Abstract
We evaluated three packing systems (PARC boxes, "GT" screen towers and "MX" screen towers) for the emergence and sexual maturation of sterile fruit flies, at three adult fly densities (1, 1.2 and 1.3 fly/cm²) and three food types. At the lowest density, results showed no significant differences in the longevity and flight ability of adult Anastrepha ludens (Loew) and Anastrepha obliqua Macquart among the three packing systems. Higher densities resulted in a decrease in these parameters. In the evaluation of the three food types, no significant differences were found either on longevity or flight ability of A. ludens. However, the greatest longevity for both sexes A. obliqua was obtained with commercial powdered Mb® and the mix of sugar, protein and corn starch on paper (SPCP) food types. The highest value for flight ability in A. obliqua males was obtained with powdered Mb® and SPCP food types, and for females with Mb® powdered food. Our data indicated that GT and MX screen tower packing systems are an alternative to the PARC boxes, since they were suitable for adult fly sexual maturation without any harm to their longevity or flight ability. The tested foods were equivalent in both fruit fly species, with the exception of the agar type for A. obliqua, which yielded the lowest biological parameters evaluated. Our results contribute to the application of new methods for the packing and release of sterile flies in large-scale programs.
Longevity; flight ability; emergence tower; Anastrepha ludens; Anastrepha obliqua
BIOLOGICAL CONTROL
Chilled packing systems for fruit flies (Diptera: Tephritidae) in the sterile insect technique
Emilio Hernández; Arseny Escobar; Bigail Bravo; Pablo Montoya
Programa Moscafrut, SAGARPA IICA, Central Poniente 14, Col Centro, 30700 Tapachula, Chiapas, México
ABSTRACT
We evaluated three packing systems (PARC boxes, "GT" screen towers and "MX" screen towers) for the emergence and sexual maturation of sterile fruit flies, at three adult fly densities (1, 1.2 and 1.3 fly/cm2) and three food types. At the lowest density, results showed no significant differences in the longevity and flight ability of adult Anastrepha ludens (Loew) and Anastrepha obliqua Macquart among the three packing systems. Higher densities resulted in a decrease in these parameters. In the evaluation of the three food types, no significant differences were found either on longevity or flight ability of A. ludens. However, the greatest longevity for both sexes A. obliqua was obtained with commercial powdered Mb® and the mix of sugar, protein and corn starch on paper (SPCP) food types. The highest value for flight ability in A. obliqua males was obtained with powdered Mb® and SPCP food types, and for females with Mb® powdered food. Our data indicated that GT and MX screen tower packing systems are an alternative to the PARC boxes, since they were suitable for adult fly sexual maturation without any harm to their longevity or flight ability. The tested foods were equivalent in both fruit fly species, with the exception of the agar type for A. obliqua, which yielded the lowest biological parameters evaluated. Our results contribute to the application of new methods for the packing and release of sterile flies in large-scale programs.
Key words: Longevity, flight ability, emergence tower, Anastrepha ludens, Anastrepha obliqua
The Sterile Insect Technique (SIT) was first successfully applied in the 1950s against the New World screwworm, Cochliomyia hominivorax (Coquerel), leading to the eradication of this pest from the USA, Mexico, and Central America after 43 years (Klassen & Curtis 2005). Currently, the technique is more broadly used against Tephritidae fruit flies (Enkerlin 2005). A successful example of the SIT is the program against the Mediterranean fruit fly, Ceratitis capitata (Wied.), established in the 1970s in order to prevent the invasion of this pest in southeastern Mexico (Hendrichs et al 1983). In Mexico, the SIT has been applied against Anastrepha ludens (Loew) since 1994 (Reyes et al 2000), and Anastrepha obliqua (Macquart) since 2001 (Artiaga-López et al 2004).
The final step in the SIT is the release of sterile flies in the field. For this purpose, flies are packed at their pupal stage in diverse containers, such as PARC (Plastic Adult Release Container) emergence boxes or towers for their emergence and sexual maturation, and finally released in the field using the "chilled adult" system (Villaseñor 1985, Salvato et al 2004, Dowell et al 2005, Shelly et al 2006). This release system is the most commonly used since it favors a more homogeneous distribution of the sterile material in the field and avoids the dissemination of undesirable residue (i.e., the remains of bags and pupae) during the release process.
