Abstract
This is the first time the karyotype of the Brazilian squirrel Sciurusaestuans ingrami, with 2n = 40, is described. The karyotype of this species comprises 18 pairs of biarmed and one minute pair of acrocentric autosomes, a medium-sized submetacentric X and a medium-sized acrocentric Y. Four pairs have an interstitial secondary constriction, co-located with nucleolar organizer regions (NORs), identified by silver-staining technique and fluorescent in situ hybridization (FISH) with ribosomal 18S/28S probes. The occurrence of multiple interstitial NORs is rare in rodents, and this is one of the few examples, identified by a molecular cytogenetics approach.
Squirrels; chromosomes; karyotype; NORs; Rodentia
RESEARCH ARTICLE
Multiple interstitial ribosomal sites (NORs) in the Brazilian squirrel Sciurus aestuans ingrami (Rodentia, Sciuridae) with 2n = 40. An overview of Sciurus cytogenetics
Valéria FagundesI; Alexandre Uarth ChristoffII, III; Renata Cecília Amaro-GhilardIV; Daniel R. ScheiblerIII; Yatiyo Yonenaga-YassudaIV
IUniversidade Federal do Espírito Santo, Centro de Ciências Humanas e Naturais, Departamento de Ciências Biológicas, Vitória, ES, Brazil
IIUniversidade Federal do Rio Grande do Sul, Instituto de Biociências, Museu de Ciências Naturais, Canoas, RS, Brazil
IIIUniversidade Luterana do Brasil, Centro de Ciências Naturais e Exatas, Departamento de Biologia, Porto Alegre, RS, Brazil
IVUniversidade de São Paulo, Instituto de Biociências, Departamento de Biologia, São Paulo, SP, Brazil
Correspondence Correspondence to V. Fagundes Universidade Federal do Espírito Santo, Centro de Ciências Humanas e Naturais, Departamento de Ciências Biológicas Av. Marechal Campos 1468 29040-070 Maruípe, Vitória, Espírito Santo, Brazil E-mail: vfagunde@uol.com.br
ABSTRACT
This is the first time the karyotype of the Brazilian squirrel Sciurusaestuans ingrami, with 2n = 40, is described. The karyotype of this species comprises 18 pairs of biarmed and one minute pair of acrocentric autosomes, a medium-sized submetacentric X and a medium-sized acrocentric Y. Four pairs have an interstitial secondary constriction, co-located with nucleolar organizer regions (NORs), identified by silver-staining technique and fluorescent in situ hybridization (FISH) with ribosomal 18S/28S probes. The occurrence of multiple interstitial NORs is rare in rodents, and this is one of the few examples, identified by a molecular cytogenetics approach.
Key words: Squirrels, chromosomes, karyotype, NORs, Rodentia.
Introduction
Squirrels of the genus Sciurus Linnaeus (1758), especially those from Central America, present a wide geographical variation in pelage coloration. Systematics of this group is rather puzzling. Currently, 50 genera and 273 species of squirrels are recognized in the world. Only 4 genera and 19 species from Neotropical forests were known. Hoffmann et al. (1993) recognized four species in Brazil: S. ignitus (westernmost region), S. igniventris (Amazon region), S. spadiceus (southern Amazon), and S. aestuans. There are some taxa included in S. aestuans which have been considered as full species by some authors:S. a. aestuans, found mainly in the northern Amazon; S. a. gilvigularis, from the southern Amazon and Venezuela; S. a. alphonsei, found along the coast, from southern Pará to Pernambuco; and S. a. ingrami, found all along eastern Brazil, from Bahia to Rio Grande do Sul (Emmons, 1997).
Chromosome data on Brazilian squirrels are scarce in the literature. Lima and Langguth (2002) described the karyotypes of the Brazilian species S. alphonsei and S. spadiceus, with 2n = 40 and FN = 76. North-American squirrels display different degrees of chromosomal variation. Terrestrial squirrels show a striking diversity, mainly due to Robertsonian rearrangements and pericentric inversions (2n = 30 to 50). In contrast, tree squirrels of the genus Sciurus exhibit a remarkable chromosomal homogeneity, with a 2n = 40 karyotype. Species from the Brazilian Atlantic forest (S. alphonsei)and Amazon forest (S. spadiceus) were observed to have 2n = 40 and FN = 76 (Lima and Langguth, 2002). Only one South-American squirrel (Sciurusgranatensis) was described with a 2n = 42 karyotype (Nadler and Hoffmann, 1970).
