Abstract
A total of 132 flounder specimens (60 Paralichthys isosceles, 36 Paralichthys patagonicus and 36 Xystreurys rasile) were collected off the coast of the state of Rio de Janeiro, Brazil. The fish were measured, necropsied, and had their organs investigated for hemiurid and lecithasterid digenean trematodes and camallanid and cucullanid nematodes. Taxonomic identification of the parasites was based on morphological and morphometric characters and was conducted using bright-field and scanning electron microscopies. The trematodes Lecithochirium monticellii and Aponurus laguncula were found parasitizing P. isosceles, P. patagonicus and X. rasile while the nematodes Procamallanus (Spirocamallanus) halitrophus and Cucullanus bonaerensis were found parasitizing P. isosceles and X. rasile and P. isosceles, P. patagonicus and X. rasile, respectively. Parasite indices of prevalence, mean intensity, mean abundance, and range of infection, as well as infection site, were evaluated for each parasite species. This study allowed to evidence the first occurrence of P. patagonicus by L. monticellii; X. rasile by A. laguncula and P. (S.) halitrophus; and P. isosceles and P. patagonicus by C. bonaerensis in the Western South Atlantic Ocean.
Keywords:
Hemiuridae; Lecithasteridae; Camallanidae; Cucullanidae; flounders; Brazil
Resumo
Um total de 132 espécimes de linguados (60 Paralichthys isosceles, 36 Paralichthys patagonicus e 36 Xystreurys rasile) foram coletados na costa do Estado do Rio de Janeiro, Brasil. Os peixes foram medidos, necropsiados e tiveram seus órgãos investigados para o encontro de trematodeos digenéticos, das famílias Hemiuridae e Lecithasteridae, e nematoides das famílias Camallanidae e Cucullanidae. A identificação taxonômica dos parasitos foi baseada nos caracteres morfológicos e morfométricos, usando-se as microscopias de campo claro e eletrônica de varredura. Os trematódeos, Lecithochirium monticellii e Aponurus laguncula, foram encontrados parasitando P. isosceles, P. patagonicus e X. rasile, enquanto os nematoides, Procamallanus (Spirocamallanus) halitrophus e Cucullanus bonaerensis, foram encontrados parasitando P. isosceles e X. rasile e P. isosceles, P. patagonicus e X. rasile, respectivamente. Os índices parasitários de prevalência, intensidade média, abundância média e amplitude de variação da intensidade de infeção, bem como os sítios de infecção foram avaliados para cada espécie de parasito. Este é o primeiro registro de P. patagonicus por L. monticellii; X. rasile por A. laguncula e P. (S.) halitrophus; e de P. isosceles e P. patagonicus por C. bonaerensis no Oceano Atlântico Sul Ocidental.
Palavras-chave:
Hemiuridae; Lecithasteridae; Camallanidae; Cucullanidae; linguados; Brasil
Introduction
The flounder species Paralichthys isosceles Jordan, 1890, P. patagonicus Jordan, 1889 and Xystreurys rasile (Jordan, 1891) (Paralichthyidae) represent important primary fishery resources in the coastal waters of Brazil (Figueiredo & Menezes, 2000Figueiredo JL, Menezes NA. Manual de peixes marinhos do sudeste do Brasil. VI. Teleostei (5). São Paulo: Museu de Zoologia, USP; 2000.). According to Cerqueira et al. (1997)Cerqueira VR, Mioso R, Macchiavello JAG, Brügger AM. Ensaios de indução de desova do linguado (Paralichthys orbignyanus Valenciennes, 1839). Bol Inst Pesca 1997; 24(special): 247-254., Massa et al. (2005)Massa AE, Palacios DL, Paredi ME, Crupkin M. Postmortem changes in quality indices of ice-stored flounder (Paralichthys patagonicus). J Food Biochem 2005; 29(5): 570-590. http://dx.doi.org/10.1111/j.1745-4514.2005.00050.x.
http://dx.doi.org/10.1111/j.1745-4514.20...
and Bernardes et al. (2005)Bernardes RA, Figueiredo JL, Rodrigues AR, Fischer LG, Vooren CM, Haimovici M, et al. Peixes da zona econômica exclusiva da região sudeste-sul do Brasil: Levantamento com armadilhas, pargueiras e rede de arrasto de fundo. São Paulo: Editora da Universidade de São Paulo; 2005., the flounder fishery is referred to as “fine fishing” due to high commercial interest, meat quality and market price and because of broad sale in domestic and foreign markets.