The packing units, by design, differ in volume and resting area that causes variations in fly density, a factor that may affect the quality of the released males. In the PARC boxes, flies are packed in paper bags, increasing the resting surface (Dowell et al 2005). The Guatemala (GT)-type towers have conduits integrated within the sieve frame and a ventilation area measuring 9,504 cm2. In the Mexico (MX)-type towers, pupae are packed in paper bags, and the units are twice the height of the Guatemalan type, achieving a ventilation area measuring 14,938 cm2, which permits a more efficient dissipation of the moisture and heat generated by the flies, reducing moisture condensation when the towers are chilled (Shelly et al 2006).
As adults undergo sexual maturation in the packing units, the flies must be fed prior to their release aid on the maintenance of their energy reserves (Warburg & Yuval 1996). At present, most fruit fly packing and releasing centers provide sugar and water (Barnes et al 2004, Dantas et al 2004, Smallridge & Hopkins 2004). Some also add hydrolyzed protein, which makes several amino acids available to adult nutrition (Kaspi & Yuval 2000, Kaspi et al 2000), production of sexual pheromones or for increasing adult mass and inducing more efficient mating by males (Yuval et al 2002, Maor et al 2004). The food given in packing centers is a mixture available as (1) powder, (2) gel and (3) solid paste on paper. The only commercially available formula is the Mb® powdered diet (Mubarqui® Aerial Services, Ciudad Victoria, Tamaulipas, México), made from amaranth flour, peanuts and sugar, containing a total 10.19% of protein.
The objective of this study was to evaluate different packing systems for fruit flies release, determining the effect of adult density and the three types of food on the longevity and flight ability of A. ludens and A. obliqua adults held during emergence and sex maturation in PARC boxes, Guatemala-type screen towers, and Mexico-type screen towers.
Our working hypotheses were: (1) if the packing units showed a difference in the ventilation and dissipation of metabolic heat, the density of the adults in the container might cause differences in longevity and flight ability in adults after being chilled for release, and (2) if the food provided is balanced with respect to protein and carbohydrate sources, there would not be significant differences in longevity or flight ability among the flies, irrespective of diet. This study is the first to discuss the different types of emergence towers used in the application of the SIT in Anastrepha fruit flies.
Material and Methods
Specimens. Adult of A. ludens and A. obliqua were obtained from the material produced at the Fruit Flies Facility (Moscafrut Plant) DGSV-SAGARPA located in Metapa de Domínguez, Chiapas, in accordance with Stevens (1991) and Artiaga-López et al (2004), respectively. The packing of the flies used in the experiment was carried out at the Chilled Adult Packing Center DGSV-SAGARPA, located in Tapachula, Chiapas. The experimental work was performed in two different assays.
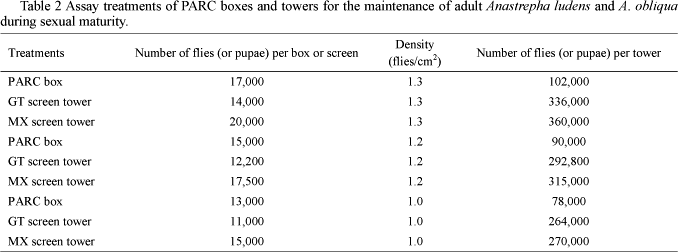
Effect of the packing type and fly density. The units for packing and sexual maturation were assayed in a three-factor experimental design. The packing system factor accounted for the PARC box, GT-type screen tower, and MX-type screen tower (Mubarqui® Aerial Services, Ciudad Victoria, Tamaulipas, México) (Table 1). The density factor was evaluated at densities of 1, 1.2 and 1.3 flies/cm2 (Table 2). The third factor was the sex (male-female). There were a total of nine treatments, which were replicated twelve times.
In the PARC boxes (60 x 33 x 48 cm), pupae were distributed among six Kraft-paper bags (26 x 20 x 11 cm) (Dowell et al 2005), and food was placed vertically in strips between the bags. Pupae were placed on the screens of the GT towers in polycarbonate containers (68.5 x 3 x 3 cm), which were placed on the sides and attached to the screen that serve as a lid. Food was placed on the screen base. In the MX towers, pupae were placed in two Kraft-paper bags (11 x 20 x 11 cm).
Each replicate corresponded to a separate lot of flies. The towers with emerged adult A. ludens and A. obliqua were kept for seven days in the emergence room at 21°C and 70% RH, and adults were fed as they matured sexually. Flies were chilled at 3°C for 45 min as described by Dowell et al (2005). After chilling, samples were taken in order to determine longevity under conditions of stress, without water and food, as well as flight ability (FAO/IAEA/USDA 2003).
The food used consisted of sugar + protein + cornstarch on paper (SPCP). A mixture of 40 ml water, 74.61 g sugar, 4.2 g hydrolyzed protein, 4 g cornstarch (Maizena®, Unilever de México, S. de R.L. de C.V. Tultitlán, México) and 0.05 g of nipagin was gradually heated until a homogeneous paste was made. The mixture was then brushed onto the surface of a brown towel paper. Once the food had dried, two 24 x 40 cm strips were placed inside the PARC boxes and screens. In PARC boxes, a sponge saturated with water and guar gum was placed on the lid to avoid dripping, while in the screen towers, only the sponge was placed on the screen that serves as a lid.
Type of food. The type of food used in the emergence tower screens was evaluated using a completely random design, with three treatments: (1) sugar + protein + cornstarch on paper (SPCP), (2) agar with sugar, protein and nipagin, and (3) the commercially prepared formula, powdered Mb®.
The SPCP was prepared as described in the previous section and was applied to the surface of a 24 x 50 cm strip of brown towel paper. A sponge saturated with water + guar gum was placed on the screen that serves as a lid to avoid dripping. Each screen was fitted with a piece of brown paper providing 1200 cm2 of surface area for fly feeding. For all foods, a density of 15,000 flies per screen in the MX-type tower was used.
The agar was prepared by dissolving 4 g of agar-agar (Agarmex, Tlalnepantla, Mexico) in 421.5 ml of hot water. The mixture was left to cool down to 50°C, and 74.61 g of sugar (Ingenio Huixtla, Chiapas, México), 4.2 g of hydrolyzed protein and 0.05 g of nipagin (Mallinckrodt Specialty Chemicals Co., St. Louis, Miss.) were added. The mixture was then poured into rectangular molds (21 x 4 x 21 cm). This treatment contained water in a readily available form (agar), so no alternative source was provided. A block of agar was placed into each screen of the tower type MX, providing 1,218 cm2 of surface area for fly feeding. In the cases of PARC boxes and tower type GT, only one face of the block (the lower face = 441 cm2) was available for fly feeding.
The Mb® (Mubarqui® Aerial Services, Cd. Victoria, Tamaulipas, México) in a commercially available powder based on a mixture of amaranth and peanut flour, and refined sugar (FAO 2007). This food is 10.19% raw protein and has a pH of 6.15 I.U., an acidity of 0.133%, and a moisture content of 4.34%. As for its microbiological characteristics, the total account was 23,750 UFC/g, of which <10 UFC/g were fungus, <10 UFC/g were yeast, and <3 NMP/g were coliform type I, non-fecal bacteria. In order to use this food in the emergence containers during sexual maturation, a mixture of 50 g of the powdered food and 15 ml of water had to be prepared at room temperature. The mixture was processed manually and was applied to one of the sides of a 170 x 8 cm corrugated plastic sheet (Karton Plast type) (Carton Plast S. A. de C. V., Ecatepec, Edo. de México), providing a surface area of 1360 cm2. Water was made available through a water-saturated Mubarqui® sponge, which was laid on the screens.
Data collection. In both experiments, adult longevity under stress conditions (no food or water) and flight ability were assessed. In order to determine longevity, 600 adults (Hernández et al 2005) were taken from each PARC box (six samples of 100 per trial). Samples were placed in 25 x 150 mm Petri dishes, as per the Quality Control Manual (FAO/IAEA/USDA 2002). In the case of the screen towers, 100-adult samples were systematically taken from the screens ordered by the numbers 2, 6, 10, 14, 18 and 22 in the respective tower, for a total of six samples of 100 per trial. Under stress conditions, the number of dead flies was quantified every 24h until 50% mortality rate was observed.