It is difficult to assess the basic or ancestral karyotype of Sciuridae, because centric fusions cannot be distinguished from fissions, but it is generally accepted that South-American sciurids evolved from ancestral North-American stock (Nadler and Hoffmann, 1970). In this paper, we present the karyotype of two Brazilian S. a. ingrami (2n = 40) females and one male, which presented a pattern of multiple interstitial NORs that is unusual in rodents.
Material and Methods
Our sample is composed of three Brazilian specimens: one female from the southernmost region, city of Venâncio Aires, state of Rio Grande do Sul (29°36'W, 52°19'S); and two specimens from the southeastern region, state of São Paulo: one female from the city of Piedade (23°42'W, 45°25'S), and one male from the city of Juquitiba (23°55'W, 47°04'S). Metaphase preparations were obtained from bone marrow, after in vivo injection of colchicine, and from fibroblasts of tail biopsy (one female), cultured in Dulbecco's Modified Eagle medium supplemented with 20% fetal bovine serum, according to conventional procedures. Metaphase cells were spread onto clean slides, air-dried, and stored at -20 °C until use. Slides were stored for several months. C-banding and silver-nitrate staining were performed according to routine techniques. We were unable, however, to obtain G-banding patterns with a good resolution.
We also mapped ribosomal genes, using in situ hybridization with a probe containing 18/28S rDNA of Xenopus laevis (HM456). Ag-staining was performed according to Howell and Black (1980), with some modifications. For FISH, slides were treated with RNAse and pepsin, denatured (70% formamide/2xSSC at 70 °C for 5 min), and 200 ng of biotinylated probe (50 mL) in a hybridization mixture (50%formamide/2xSSC, 10% dextran sulfate) were applied onto the slides. Hybridization was performed overnight in a moist chamber at 37 °C. FITC-avidin and biotinylated anti-avidin antibody (Vector) were used to detect the probe hybridization signals. After detection, slides were mounted in antifade (Vectashield) staining solution containing propidium iodide (0.5 mL/mL) and DAPI (0.8 mL/mL). Metaphases were analyzed in a Zeiss Axiophot microscope, using dual-band pass filter, and photographed with 400 ISO film (Fuji).
For the localization of telomeric sequences, FISH of the digoxigenin-labeled (TTAGGG)n oligomer (Oncor Inc.) was performed following the recommended protocol. Chromosomes were photographed using ASA 400 Kodak Kodachrome film.
Results
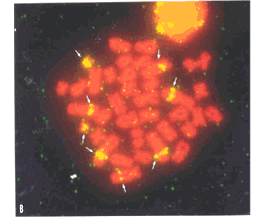
Eighteen biarmed chromosome pairs of gradually varying sizes and one minute acrocentric pair (n.19) of autosomes, one medium-sized submetacentric X, similar in size to pair n. 5, and the single medium-sized acrocentric Y compose the karyotype of S. a. ingrami (2n = 40, FN = 74). Eight secondary constrictions were observed interstitially on the long arms of four chromosome pairs: the six large submetacentrics of pairs n. 1, 2, and 3, and the two small submetacentrics of pair n. 17 (Figure 1).
C-banding revealed slightly stained pericentromeric heterochromatin on some pairs of autosomes (1, 2, 3, 4, 6, 8, 9, 10, and 14) and on the X chromosome. Heterochromatin is also observed at the secondary constriction of the small submetacentric chromosome 17 (Figure 2). The Y chromosome is not heterochromatic (data not shown). Figure 3a shows unbanded chromosomes after DAPI staining, which could explain the absence of G-banding after trypsin treatment.
Silver nitrate staining revealed Ag-NORs co-located to secondary constrictions in pairs 1, 2, 3, and 17. In 20 metaphases analyzed from one female, there was a variation from 5 to 7 Ag-NORs per cell, their frequency being of 100% on pairs 1 and 3, 97.5% on pair 17, and 22.5% on pair 2. The FISH technique showed 8 signals, which were coincident with secondary constrictions in all cells analyzed (Figure 3b). Localization of telomeric sequences revealed signals restricted to termini of all chromosomes only (Figure 3c).
Discussion
Chromosome data on squirrels from the New World are scarce. Most studies have focused on Eurasian, Japanese and South African squirrels. This is the second paper presenting chromosomal data of a Brazilian squirrel.
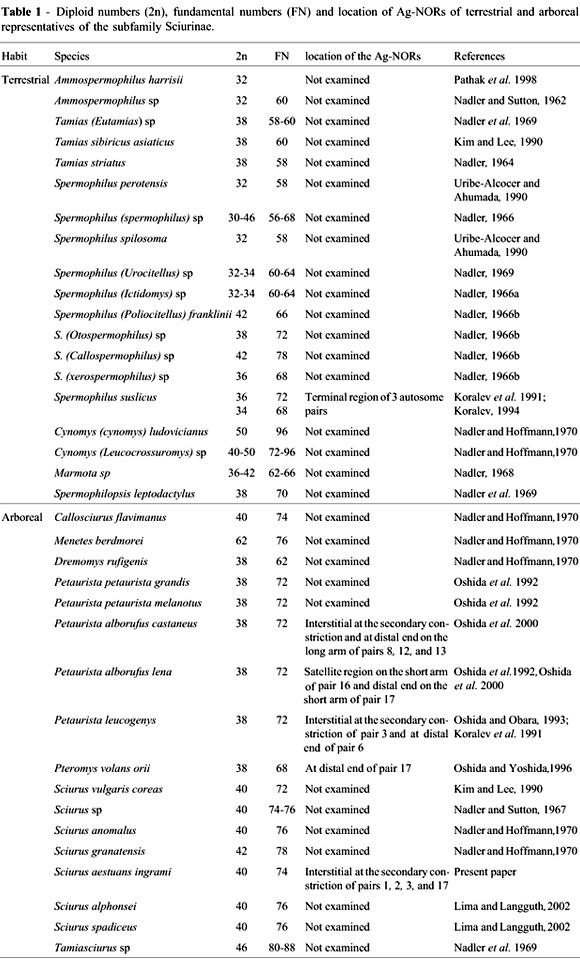
Arboreal squirrels were thought to present a striking karyotype homogeneity (Nadler and Hoffmann, 1970), but a review of squirrel chromosome data, presented in Table 1, showed that there is no such homogeneity. The diploid number 40 is a general feature in animals of the genus Sciurus, but their karyotypes are clearly different, as shown by conventional staining, C-banding and Ag-NOR patterns.
The karyotype of S. a. ingrami (2n = 40, NF = 74) differs from those of the Brazilian species S. alphonsei and S. spadiceus (2n = 40, FN = 76) in that its chromosome pair 19 is acrocentric instead of metacentric. This variation could be due to a pericentric inversion occurred in this pair. Chromosome pairs 1, 2, and 3 of S. a. ingrami presented interstitial secondary constrictions on the long arms, whereas, in the two Brazilian species, they were observed only in pair 1. The highly condensed morphology of the chromosomes of S. spadiceus and S. alphonsei may have been an impediment for the visualization of secondary constrictions. The X and Y chromosomes seem to be similar in these species. S. a. ingrami differs from the Venezuelan species S. granatensis (2n = 42, FN = 78) basically by the presence of 19 meta/submetacentric autosomes and one small acrocentric in the latter.
As compared to European/Asian species, S. a. ingrami differs from theIranian S. anomalus (2n = 40, NF = 76) basically by the presence of one minute acrocentric and four pairs of chromosomes with secondary constrictions, in contrast with one small subtelocentric in the latter. S. a. ingrami differs from the Korean S. vulgaris coreae (2n = 40, NF = 72) by the presence of 17 meta/submetacentrics and two acrocentric pairs of autosomes in the latter. NORs were not described in the species S. anomalus,S. granatensis and S. vulgaris coreae. Regarding heterochromatin, S. vulgaris coreae presented blocks at the centromeres and telomeres of all autosomes, whereas only some weak signals were observed in 10 pairs of chromosomes of the S. a. ingrami presented here.
Pericentric inversion seems to be the most frequent mechanism to explain the differences among squirrel karyotypes.
The telomeric DNA distribution in the genome of the antelope ground squirrel Ammospermophilus harrisii (2n = 32), which presented large C-banded regions, showed that all C-banded segments, except a few intercalary segments, hybridized to this DNA (Pathak et al., 1998). S. a. ingrami presented some pericentromeric heterochromatin and absence of signals on non-telomeric sites.
The presence of multiple interstitial (nucleolus organizer regions) NORs coincident with secondary constrictions, as observed in S. a. ingrami, is rare in rodents. Examples are the giant flying squirrels Petaurista alborufus castaneus and P. a. lena, with secondary constrictions on two chromosome pairs (8 and 13) and Ag-NORs, detected interstitially and at the distal end of pairs 8, 12, and 13 (Oshida et al., 2000).
Ag-NORs were analyzed in some squirrel species (Table 1). Some data on arboreal squirrels were found in the literature, revealing interstitial and terminal Ag-NORs. The spotted ground squirrel Spermophilus suslicus (2n = 36 and 2n = 34) had one interstitial (pair 3) and two telomeric (pairs 6 and 9) Ag-NORs (Koralev, 1994). The Siberian flying squirrel Pteromys volans orii (2n = 38, NF = 68) presented one pair of terminal Ag-NORs on the short arm of chromosome 17. A size variation of Ag-NORs was observed, indicating a variable NOR activity in this species (Oshida and Yoshida, 1996).
The use of FISH allowed us to detect eight chromosomes with NORs in Sciurus a. ingrami, while silver staining revealed only seven, suggesting that these techniques should be used together, to the best understanding of the variability and differential activity of ribosomal genes.
Acknowledgments
Grants to support this study were provided by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ).
Editor: Fausto Foresti
Received: May 6, 2002
Accepted: May 5, 2003
- Emmons LE and Feer F (1997) Neotropical rainforest mammals. A field guide. 2nd ed. University of Chicago. Chicago.
- Hoffmann RS, Anderson CG, Thorington RW Jr and Heaney LR (1993) Family Sciuridae. In: Wilson DE and Reeder M (eds) Mammals species of the world: a taxonomic and geographic reference. 2nd ed. Smithsonian Institute Press, Washington DC, pp 419-465.
- Kim JBK and Lee HYA (1990) Comparative study in Korean squirrels: I. Karyotype analysis of Sciurus vulgaris coreae and Tamias sibiricus asiaticus by conventional Giemsa staining and C-banding method. Korean Journal of Zoology 33:222-230.
- Koralev VP (1994) Chromosomal differentiation of spotted ground squirrel Spermophilus suslicus Guld. 1770. Polish Ecological Studies 20:537-541.
- Koralev VP, Lyapunova EA and Vorontsov NN (1991) Increased variability of cytogenetic parameters in artificial population of spotted ground squirrel Spermophilus suslicus in the Elbrus region (USSR). Genetika 27:154-159.
- Lima, JFS and Langguth A (2002) Karyotypes of Brazilian squirrels: Sciurus spadiceus and Sciurus alphonsei (Rodentia, Sciuridae). Folia Zool 51:201-204.
- Nadler CF (1966a)Chromosomes and systematics of American ground squirrels of subgenus Spermophilus J Mammalogy 47:579
- Nadler CF (1966b) Chromosomes of Spermophilus franklini and taxonomy of the ground squirrel genus Spermophilus Syst Zool 15:199-206.
- Nadler CF (1968) Chromosomes of Spermophilus townsendi (Rodentia - Sciuridae) and report of a new subspecies. Cytogenetics 7:144.
- Nadler CF and Hoffmann RS (1970) Chromosomes of some Asian and South American squirrels (Rodentia, Sciuridae). Experientia 26:1383-1386.
- Nadler CF, Hoffmann RS and Lay DM (1969) Chromosomes of the Asian chipmunk Eutamias sibiricus Laxmann (Rodentia: Sciuridae). Experientia 25:868-869
- Nadler CF and Sutton DA (1962) Mitotic chromosomes of some North American Sciuridae. Proc Soc Exp Biol Med 110:36.
- Nadler CF and Sutton DA (1967) Chromosome of some squirrels (Mammalia - Sciuridae) from the genera Sciurus and Glaucomys Experientia 23:249-251.
- Oshida T and Obara Y (1993) C-band variation in the chromosomes of the Japanese giant flying squirrel, Petaurista leucogenys Journal of the Mammalogical Society of Japan 18:61-67.
- Oshida T, Obara Y, Lin LK and Yoshida MC (2000) Comparison of banded karyotypes between two subspecies of the red and white giant flying squirrel Petaurista alborufus (Mammalia, Rodentia). Caryologia 53:261-267.
- Oshida T, Satoh H and Obara Y (1992) A preliminary note on the karyotypes of giant flying squirrels Petaurista alborufus and Petaurista petaurista Journal of the Mammalogical Society of Japan 16:59-69.
- Oshida T and Yoshida MC (1996) Banded karyotypes and the localization of ribosomal RNA genes of Eurasian flying squirrel, Pteromys volans orii (Mammalia, Rodentia). Caryologia 49:219-225.
- Pathak SA, Dolhonde JA and Multani AS (1998) Amplification of telomeric DNA and the extent of karyotypic evolution. Cytobios 93:141-146.
- Uribe-Alcocer M, Castro-Perez A, Arreguin-Espinosa J (1989) "G" banding in Spermophilus spilosoma cabrerai (Dalquest, 1951) (Rodentia: Sciuridae) and its taxonomic implication. Annales Institute Biologia Universidad Nacional Aut Mexico Serie Zoologica. 60:433-448.
- Uribe-Alcocer M and Ahumada MA (1990) The karyotype of Spermophilus perotensis (Sciuridae; Rodentia). Cytobios 64:87-92.
Publication Dates
-
Publication in this collection
29 Sept 2003 -
Date of issue
2003
History
-
Received
06 May 2002 -
Accepted
05 May 2003