Digenean trematodes present a certain degree of specificity in relation to their site of infection and their definitive host, although some species may infect different sites, such as adult individuals of species of the families Hemiuridae and Lecithasteridae when parasitizing the digestive system of fish (Eiras et al., 2006Eiras JC, Takemoto RM, Pavanelli GC. Métodos de estudo e técnicas laboratoriais em parasitologia de peixes. 2ª ed. Maringá: Eduem; 2006.).
Parasitic nematodes are important pathogens associated with human and non-human animal health (Acha & Szyfres, 2003Acha PN, Szyfres B. Zoonoses and communicable diseases common to man and animals. Vol. III. Parasitoses. 3rd ed. Washington, DC: PAHO; 2003. Scientific and Technical Publication; no. 580.). Nematodes inhabit hosts of fresh, brackish and marine waters around the world, and some are known to be agents of serious fish diseases that cause considerable losses to the fishing industry (Klimpel & Palm, 2011Klimpel S, Palm HW. Anisakid Nematode (Ascaridoidea) life cycles and distribution: increasing zoonotic potential in the time of climate change? In: Mehlhorn H, editor. Progress in parasitology. Berlin: Springer; 2011. p. 201-221. (Parasitology Research Monographs, vol. 2). http://dx.doi.org/10.1007/978-3-642-21396-0_11
http://dx.doi.org/10.1007/978-3-642-2139...
). Adult nematodes of the families Camallanidae and Cucullanidae are commonly found parasitizing the intestine of various fish, their definitive hosts (Williams & Jones, 1994Williams H, Jones A. Parasitic worms of fish. London: Taylor & Francis; 1994. http://dx.doi.org/10.1201/b12595.
http://dx.doi.org/10.1201/b12595...
; Lanfranchi et al., 2004Lanfranchi AL, Timi JT, Sardella NH. Cucullanus bonaerensis n. sp. (Nematoda: Cucullanidae) parasitizing Urophycis brasiliensis (Pisces: Phycidae) from Argentinean Waters. J Parasitol 2004; 90(4): 808-812. http://dx.doi.org/10.1645/GE-3276. PMid:15357074.
http://dx.doi.org/10.1645/GE-3276...
; Cárdenas & Lanfredi, 2005Cárdenas MQ, Lanfredi RM. Further description of Procamallanus (Spirocamallanus) halitrophus comb. n. (Nematoda: Camallanidae) from flounder off the Brazilian coast by light and scanning electron microscopy. J Parasitol 2005; 91(3): 606-613. http://dx.doi.org/10.1645/GE-3422. PMid:16108554.
http://dx.doi.org/10.1645/GE-3422...
).
Studies, including morphological and ecological surveys, have reported some groups of helminths parasitizing the flounder species P. isosceles, P. patagonicus and X. rasile (Paralichthyidae) in Brazil (Felizardo et al., 2009aFelizardo NN, Knoff M, Pinto RM, Gomes DC. Larval anisakid nematodes of the flounder, Paralichthys isosceles Jordan, 1890 (Pisces: Teleostei) from Brazil. Neotrop Helminthol 2009a; 3(2): 57-64., bFelizardo NN, Menezes RC, Tortelly R, Knoff M, Pinto RM, Gomes DC. Larvae of Hysterothylacium sp. (Nematoda: Anisakidae) in the sole fish Paralichthys isosceles Jordan, 1890 (Pisces: Teleostei) from the littoral of the state of Rio de Janeiro, Brazil. Vet Parasitol 2009b; 166(1-2): 175-177. http://dx.doi.org/10.1016/j.vetpar.2009.08.004. PMid:19713041.
http://dx.doi.org/10.1016/j.vetpar.2009....