To determine flight ability, we used the PARC unit where we collected the chilled flies. The PARC boxes were exposed to external environmental conditions and sun light for 30 min while the flies awoke from the chilling and finally flew away. Flight ability was estimated from the difference in weight (g) between the full packing unit and the fly residue that remained in the packing unit after the flyers had left the PARC box. The non-flyers, deformed flies, and dead flies remained at the bottom of the PARC box and were referred to as the "residue." The total residue was weighed and flies of both sexes were counted. When the residue was greater than 15 g, three samples of 3 g each were taken, and the number of flies per gram was estimated, thus providing estimates of the number of flies remaining in the residue. Therefore, flight ability is presented as the percentage of flies that flew out of the PARC box.
Flight ability (FA) was calculated as follows: FA = [(NEF NFR) / NEF] x 100, where NEF is the Number of Emerged Flies per parking unit, and NFR is the Number of Flies in the Residue. Previously, NEF = NP x ER, where NP is the Number of Pupa, and ER the Emergence Ratio; NFR = GR x NFG, where GR is the Grams of Residue, and NFG is the Number of Flies in one Gram.
Statistical analysis. Longevity and flight ability had to be transformed from percentages into arc-sine degrees as radians of the square root of the ratio X' = Sen-1 "X, where X is the original value as a ratio (percentage /100) (Underwood 2005). Using the transformed data, a variance analysis was undertaken, and the separation of means was performed in accordance with Tukey's Test (P < 0.05) (Zar 1984). JMP Statistical Discovery Software (SAS Institute 2003) was used for analysis. For both species, A. ludens and A. obliqua, analyses were conducted by sex.
Results
Effect of the packing unit and density. There was no difference in longevity between sexes in either species regarding the effect of the packing unit and density: A. ludens (F17, 198 = 1.51, P = 0.0932) and A. obliqua (F17, 198 = 1.18, P = 0.2803). Flight ability (%) results indicated a significant difference between sexes in both species: A. ludens (F17, 198 = 11.46, P < 0.001) and A. obliqua (F17, 198 = 8.62, P < 0.001).
Anastrepha ludens. The longevity of males showed no significant difference when they were kept in GT screen towers and MX screen towers at densities of 1, 1.2 and 1.3 flies / cm2, only showed difference when were kept in PARC boxes and the highest longevity was observed in males held at a density of 1 fly / cm2 (F2, 33 = 154.14, P < 0.001) (Table 3). The longevity of females showed the same performance, since that highest average of longevity was observed in females that were kept in PARC boxes at a density of 1 fly / cm2 (F2, 33 = 4.69, P = 0.0161) (Table 3).
Males flight ability at the densities of 1 and 1.2 flies/cm2 not showed significant differences when they were kept in GT and MX screen towers, but when they were kept in PARC boxes the highest flight ability were observed at a density of 1 fly / cm2 and the lowest was for males kept at the density of 1 fly / cm2 (F2, 33 = 5.40, P = 0.0093). Females flight ability showed no significant difference when they were kept in GT screen tower at 1, 1.2 and 1.3 flies / cm2, however they showed significant difference when they were kept in PARC boxes and MX screen tower, considering only the last two packing unit, the highest average was observed in males kept at 1 and 1.2 flies / cm2 and the lowest at 1.3 flies / cm2 (F2, 33 = 10.43, P = 0.0003) (Table 4).
Anastrepha obliqua. The longevity of males showed no significant difference when they were kept in GT screen towers and MX screen towers at densities of 1, 1.2 and 1.3 flies / cm2, only showed difference when were kept in PARC boxes and the highest longevity was observed in males held at a density of 1 fly / cm2 and the lowest at 1.3 flies / cm2 (F2, 33 = 3.47, P = 0.043) (Table 5). The longevity of females showed the same performance, the highest average of longevity was observed in females that were kept in PARC boxes at a density of 1 fly / cm2 and the lowest at 1.3 flies / cm2 (F2, 33 = 14.93, P < 0.0001) (Table 5).
Males flight ability at the densities of 1, 1.2 and 1.3 flies/cm2 showed no significant differences when they were kept in GT screen towers, but the difference was significant when they were kept in PARC boxes and MX screen tower; the highest flight ability were observed at a density of 1 fly / cm2 in PARC boxes and the lowest was for males kept at the density of 1.3 flies / cm2 in MX screen tower (F2, 33 = 23.54, P < 0.001) (Table 6). Females flight ability showed no significant difference when they were kept in GT screen tower at 1, 1.2 and 1.3 flies / cm2, however difference was significant when they were kept in PARC boxes and MX screen tower (F2, 33 = 8.51, P = 0.001) (Table 6).
Food type. The type of diet did not affect adult longevity and flight ability in A. ludens, for male (F2, 33 = 0.44, P = 0.6458; F2, 33 = 2.07, P = 0.1422), and female (F2, 33 = 1.28, P = 0.2912; F2, 33 = 1.8, P = 0.1817).
The highest values for longevity in males (F2, 33 = 8.15, P = 0.0013) and females (F2, 33 = 9.91, P = 0.0.0004) of A. obliqua were obtained with the powdered Mb® diet and with the sugar + protein + corn starch/ paper. The highest flight ability for males (F2, 33 = 28.47, P < 0.0001) and females (F2, 33 = 4.23, P = 0.0232) were obtained with the Mb® diet, although not different from that obtained for males in sugar + protein + corn starch/ paper (Fig 1).
Discussion
Salvato et al (2004) and Shelly et al (2006) found no differences in fly quality using emergence towers as an alternative to PARC boxes for packing and sexual maturation. Our data indicated that longevity and flight ability were not affected by the different types of packing units compared, which implies that differences in resting areas and ventilation do not have any significant effects on these parameters. However, the lower density of adult flies per packing unit (1 fly/cm2), consistently produced the best results in longevity and flight ability for both fruit fly species, suggesting that the crowding could play an important role in the final performance of confined insects when released in the field.
Similar results were observed by Liedo et al (2007) and Díaz-Fleischer et al (2009), who evaluated cage density under laboratory conditions and found that low densities resulted in individuals with increased mating competitiveness. In the same sense, the highest flight ability for A. ludens was obtained in MX screen towers, as they have a larger ventilation area. This is in agreement with what Dowell et al (2005) previously noted: humidity control during emergence and chilling is of great importance (see also Broufas et al 2009), since high humidity (e.g., 94%), can increase water condensation upon chilling, causing the insects to get stuck to the walls of the container, where they may die or have their legs and/or wings amputated.
A "tower" of PARC boxes contains six units measuring 0.60 x 0.48 x 0.33 m (length x width x height), providing a surface area of 0.29 m2. A GT emergence tower is made up of 24 screens measuring 0.66 x 0.72 x 0.04 m, with a surface area of 0.48 m2. An MX tower consists of 18 screens measuring 0.77 x 0.77 x 0.10 m, giving a surface area of 0.59 m2. Accordingly, a GT tower is equivalent to 1.6 PARC box "towers", and an MX tower is equivalent to ≅ 2 PARC box "towers". At a density of 1 fly / cm2, 1.6 PARC towers hold 124,800 flies, while ≅ 2 PARC towers would hold 156,000 flies. GT towers hold 264,000 flies, while the MX towers hold 270,000. The MX tower packing unit, loaded with 15,000 pupae, can hold a greater number of flies per packing unit when compared to what is currently in use in the release facilities in Mission, Texas (USA) or at Montemorelos, N.L., where 14,000 and 7,000 A. ludens pupae are loaded per screen, at maximum and minimum densities, respectively. If we compare the occupied area for an MX tower and two PARC box towers, the former packing unit permits the release of 114,000 more flies than the latter, while a GT tower compared to 1.6 PARC box towers permits the release of 139,000 more flies.
The absence of any significant difference in longevity or flight ability in A. ludens indicates that all three types of food provide the nutrients necessary for adults to survive and fly, although the highest values for flight ability were obtained with the balanced powdered diet, Mb®. In the case of A. obliqua, longevity was lower when nutrients were provided through the agar type food, but no differences were found between the balanced powdered diet (Mb®) and sugar + protein + corn starch/paper diet. These results contrast with the report of Cresoni-Pereira & Zucoloto (2001), who determined that the optimum protein concentration in the adult diet runs between 2.5% and 6.5%, and the values above 8.5% reduced longevity of female A. obliqua, since the powdered Mb® diet contains 10.19% protein. This suggests that it would be beneficial to re-evaluate the dose given to this species of this type of food, which seems to be promising if we consider that it contains natural protein from amaranth, peanut and egg, which apparently have not had detrimental effects on longevity in A. obliqua.
Our results support the idea that adult density per packing unit must be carefully determined in order to release insects with better performance in the field. In congruence with Salvato et al (2004) and Shelly et al (2006), we also conclude that the GT and MX screen tower packing systems are alternatives to PARC boxes, since they supply the conditions required to reach sexual maturity of the sterile flies without negative effects on longevity or flight ability. The three types of food were found to be equivalent from the perspective of the evaluated parameters (except for agar type food in A. obliqua), since they provide to adults the nutrients necessary to achieve high longevity and flight ability. These findings provide for greater options and support the application and development of new methods in the packing and release of sterile flies in large-scale programs.
Acknowledgments
We thank Lucy Tirado and the staff at the CEAF Tapachula for lending their facilities and support for this study. We are also grateful to Julio Dominguez, Eduardo Solís and Trinidad Artiaga (Moscafrut Facility) for providing the biological material and to Mubarqui® Aerial Services, Cd. Victoria, Tamps., México, for providing the food marked Mb®.
Received 17/III/08.
Accepted 10/II/10.
Edited by Dori E Nava ESALQ/USP
- Artiaga-López T, Hernández E, Dominguez J, Moreno D S, Orozco-Dávila D (2004) Mass-production of Anastrepha obliqua at the Moscafrut Fruit Fly Facility, Mexico, p.389-392. In Brian B N (ed) Proceedings of the 6th International Symposium on Fruit Flies of Economic Importance. Johannesburg, Ultra Litho (Pty) Ltd, 509p.
- Barnes B N, Eyles D K, Franz G (2004) South Africa´s fruit fly SIT programme- the Hex River Valley pilot project and beyond, p.131-141. In Brian B N (ed) Proceedings of the 6th International Symposium on Fruit Flies of Economic Importance. Johannesburg, Ultra Litho (Pty) Ltd, 509p.
- Broufas G D, Pappas M L, Koveos D S (2009) Effect of relative humidity on longevity, ovarian maturation, and egg production in the olive fruit fly (Diptera: Tephritidae). Ann Entomol Soc Amer 102: 70-75.
- Cresoni-Pereira C, Zucoloto F S (2001) Influence of quantities of brewer yeast on the performance of Anastrepha obliqua wild females (Diptera: Tephritidae). Iheringia, Série Zool 91: 53-60.
- Dantas L, Pereira R, Silva N, Rodrigues A, Costa R (2004) The SIT control programme against Medfly on Madeira Island, p.127-130. In Brian B N (ed) Proceedings of the 6th International Symposium on Fruit Flies of Economic Importance. Johannesburg, Ultra Litho (Pty) Ltd, 509p.
- Díaz-Fleischer F, Arredondo J, Aluja M (2009) Enriching early adult environment affects the copulation behaviour of a tephritid fly. J Exp Biol 212: 2120-2127.
- Dowell R V, Worley J, Gomes P J (2005) Sterile insect supply, emergence, and release, p.297-324. In Dyck V A, Hendrichs J, Robinson A S (eds) Sterile insect technique. Principles and practice in area-wide integrated pest management. The Netherlands, Springer, 787p.
- Enkerlin W (2005) Impact of fruit flies programmes using the sterile insect technique, p.651-676. In Dyck V A, Hendrichs J, Robinson A S (eds) Sterile insect technique. Principles and practice in area-wide integrated pest management. The Netherlands, Springer, 787p.
- FAO Food and Agriculture Organization of the United Nations (2007) Guidance for packing, shipping, holding and release of sterile flies in area-wide fruit fly control programmes, FAO Plant production and protection paper 190, Rome, Italy, 134p.
- FAO/IAEA/USDA (2003) Manual for product quality control and shipping procedures for sterile mass-reared tephritid fruit flies, version 5.0. Vienna, Austria, IAEA, 85p.
- Hernández E, Flores-Breceda S, Sosa-Iturbe M de L, Ezquivel H (2005) Tamaño de unidad muestral y número de repeticiones para la estimación de los parámetros de desarrollo de Anastrepha obliqua y Anastrepha ludens (Diptera: Tephritidae). Folia Entomol Mex 44: 155-164.
- Hendrichs J, Ortiz G, Liedo P, Schwartz A (1983) Six years of successful medfly program in Mexico and Guatemala, p.353-365. In Cavalloro R (ed) Proceedings Symposium: Fruit Flies of Economic Importance. CEC/IOBC International Symposium, 16-19 November 1982. Athens, Greece. Rotterdam, The Netherlands, A A Balkeman, 642p.
- Kaspi R, Taylor P W, Yuval B (2000) Diet and size influence sexual advertisement and copulatory success of males in Mediterranean fruit fly leks. Ecol Entomol 5: 279-284.
- Kaspi R, Yuval B (2000) Post-teneral protein feeding improves sexual competitiveness but reduces longevity of mass-reared sterile male Mediterranean fruit flies (Diptera: Tephritidae). Ann Entomol Soc Amer 93: 949-955.
- Klassen W, Curtis C F (2005) History of the sterile insect technique, p.3-36. In Dyck V A, Hendrichs J, Robinson A S (eds) Sterile insect technique. Principles and practice in area-wide integrated pest management. The Netherlands, Springer, 787p.
- Liedo P, Salgado S, Oropeza A, Toledo J (2007) Improving mating performance of mass-reared sterile Mediterranean fruit flies (Diptera: Tephritidae) through changes in adult holding conditions: demography and mating competitiveness. Fla Entomol 90: 33-40.
- Maor M, Kamensky B, Shloush S, Yuval B (2004) Effects of post-teneral diet on foraging success of sterile male Mediterranean fruit flies. Entomol Exp Appl 110: 225230.
- Reyes J, Santiago G, Hernández P (2000) The Mexican fruit fly eradication programme, p.377-380. In Tan K H (ed) Area-wide control of fruit flies and other insect pests. Malaysia, Penang, Penerbit Universiti Sains, 782p.
- Salvato M, Holler T, Worley J, Stewart J (2004) Efficacy of tower Medfly eclosion systems. Biocontrol Sc Tech 14: 77-80.
- SAS Institute (2003) JMP statistical discovery software, version 5.0.1. SAS Institute Inc., Cary, North Carolina.
- Shelly T E, Holler T C, Stewart J L (2006) Mating competitiveness of mass-reared males of the Mediterranean fruit fly (Diptera:Tephritidae) from eclosion towers. Fla Entomol 89: 380-387.
- Smallridge C J, Hopkins D C (2004) Preventative sterile fly release for the management of a Mediterranean fruit fly outbreak in South Australia, p.213-215. In Brian B N (ed) Proceedings of the 6th International Symposium on Fruit Flies of Economic Importance. Johannesburg, Ultra Litho (Pty) Ltd, 509p.
- Stevens L (1991) Manual of standard operating procedures (SOP) for the mass-rearing and sterilization of the Mexican fruit fly, Anastrepha ludens (Loew). USDA-APHIS, South Central Region, Mission Texas, 39p.
- Underwood A J (2005) Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge, 504p.
- Villaseñor C A (1985) Comparación de tres sistemas de liberación aérea para mosca del Mediterráneo estéril, Ceratitis capitata (Wied.). Tesis, Universidad Autónoma de Chiapas, Huehuetán, Chiapas, 95p.
- Warburg M S, Yuval B (1996) Effects of diet and activity on lipid levels of adult Mediterranean fruit flies. Physiol Entomol 21: 151-158.
- Yuval B, Kaspi R, Fielf S A, Blay S, Taylor P (2002) Effects of pos-teneral nutrition on reproductive success of male Mediterranean fruit flies (Diptera: Tephritidae). Fla Entomol 85: 165-170.
- Zar J H (1984) Biostatistical analysis. New Jersey Prentice-Hall, 718p.
Publication Dates
-
Publication in this collection
14 Sept 2010 -
Date of issue
Aug 2010
History
-
Received
17 Mar 2008 -
Accepted
10 Feb 2010