Abstract
The kinetic analysis of Bacillus thuringiensis var. israelensis (Bti) growth is often hindered by the impossibility of using conventional methods to measure biomass concentration in natural raw-material-based media, which normally have a high content of insoluble solids. In this work, application of a mathematical model based on respiratory parameters in the estimation of Bti biomass concentration was evaluated. The values obtained for the respiratory parameters true cell yield from oxygen consumption and maintenance coefficient for oxygen (mO) were 0.091 g cell/mmol O2 and 2.1 mmol O2/g cell/hour, respectively. With these constants and the oxygen uptake rates obtained from oxygen balance calculations, cell concentrations of Bti were estimated in batch runs with initial glucose concentrations from 10 to 80 g/L and were found to be in good agreement with experimental data.
Bacillus thuringiensis var. israelensis; Quantification of cell concentration; Oxygen balance; Respiratory parameters
BIOTECHNOLOGY
Indirect estimation of Bacillus thuringiensis var. israelensis biomass concentration using oxygen balance data
M. M. SilveiraI, * * To whom correspondence should be addressed ; M. A. B. MolinaII
IUniversidade de Caxias do Sul, Phone/Fax:+(55) (54) 218-2149, Cx. Postal 1352, CEP 95001-970, Caxias do Sul - RS, Brazil. E-mail-mmsilvei@ucs.br
IIUniversidade Estadual do Norte Fluminense, Centro de Biociências e Biotecnologia. Av. Alberto Lamego 2000, CEP 28013-600, Campos dos Goytacazes, RJ - Brazil
ABSTRACT
The kinetic analysis of Bacillus thuringiensis var. israelensis (Bti) growth is often hindered by the impossibility of using conventional methods to measure biomass concentration in natural raw-material-based media, which normally have a high content of insoluble solids. In this work, application of a mathematical model based on respiratory parameters in the estimation of Bti biomass concentration was evaluated. The values obtained for the respiratory parameters true cell yield from oxygen consumption and maintenance coefficient for oxygen (mO) were 0.091 g cell/mmol O2 and 2.1 mmol O2/g cell/hour, respectively. With these constants and the oxygen uptake rates obtained from oxygen balance calculations, cell concentrations of Bti were estimated in batch runs with initial glucose concentrations from 10 to 80 g/L and were found to be in good agreement with experimental data.
Keywords: Bacillus thuringiensis var. israelensis; Quantification of cell concentration; Oxygen balance; Respiratory parameters.
INTRODUCTION
The growing worldwide concern over the hazardous effects of chemical insecticides on human health and the environment has greatly increased the use of biological insecticides as a safe alternative for controlling agricultural plagues and human-disease vectors. Of the biological insecticides, those based on the endotoxins of Bacillus thuringiensis var. israelensis (Bti) H14 are considered particularly important since they are utilised for controlling larvae of mosquitoes of the families Culicidae, which can serve as vectors of diseases such as malaria and dengue fever, and Simulidae, which could be related to the transmission of viruses, filariae and protozoa (Ruas Neto and Oliveira, 1985).
Although in the last few years the use of Bti-based insecticides has been increasing all over the world, relatively few scientific articles concerning the kinetics of this fermentative process have been published. In part, this is due to the normally high insoluble solids content in media formulated with natural raw material, which hinders determination of biomass concentrations by conventional methods.
Kraemer-Schafhalter and Moser (1996) proposed a non-structured mathematical model based on several parameters, such as the concentration of dissolved oxygen and organic acids formed during cultivation, for simulation of B. thuringiensis var. israelensis growth. This mathematical model showed good agreement with experimental results but, according to the authors, is dependent on medium composition and type of bioreactor utilised. Later, Berbert-Molina et al. (2001) defined some empirical equations for the mathematical description of Bti growth in which easily measured variables, such as the biomass yield from glucose consumption and the oxygen uptake rate, were involved. However, since Bti preferentially utilises the protein-nitrogen source to grow (Mignone and Avignone-Rossa, 1996; Kraemer-Schafhalter and Moser, 1996), application of this method depends on the carbon-nitrogen rate of each specific medium.