, 2010Felizardo NN, Torres EJL, Fonseca MCG, Pinto RM, Gomes DC, Knoff M. Cestodes of the flounder Paralichthys isosceles Jordan, 1890 (Osteichthyes - Paralichthyidae) from the State of Rio de Janeiro, Brazil. Neotrop Helminthol 2010; 4(2): 113-125., 2011Felizardo NN, Justo MC, Knoff M, Fonseca MCG, Pinto RM, Gomes DC. Juvenile didymozoids of the types, Torticaecum and Neotorticaecum (Didymozoidae: Digenea), from new marine fish hosts (Pisces: Teleostei) in the neotropical region of Brazil. J Helminthol 2011; 85(3): 270-275. http://dx.doi.org/10.1017/S0022149X10000507. PMid:20854704.
http://dx.doi.org/10.1017/S0022149X10000...
, 2018Felizardo NN, Knoff M, Diniz JB, Torres EJL, Calixto FAA, São Clemente SC. Pterobothrium crassicolle parasitizing Paralichthys orbignyanus (Osteichthyes, Paralichthyidae) in Brazil. An Acad Bras Cienc 2018; 90(2): 1605-1610. http://dx.doi.org/10.1590/0001-3765201820170323. PMid:29768571.
http://dx.doi.org/10.1590/0001-376520182...
; Fonseca et al., 2012Fonseca MCG, São Clemente SC, Felizardo NN, Gomes DC, Knoff M. Trypanorhyncha cestodes of hygienic-sanitary importance infecting flounders Paralichthys patagonicus Jordan, 1889 and Xystreurys rasile (Jordan, 1891) of the Neotropical region, Brazil. Parasitol Res 2012; 111: 865-874. http://dx.doi.org/10.1007/s00436-012-2912-z. PMid:22488201.
http://dx.doi.org/10.1007/s00436-012-291...
, 2016Fonseca MCG, Knoff M, Felizardo NN, Di Azevedo MIN, Torres EJL, Gomes DC, et al. Integrative taxonomy of Anisakidae and Raphidascarididae (Nematoda) in Paralichthys patagonicus and Xystreurys rasile (Pisces: teleostei). Int J Food Microbiol 2016; 235: 113-124. http://dx.doi.org/10.1016/j.ijfoodmicro.2016.07.026. PMid:27491056.
http://dx.doi.org/10.1016/j.ijfoodmicro....
, 2019Fonseca MCG, Knoff M, Felizardo NN, Torres EJL, Di Azevedo MIN, Gomes DC, et al. Acanthocephalan parasites of the flounder species Paralichthys isosceles, Paralichthys patagonicus and Xystreurys rasile from Brazil. Rev Bras Parasitol Vet 2019; 28(3): 346-359. http://dx.doi.org/10.1590/s1984-29612019031. PMid:31215609.
http://dx.doi.org/10.1590/s1984-29612019...
; Knoff et al., 2012Knoff M, Felizardo NN, Iniguez AM, Maldonado JRA Jr, Torres EJL, Pinto RM, et al. Genetic and morphological characterisation of a new species of the genus Hysterothylacium (Nematoda) from Paralichthys isosceles Jordan, 1890 (Pisces: Teleostei) of the Neotropical region, State of Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 2012; 107(2): 186-193. http://dx.doi.org/10.1590/S0074-02762012000200006. PMid:22415256.
http://dx.doi.org/10.1590/S0074-02762012...
; Alarcos et al., 2016Alarcos AJ, Pereira NA, Taborda NL, Luque JL, Timi JT. Parasitogical evidence of stocks of Paralichthys isosceles (Pleuronectiformes: Paralichthyidae) at small end geographical scales in South American Atlantic coasts. Fish Res 2016; 173(3): 221-228. http://dx.doi.org/10.1016/j.fishres.2015.07.018.
http://dx.doi.org/10.1016/j.fishres.2015...
; Eiras et al., 2016Eiras JC, Velloso AL, Pereira J Jr. Parasitos de peixes marinhos da América do Sul. Rio Grande: Ed. FURG; 2016.; Justo et al., 2017Justo MCN, Fernandes BMM, Knoff M, Cárdenas MQ, Cohen SC. Checklist of Brazilian Cestoda. Neotrop Helminthol 2017; 11(1): 187-282.) including species of trematodes and nematodes. The present paper continues these studies on flounders occurring off of the Brazilian coast. Thus, aiming to continue the study of these helminths was investigated the presence of hemiurid and lecithastherid digenean trematodes and camallanid and cucullanid nematodes parasitizing paralichthyid flounders from off the coast of the state of Rio de Janeiro, Brazil. These helminths were identified morphologically and morphometrically by bright-field and scanning electron microscopies, calculated their parasitic indices and determined their sites and ranges of infection.
Material and Methods
Host fish and parasite sampling
One hundred and thirty-two flounders were acquired: 60 P. isosceles, mean length 37.5 cm (16.4-68.9 cm), mean weight 39.1 g (25.0-42.0 g); 36 P. patagonicus, mean length 39.2 cm (28.5-59.0 cm), mean weight 747.3 g (280.0-2530.0 g); and 36 X. rasile, mean length 29.7 cm (14.0-51.0 cm) and mean weight 371.3 g (25.0-1440.0 g). The fish were obtained in small markets selling only fish caught offshore of the municipalities of Cabo Frio (22º52'46” S, 42º01'07” W), Niterói (22º53'00” S, 43º06'13” W), Rio de Janeiro (22°54'13” S, 43°12'35” W), and Angra dos Reis (23º00'24” S, 44º19'05” W), of the state of Rio de Janeiro (RJ), Brazil. Fish were transported on ice to the laboratory to investigate the presence of helminths. The fish were identified according to Nakamura et al. (1986)Nakamura I, Inada T, Takeda M, Hatanaka H. Important fishes trawled off Patagonia. Tokyo: JAMARC; 1986. and Figueiredo & Menezes (2000)Figueiredo JL, Menezes NA. Manual de peixes marinhos do sudeste do Brasil. VI. Teleostei (5). São Paulo: Museu de Zoologia, USP; 2000.. After necropsy, internal organs were placed in Petri dishes with 0.65% NaCl solution to evaluate the presence of hemiurid and lecisthasterid trematodes and camallanid and cucullanid nematodes. Trematodes were fixed in AFA (alcohol, formalin, and acetic acid), preserved in 70% ethanol, stained in Langeron´s carmine or in Harris’ hematoxylin, clarified in beechwood creosote, and preserved as whole mounts on Canada balsam. Nematodes were fixed in AFA, preserved in 70% ethanol and later clarified with Amman's lactophenol (Knoff & Gomes, 2012Knoff M, Gomes DC. Metodologia básica para coleta e o processamento de helmintos parasitos. In: Molinaro EM, Caputo LFG, Amendoeira MRR, editors. Conceitos e métodos para a formação de profissionais em laboratórios de saúde. Rio de Janeiro: EPSJV, IOC; 2012. (vol. 5).).
Morphological identification and parasitic indices
Hemiurid species were identified using Looss (1908)Looss A. Beiträge zur Systematik der Distomen. Zur Kenntnis der Familie Hemiuridae. Zool Jahrb (Syst) 1908; 26: 63-180., lecithasterid species using Skrjabin & Guschanskaja (1955)Skrjabin KI, Guschanskaja LK. Suborder Hemiurata (Markevitsch, 1951) Skrjabin et Guschanskaja, 1954. Second part. In: Skrjabin KI, editor. Trematodes of animals and man. Moscow: Akademii Nauk SSSR; 1955. p. 337-643. (vol. 10)., camallanid species using Fusco & Overstreet (1978)Fusco AC, Overstreet RM. Spirocamallanus cricotus sp. n. and S. halitrophus sp. n. (Nematoda: Camallanidae) from fishes in the northern Gulf of Mexico. J Parasitol 1978; 64(2): 239-244. http://dx.doi.org/10.2307/3279663. PMid:641665.
http://dx.doi.org/10.2307/3279663...
and Cárdenas & Lanfredi (2005)Cárdenas MQ, Lanfredi RM. Further description of Procamallanus (Spirocamallanus) halitrophus comb. n. (Nematoda: Camallanidae) from flounder off the Brazilian coast by light and scanning electron microscopy. J Parasitol 2005; 91(3): 606-613. http://dx.doi.org/10.1645/GE-3422. PMid:16108554.
http://dx.doi.org/10.1645/GE-3422...
and cucullanid species using Lanfranchi et al. (2004)Lanfranchi AL, Timi JT, Sardella NH. Cucullanus bonaerensis n. sp. (Nematoda: Cucullanidae) parasitizing Urophycis brasiliensis (Pisces: Phycidae) from Argentinean Waters. J Parasitol 2004; 90(4): 808-812. http://dx.doi.org/10.1645/GE-3276. PMid:15357074.
http://dx.doi.org/10.1645/GE-3276...
. The taxonomic classification of digenean trematodes was according to Gibson et al. (2002)Gibson DI, Jones A, Bray RA. Keys to the Trematoda. Wallingford: CABI Publishing; 2002. (vol. 1). http://dx.doi.org/10.1079/9780851995472.0000.
http://dx.doi.org/10.1079/9780851995472....
while that of nematodes followed De Ley & Blaxter (2004)De Ley P, Blaxter M. A new system for Nematoda: combining morphological characters with molecular trees, and translating clades into ranks and taxa. In: Cook R, Hunt DJ, editors. Nematology monographs and perspectives. Leiden: EJ Brill; 2004. p. 633-653.. Samples were analyzed using an Olympus BX-41 bright-field microscope and images captured using a Canon Power Shot A640 digital camera coupled to a Zeiss Axiophot microscope using a Nomarski’s differential interference contrast (DIC) apparatus. Illustrations were made using a drawing tube connected to the microscope. Measurements are provided in millimeters (mm) unless otherwise indicated, with averages in parentheses. Scanning electron microscopy (SEM) was used to elucidate topographic details of specimens of Cucullanus, with processing according to Lopes Torres et al. (2013)Lopes Torres EJ, Souza W, Miranda K. Comparative analysis of Trichuris muris surface using conventional, low vacuum, environmental and field emission scanning electron microscopy. Vet Parasitol 2013; 196(3-4): 409-416. http://dx.doi.org/10.1016/j.vetpar.2013.02.026. PMid:23537947.
http://dx.doi.org/10.1016/j.vetpar.2013....
. Samples for SEM were fixed in Karnovsky solution, dehydrated in an ethanol series (70-100%), CO2 critical-point dried, coated in gold and examined and photographed using JEOL SM - 25 SII scanning electron microscope under an acceleration voltage of 15 kV. The parasitic indices of prevalence (P), mean intensity (MI) and mean abundance (MA) were calculated according to Bush et al. (1997)Bush AO, Lafferty KD, Lotz JM, Shostak AW. Revisited. J Parasitol 1997; 83(4): 575-583. http://dx.doi.org/10.2307/3284227. PMid:9267395.
http://dx.doi.org/10.2307/3284227...
. Range of infection (RI) and sites of infection (SI) are also presented. Representative specimens were deposited in the Helminthological Collection of the Oswaldo Cruz Institute (CHIOC), FIOCRUZ, Rio de Janeiro, RJ, Brazil.
Results
A total of 1,127 parasite specimens were collected from the sampled flounders, with 783 digenean trematodes of two species of two families (398 Hemiuridae, 385 Lecithasteridae) and 344 nematodes of two species of two families (46 Camallanidae, 298 Cucullanidae). The total number of helminths per flounder species were: 401 from P. isosceles, 584 from P. patagonicus and 142 from X. rasile. The species taxonomic identifications follow.
Platyhelminthes Minot, 1876, Rabditophora Ehlers, 1985, Neodermata Ehlers, 1985, