kimber-Trojnar Ż, Marciniak B, Oleszczuk J, Leszczyńska-Gorzelak B. Fetal programming of the metabolic syndrome. Taiwan J Obstet Gynecol. 2017;56(2):133-8. http://dx.doi.org/10.1016/j.tjog.2017.01.001 PMid:28420495.
» http://dx.doi.org/10.1016/j.tjog.2017.01.001 - Matheson H, Veerbeek JHW, Charnock-Jones DS, Burton GJ, Yung HW. Morphological and molecular changes in the murine placenta exposed to normobaric hypoxia throughout pregnancy. J Physiol. 2016;594(5):1371-88. http://dx.doi.org/10.1113/JP271073 PMid:26278110.
» http://dx.doi.org/10.1113/JP271073 - McMillen IC, Edwards LJ, Duffield J, Muhlhausler BS. Regulation of leptin synthesis and secretion before birth: implications for the early programming of adult obesity. Reproduction. 2006;131(3):415-27. http://dx.doi.org/10.1530/rep.1.00303 PMid:16514185.
» http://dx.doi.org/10.1530/rep.1.00303 - Molnär M, Söto T, Tóth T, Hertelendy F. Prolonged blockade of nitric oxide synthesis in gravid rats produces sustained hypertension, proteinuria, thrombocytopenia, and intrauterine growth retardation. Am J Obstet Gynecol. 1994;170(5):1458-66. http://dx.doi.org/10.1016/S0002-9378(13)90488-9 PMid:7909994.
» http://dx.doi.org/10.1016/S0002-9378(13)90488-9 - Morris MJ, Chen H. Established maternal obesity in the rat reprograms hypothalamic appetite regulators and leptin signaling at birth. Int J Obes. 2009;33(1):115-22. http://dx.doi.org/10.1038/ijo.2008.213 PMid:18982008.
» http://dx.doi.org/10.1038/ijo.2008.213 - Morrison JL, Botting KJ, Darby JRT, David AL, Dyson RM, Gatford KL, Gray C, Herrera EA, Hirst JJ, Kim B, Kind KL, Krause BJ, Matthews SG, Palliser HK, Regnault TRH, Richardson BS, Sasaki A, Thompson LP, Berry MJ. Guinea pig models for translation of the developmental origins of health and disease hypothesis into the clinic. J Physiol. 2018;596(23):5535-69. http://dx.doi.org/10.1113/JP274948 PMid:29633280.
» http://dx.doi.org/10.1113/JP274948 - Natale BV, Mehta P, Vu P, Schweitzer C, Gustin K, Kotadia R, Natale DRC. Reduced Uteroplacental Perfusion Pressure (RUPP) causes altered trophoblast differentiation and pericyte reduction in the mouse placenta labyrinth. Sci Rep. 2018;8(1):17162. http://dx.doi.org/10.1038/s41598-018-35606-x PMid:30464252.
» http://dx.doi.org/10.1038/s41598-018-35606-x - Neri C, Edlow AG. Effects of maternal obesity on fetal programming: molecular approaches. Cold Spring Harb Perspect Med. 2015;6(2):a026591. http://dx.doi.org/10.1101/cshperspect.a026591 PMid:26337113.
» http://dx.doi.org/10.1101/cshperspect.a026591 - Niu Y, Kane AD, Lusby CM, Allison BJ, Chua YY, Kaandorp JJ, Nevin-Dolan R, Ashmore TJ, Blackmore HL, Derks JB, Ozanne SE, Giussani DA. Maternal allopurinol prevents cardiac dysfunction in adult male offspring programmed by chronic hypoxia during pregnancy. Hypertension. 2018;72(4):971-8. http://dx.doi.org/10.1161/HYPERTENSIONAHA.118.11363 PMid:30354714.
» http://dx.doi.org/10.1161/HYPERTENSIONAHA.118.11363 - Olive EL, Xiao E, Natale DR, Fisher SA. Oxygen and lack of oxygen in fetal and placental development, feto–placental coupling, and congenital heart defects. Birth Defects Res. 2018;110(20):1517-30. http://dx.doi.org/10.1002/bdr2.1430 PMid:30576091.
» http://dx.doi.org/10.1002/bdr2.1430 - Ozanne SE, Lewis R, Jennings BJ, Hales CN. Early programming of weight gain in mice prevents the induction of obesity by a highly palatable diet. Clin Sci. 2004;106(2):141-5. http://dx.doi.org/10.1042/CS20030278 PMid:14507258.
» http://dx.doi.org/10.1042/CS20030278 - Pampanini V, Jahnukainen K, Sahlin L, Germani D, Puglianiello A, Cianfarani S, Söder O. Impact of uteroplacental insufficiency on ovarian follicular pool in the rat. Reprod Biol Endocrinol. 2019;17(1):10. http://dx.doi.org/10.1186/s12958-019-0453-3 PMid:30630482.
» http://dx.doi.org/10.1186/s12958-019-0453-3 - Peeters LLH, Sheldon RE, Jones MD Jr, Makowski EL, Meschia G. Blood flow to fetal organs as a function of arterial oxygen content. Am J Obstet Gynecol. 1979;135(5):637-46. http://dx.doi.org/10.1016/S0002-9378(16)32989-1 PMid:507116.
» http://dx.doi.org/10.1016/S0002-9378(16)32989-1 - Perrone S, Santacroce A, Picardi A, Buonocore G. Fetal programming and early identification of newborns at high risk of free radical-mediated diseases. World J Clin Pediatr. 2016;5(2):172-81. http://dx.doi.org/10.5409/wjcp.v5.i2.172 PMid:27170927.
» http://dx.doi.org/10.5409/wjcp.v5.i2.172 - Petry CJ, Dorling MW, Wang CL, Pawlak DB, Ozanne SE. Catecholamine levels and receptor expression in low protein rat offspring. Diabet Med. 2000;17(12):848-53. http://dx.doi.org/10.1046/j.1464-5491.2000.00392.x PMid:11168327.
» http://dx.doi.org/10.1046/j.1464-5491.2000.00392.x - Phuthong S, Reyes-Hernández CG, Rodríguez-Rodríguez P, Ramiro-Cortijo D, Gil-Ortega M, González-Blázquez R, González MC, López de Pablo AL, Arribas SM. Sex differences in placental protein expression and efficiency in a rat model of fetal programming induced by maternal undernutrition. Int J Mol Sci. 2020;22(1):237. http://dx.doi.org/10.3390/ijms22010237 PMid:33379399.
» http://dx.doi.org/10.3390/ijms22010237 - Redman CWG. Preeclampsia: a multi-stress disorder. Rev Med Interne. 2011;32(Suppl. 1):S41-4. http://dx.doi.org/10.1016/j.revmed.2011.03.331 PMid:21530020.
» http://dx.doi.org/10.1016/j.revmed.2011.03.331 - Risal S, Manti M, Lu H, Fornes R, Larsson H, Benrick A, Deng Q, Cesta CE, Rosenqvist MA, Stener-Victorin E. Prenatal androgen exposure causes a sexually dimorphic transgenerational increase in offspring susceptibility to anxiety disorders. Transl Psychiatry. 2021;11(1):45. http://dx.doi.org/10.1038/s41398-020-01183-9 PMid:33441551.
» http://dx.doi.org/10.1038/s41398-020-01183-9 - Risal S, Pei Y, Lu H, Manti M, Fornes R, Pui HP, Zhao Z, Massart J, Ohlsson C, Lindgren E, Crisosto N, Maliqueo M, Echiburu B, Ladron de Guevara A, Sir-Petermann T, Larsson H, Rosenqvist MA, Cesta CE, Benrick A, Deng Q, Stener-Victorin E. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med. 2019;25(12):1894-904. http://dx.doi.org/10.1038/s41591-019-0666-1 PMid:31792459.
» http://dx.doi.org/10.1038/s41591-019-0666-1 - Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30(Suppl. A):32-7. http://dx.doi.org/10.1016/j.placenta.2008.11.009 PMid:19070896.
» http://dx.doi.org/10.1016/j.placenta.2008.11.009 - Rosario FJ, Jansson N, Kanai Y, Prasad PD, Powell TL, Jansson T. Maternal protein restriction in the rat inhibits placental insulin, mTOR, and STAT3 signaling and down-regulates placental amino acid transporters. Endocrinology. 2011;152(3):1119-29. http://dx.doi.org/10.1210/en.2010-1153 PMid:21285325.
» http://dx.doi.org/10.1210/en.2010-1153 - Rosario FJ, Kanai Y, Powell TL, Jansson T. Increased placental nutrient transport in a novel mouse model of maternal obesity with fetal overgrowth. Obesity. 2015;23(8):1663-70. http://dx.doi.org/10.1002/oby.21165 PMid:26193061.
» http://dx.doi.org/10.1002/oby.21165 - Rosario FJ, Powell TL, Jansson T. Activation of placental insulin and mTOR signaling in a mouse model of maternal obesity associated with fetal overgrowth. Am J Physiol Regul Integr Comp Physiol. 2016;310(1):R87-93. http://dx.doi.org/10.1152/ajpregu.00356.2015 PMid:26491103.
» http://dx.doi.org/10.1152/ajpregu.00356.2015 - Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EHJM, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51(2):383-92. http://dx.doi.org/10.1161/HYPERTENSIONAHA.107.101477 PMid:18086952.
» http://dx.doi.org/10.1161/HYPERTENSIONAHA.107.101477 - Santillan MK, Pelham CJ, Ketsawatsomkron P, Santillan DA, Davis DR, Devor EJ, Gibson-Corley KN, Scroggins SM, Grobe JL, Yang B, Hunter SK, Sigmund CD. Pregnant mice lacking indoleamine 2,3-dioxygenase exhibit preeclampsia phenotypes. Physiol Rep. 2015;3(1):e12257. http://dx.doi.org/10.14814/phy2.12257 PMid:25602015.
» http://dx.doi.org/10.14814/phy2.12257 - Santillan MK, Santillan DA, Scroggins SM, Min JY, Sandgren JA, Pearson NA, Leslie KK, Hunter SK, Zamba GKD, Gibson-Corley KN, Grobe JL. Vasopressin in preeclampsia: a novel very early human pregnancy biomarker and clinically relevant mouse model. Hypertension. 2014;64(4):852-9. http://dx.doi.org/10.1161/HYPERTENSIONAHA.114.03848 PMid:25001273.
» http://dx.doi.org/10.1161/HYPERTENSIONAHA.114.03848 - Sarli PM, Manousopoulou A, Efthymiou E, Zouridis A, Potiris A, Pervanidou P, Panoulis K, Vlahos N, Deligeoroglou E, Garbis SD, Eleftheriades M. Liver proteome profile of growth restricted and appropriately grown newborn Wistar rats associated with maternal undernutrition. Front Endocrinol. 2021;12:684220. http://dx.doi.org/10.3389/fendo.2021.684220 PMid:34127923.
» http://dx.doi.org/10.3389/fendo.2021.684220 - Sathishkumar K, Elkins R, Chinnathambi V, Gao H, Hankins GDV, Yallampalli C. Prenatal testosterone-induced fetal growth restriction is associated with down-regulation of rat placental amino acid transport. Reprod Biol Endocrinol. 2011;9(1):110. http://dx.doi.org/10.1186/1477-7827-9-110 PMid:21812961.
» http://dx.doi.org/10.1186/1477-7827-9-110 - Sferruzzi-Perri AN, Camm EJ. The programming power of the placenta. Front Physiol. 2016;7:33. http://dx.doi.org/10.3389/fphys.2016.00033 PMid:27014074.
» http://dx.doi.org/10.3389/fphys.2016.00033 - Siragher E, Sferruzzi-Perri AN. Placental hypoxia: what have we learnt from small animal models? Placenta. 2021;113:29-47. http://dx.doi.org/10.1016/j.placenta.2021.03.018 PMid:34074553.
» http://dx.doi.org/10.1016/j.placenta.2021.03.018 - Skeffington KL, Higgins JS, Mahmoud AD, Evans AM, Sferruzzi-Perri AN, Fowden AL, Yung HW, Burton GJ, Giussani DA, Moore LG. Hypoxia, AMPK activation and uterine artery vasoreactivity. J Physiol. 2016;594(5):1357-69. http://dx.doi.org/10.1113/JP270995 PMid:26110512.
» http://dx.doi.org/10.1113/JP270995 - Strakovsky RS, Zhang X, Zhou D, Pan YX. Gestational high fat diet programs hepatic phosphoenolpyruvate carboxykinase gene expression and histone modification in neonatal offspring rats. J Physiol. 2011;589(Pt 11):2707-17. http://dx.doi.org/10.1113/jphysiol.2010.203950 PMid:21486814.
» http://dx.doi.org/10.1113/jphysiol.2010.203950 - Strauss JF 3rd, Martinez F, Kiriakidou M. Placental steroid hormone synthesis: unique features and unanswered questions. Biol Reprod. 1996;54(2):303-11. http://dx.doi.org/10.1095/biolreprod54.2.303 PMid:8788180.
» http://dx.doi.org/10.1095/biolreprod54.2.303 - Sun M, Maliqueo M, Benrick A, Johansson J, Shao R, Hou L, Jansson T, Wu X, Stener-Victorin E. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring. Am J Physiol Endocrinol Metab. 2012;303(11):E1373-85. http://dx.doi.org/10.1152/ajpendo.00421.2012 PMid:23047983.
» http://dx.doi.org/10.1152/ajpendo.00421.2012 - Taylor PD, Samuelsson AM, Poston L. Maternal obesity and the developmental programming of hypertension: a role for leptin. Acta Physiol (Oxf). 2014;210(3):508-23. http://dx.doi.org/10.1111/apha.12223 PMid:24433239.
» http://dx.doi.org/10.1111/apha.12223 - Thaete LG, Dewey ER, Neerhof MG. Endothelin and the regulation of uterine and placental perfusion in hypoxia-induced fetal growth restriction. J Soc Gynecol Investig. 2004;11(1):16-21. http://dx.doi.org/10.1016/j.jsgi.2003.07.001 PMid:14706678.
» http://dx.doi.org/10.1016/j.jsgi.2003.07.001 - Thompson LP, Pence L, Pinkas G, Song H, Telugu BP. Placental hypoxia during early pregnancy causes maternal hypertension and placental insufficiency in the hypoxic guinea pig model. Biol Reprod. 2016;95(6):128. http://dx.doi.org/10.1095/biolreprod.116.142273 PMid:27806942.
» http://dx.doi.org/10.1095/biolreprod.116.142273 - Troisi R, Potischman N, Roberts J, Ness R, Crombleholme W, Lykins D, Siiteri P, Hoover RN. Maternal serum oestrogen and androgen concentrations in preeclamptic and uncomplicated pregnancies. Int J Epidemiol. 2003;32(3):455-60. http://dx.doi.org/10.1093/ije/dyg094 PMid:12777436.
» http://dx.doi.org/10.1093/ije/dyg094 - Turan S, Aberdeen GW, Thompson LP. Chronic hypoxia alters maternal uterine and fetal hemodynamics in the full-term pregnant Guinea pig. Am J Physiol Regul Integr Comp Physiol. 2017;313(4):R330-9. http://dx.doi.org/10.1152/ajpregu.00056.2017 PMid:28679680.
» http://dx.doi.org/10.1152/ajpregu.00056.2017 - Vargas VE, Gurung S, Grant B, Hyatt K, Singleton K, Myers SM, Saunders D, Njoku C, Towner R, Myers DA. Gestational hypoxia disrupts the neonatal leptin surge and programs hyperphagia and obesity in male offspring in the Sprague-Dawley rat. PLoS One. 2017;12(9):e0185272. http://dx.doi.org/10.1371/journal.pone.0185272 PMid:28957383.
» http://dx.doi.org/10.1371/journal.pone.0185272 - Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279(1):E83-7. http://dx.doi.org/10.1152/ajpendo.2000.279.1.E83 PMid:10893326.
» http://dx.doi.org/10.1152/ajpendo.2000.279.1.E83 - Villarroel C, Salinas A, López P, Kohen P, Rencoret G, Devoto L, Codner E. Pregestational type 2 diabetes and gestational diabetes exhibit different sexual steroid profiles during pregnancy. Gynecol Endocrinol. 2017;33(3):212-7. http://dx.doi.org/10.1080/09513590.2016.1248933 PMid:27898283.
» http://dx.doi.org/10.1080/09513590.2016.1248933 - Vomhof-DeKrey E, Darland D, Ghribi O, Bundy A, Roemmich J, Claycombe K. Maternal low protein diet leads to placental angiogenic compensation via dysregulated M1/M2 macrophages and TNFα expression in Sprague-Dawley rats. J Reprod Immunol. 2016;118:9-17. http://dx.doi.org/10.1016/j.jri.2016.08.009 PMid:27596280.
» http://dx.doi.org/10.1016/j.jri.2016.08.009 - Vucetic Z, Totoki K, Schoch H, Whitaker KW, Hill-Smith T, Lucki I, Reyes TM. Early life protein restriction alters dopamine circuitry. Neuroscience. 2010;168(2):359-70. http://dx.doi.org/10.1016/j.neuroscience.2010.04.010 PMid:20394806.
» http://dx.doi.org/10.1016/j.neuroscience.2010.04.010 - Walters KA, Bertoldo MJ, Handelsman DJ. Evidence from animal models on the pathogenesis of PCOS. Best Pract Res Clin Endocrinol Metab. 2018;32(3):271-81. http://dx.doi.org/10.1016/j.beem.2018.03.008 PMid:29779581.
» http://dx.doi.org/10.1016/j.beem.2018.03.008 - Walters KA. Androgens in polycystic ovary syndrome: lessons from experimental models. Curr Opin Endocrinol Diabetes Obes. 2016;23(3):257-63. http://dx.doi.org/10.1097/MED.0000000000000245 PMid:26866639.
» http://dx.doi.org/10.1097/MED.0000000000000245 - Walters KA. Role of androgens in normal and pathological ovarian function. Reproduction. 2015;149(4):R193-218. http://dx.doi.org/10.1530/REP-14-0517 PMid:25516989.
» http://dx.doi.org/10.1530/REP-14-0517 - Warner MJ, Ozanne SE. Mechanisms involved in the developmental programming of adulthood disease. Biochem J. 2010;427(3):333-47. http://dx.doi.org/10.1042/BJ20091861 PMid:20388123.
» http://dx.doi.org/10.1042/BJ20091861 - Warshaw ML, Johnson DC, Khan I, Eckstein B, Gibori G. Placental secretion of androgens in the rat. Endocrinology. 1986;119(6):2642-8. http://dx.doi.org/10.1210/endo-119-6-2642 PMid:3490962.
» http://dx.doi.org/10.1210/endo-119-6-2642 - Williams L, Seki Y, Vuguin PM, Charron MJ. Animal models of in utero exposure to a high fat diet: a review. Biochim Biophys Acta Mol Basis Dis. 2014;1842(3):507-19. http://dx.doi.org/10.1016/j.bbadis.2013.07.006 PMid:23872578.
» http://dx.doi.org/10.1016/j.bbadis.2013.07.006 - Zhu W, Zhu J, Liang L, Shen Z, Wang Y. Maternal undernutrition leads to elevated hepatic triglycerides in male rat offspring due to increased expression of lipoprotein lipase. Mol Med Rep. 2016;13(5):4487-93. http://dx.doi.org/10.3892/mmr.2016.5040 PMid:27035287.
» http://dx.doi.org/10.3892/mmr.2016.5040
Publication Dates
-
Publication in this collection
22 Apr 2022 -
Date of issue
2022
History
-
Received
23 Dec 2021 -
Accepted
21 Mar 2022