Zabriskie and Humphrey (1978) described a model based exclusively on respiratory parameters for the indirect quantification of microbial concentrations. This model was tested for growth of the facultative anaerobic yeast Saccharomyces cerevisiae and responded satisfactorily to situations in which only the respiratory pathway was involved. The authors reported that the model could be applicable to on-line process control, since its use depends basically on measurement of the oxygen uptake rate (OUR), which can be directly determined with simple equipment.
Although the characteristics of B. thuringiensis var. israelensis growth are more complex than those of S. cerevisiae, in the present work, the model of Zabriskie and Humphrey (1978) was tested for estimation of the cell mass of this facultative anaerobic bacterium under an unlimited oxygen supply. For this purpose, values of the variable OUR and the constant parameters true cell yield from oxygen consumption (YGO) and maintenance coefficient for oxygen (mO) were assessed.
MATERIAL AND METHODS
Microorganism
B. thuringiensis var. israelensis H-14 IPS82, obtained from Institute Pasteur (France), was used in all the experiments described in this work. The strain was maintained on nutrient agar at 4°C.
Media
LB medium, used for the preparation of the pre-inoculum, had the following composition (g/L): peptone, 10.0; yeast extract, 5.0 and NaCl, 5.0. The medium was adjusted to pH 7.0 with 2N NaOH, before sterilisation at 121°C for 20 minutes.
Modified GYS medium, used for inoculum preparation and bioreactor experiments, contained the following (g/L): glucose , 20 (inocula) or 10 to 80 (experiments); yeast extract, 12.0; (NH4)2SO4, 3.0; CaCl2.2H2O, 0.12; MgSO4.7H2O, 1.5; MnSO4.H2O, 0.09; K2HPO4, 1.5 and KH2PO4; 1.5. Concentrated glucose solutions were prepared and sterilised separately and added to the medium before inoculation.
Experimental Conditions
Pre-inocula were prepared in 125-mL Erlenmeyer flasks containing 25 mL of LB medium. These flasks were inoculated with spores of B. thuringiensis var. israelensis and statically incubated for 15 hours at 30°C. For inoculum preparation, 10 mL of pre-inoculum culture were transferred to 500-mL Erlenmeyer flasks containing 100 mL of modified GYS medium, and these flasks were incubated at 30°C for 5 to 6 hours in a New Brunswick incubator shaker model 25D (USA) at a rate of 110 min-1. An amount of this culture corresponding to 5% of the fermentation medium was used as inoculum.
Batch runs were performed in a 5-L Biostat MD bioreactor (B. Braun Biotech, Germany) containing 3.6 L of medium after inoculation. The temperature was kept at 30°C. The initial pH was 7.0 and was automatically controlled at a minimum value of 5.6 with 5M KOH. Initially, the shaking rate was set at 550 min-1 and aeration at 2 L/min; both of these parameters were occasionally varied to maintain the dissolved oxygen concentration at a minimum of 35% saturation.
Analytical Assays
Cell growth was initially followed by reading optical density of cell suspensions at 610 nm in a Shimadzu UV-160A (Japan) instrument after appropriate dilution in distilled water. These turbidimetric measurements were converted into concentration (g/L) by a correlation curve. Then, when the culture became flocculent, biomass concentrations were measured by drying cell suspensions that had been three times washed and centrifuged to a constant weight at 85°C.
Glucose was enzymatically assayed using a glucose oxidase/peroxidase test kit (Celm, Brazil).
Dissolved oxygen concentration was determined using a polarographic electrode (Mettler-Toledo, Germany).
The molar fractions of oxygen and carbon dioxide were measured in the gas at the bioreactor outlet with a Mayhak (Germany) gas analyser. By using these data, oxygen uptake rate (OUR) during the different runs was calculated as described by Wang (1985). With OUR values and the cell concentration (X) measured for the same process time, the specific oxygen uptake rates (QO2) were calculated with the following equation:
QO2 = OUR / X
RESULTS AND DISCUSSION
B. thuringiensis var. israelensis IPS82 was cultivated in modified GYS medium with initial glucose concentrations (S0) from 10 to 80 g/L. These runs were carried out with an unlimited oxygen supply, since this condition is needed for sporulation and endotoxin formation by this bacterium (Avignone-Rossa et al., 1992; Kraemer-Schafhalter and Moser, 1996; Berbert-Molina, 1998). With any S0, four well-defined growth phases, which are based on the morphological and physiological alterations occurring during cultivation, were observed: Phase I, vegetative growth; Phase II, transition to spore formation; Phase III, spore formation; Phase IV, spore maturation and cell lysis. During Phases III and IV, protein crystals, which are responsible for the toxic activity of Bti against mosquito larvae, were microscopically detected.
In this work, the discussion is focused solely on Phases I and II, in which cell growth of B. thuringiensis var. israelensis occurs. Phases III and IV were not analysed, despite their importance in the production of biological insecticide. Nevertheless, when Bti was cultivated under conditions appropriate for the medium composition and oxygen supply, it was expected that large cell concentrations at the end of Phase II would result in high titres of endotoxins in Phases III and IV. Thus, mathematical models for describing cell growth during Phases I and II could be an efficient tool for the kinetic analysis of this process.
According to Nagai (1979), the specific oxygen uptake rate (QO2) and the specific growth rate (µX) are correlated by the equation
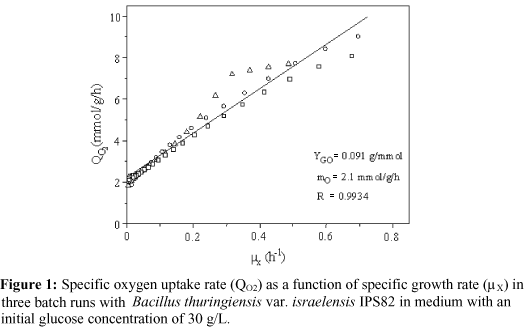
where YGO is the true cell yield from oxygen consumption and mO is the maintenance coefficient for oxygen.
From Equation 1, the values of the constants YGO and mO can be graphically determined, as suggested by Pirt (in Zabriskie and Humphrey, 1978). By this procedure, the constants were calculated for three B. thuringiensis var. israelensis IPS82 batch runs in modified GYS medium (S0 = 30 g/L) for which oxygen uptake rate (OUR) and cell concentration (X) data were available (Figure 1). The values found for YGO and mO, 0.091 g biomass/mmol O2 and 2.1 mmol O2/g biomass/h, were comparable to those measured by Moraes and co-workers (in Rowe et al., 2003): YGO = 0.066 g/mmol and mO = 3.6 mmol/g/h.
As already mentioned, all runs discussed in this work were carried out under the condition of unlimited oxygen supply to assure that the respiratory metabolism of Bti would be favoured. Thus, assuming that this facultative anaerobe could behave as an aerobic microorganism, the mathematical model formulated by Zabriskie and Humphrey (1978) for aerobic batch cultivation would be useful for estimating Bti concentration as described in the sequence.
Equation 1 can be rewritten as
where dX/dt is the biomass growth rate.
Equation 2 can be integrated using the method of trapezoids, as suggested by Zabriskie and Humphrey (1978):
where Xn and Xn-1 are the biomass concentrations at cultivation times tn and tn-1, Dt is the time interval from tn-1 to tn, and OURn and OURn-1 are the oxygen uptake rates at cultivation times tn and tn-1.
Equation 4 provides cumulative cell concentrations with time. The first value of Xn-1 must be estimated from the cell concentration in the inoculum for each batch.
With the constants YGO and mO and the values of OUR obtained from the oxygen balance, B. thuringiensis var. israelensis concentrations were estimated in the four batch experiments with different initial glucose concentrations. The time courses of cell growth in these runs are depicted in Figures 2 to 5, in which the discrete points represent the experimental results and the continuous lines are the estimated curves obtained from OUR values with Equation 4.
As shown in Figures 2 to 5, the curves derived from the application of Equation 4 show relatively good agreement with the experimental data, specially when initial glucose concentrations from 30 to 80 g/L were used. In general, deviations were found in the period of cultivation immediately before the stationary phase (Phase II). This behaviour was probably related to the drastic morphological and physiological changes that occurred during Phase II, which is characterised by the aggregation of cells to form clumps with a large number of bacteria, followed by the formation of subterminal spores in isolated cells. Despite these deviations, Bti concentration could be estimated during the exponential and stationary phases, allowing measurement of important evaluation parameters, such as the maximum specific growth rate and the biomass yield from glucose consumption.
CONCLUSIONS
The results of this work show that the respiratory mathematical model of Zabriskie and Humphrey (1978) could be used to estimate the biomass concentration of B. thuringiensis var. israelensis in spite of the morphological and physiological complexity observed during the growth of this bacterium. Further studies, however, are needed to describe the behaviour of the culture after the stationary phase (Phases III and IV), in which cell lysis and release of toxin crystals occur.
NOMENCLATURE
Received: October 20, 2004
Accepted: April 10, 2005
- Avignone-Rossa, C., Arcas, J. and Mignone, C., Bacillus thuringiensis Growth, Sporulation and d-Endotoxin Production in Oxygen Limited and Non-Limited Cultures. World Journal of Microbiology and Biotechnology, 8, 301-304 (1992).
- Berbert-Molina, M.A., Estudo da Influência do Suprimento de Oxigênio e da Concentração de Glicose sobre o Cultivo de Bacillus thuringiensis var. israelensis IPS82 em Regime Descontínuo. Ph.D. Thesis, Universidade de São Paulo (1998).
- Berbert-Molina, M.A., Silveira, M.M. and Sato, S., Cultivo de Bacillus thuringiensis var. israelensis em Meio Semi-Sintético: Simulação Matemática do Crescimento. I Congresso da Sociedade Brasileira de Biotecnologia, São Paulo, pp. 178-183 (2001).
- Kraemer-Schafhalter, A. and Moser, A., Kinetic Study of Bacillus thuringiensis var. israelensis in Lab-Scale Batch Process. Bioprocess Engineering, 14, 139-144 (1996).
- Mignone, C.F. and Avignone-Rossa, C., Analysis of Glucose Fluxes in Continuous Cultures of Bacillus thuringiensis Applied Microbiology and Biotechnology, 46, 78-84 (1996).
- Nagai, S., Energy Balance for Microbial Growth Kinetics. Advances in Biochemical Engineering, 11, 49-83 (1979).
- Rowe, G.E., Margaritis, A. and Wei, N., Specific Oxygen Uptake Rate Variations during Batch Fermentation of Bacillus thuringiensis Subspecies kurstaki HD1. Biotechnology Progress, 19, 1439-1443, 2003.
- Ruas Neto, A.L. and Oliveira, C.M., Biological Control of Culicidae and Simulidae: Bacterial Insecticides. Revista Brasileira de Malariologia e Doenças Tropicais, 37, 61-75 (1985).
- Wang, H.Y., Analysis of Fermentation Gases. In: Moo-Young, M. Comprehensive Biotechnology: The Principles, Application and Regulation of Biotechnology in Industry, Agriculture and Medicine. Pergamon Press, 423-431 (1985).
- Zabriskie, D.W. and Humphrey, D.W., Real-Time Estimation of Aerobic Batch Fermentation Biomass Concentration by Component Balancing. AIChE Journal, 24, 138-146 (1978).
Publication Dates
-
Publication in this collection
02 Jan 2006 -
Date of issue
Dec 2005
History
-
Received
20 Oct 2004 -
Accepted
10 Apr 2005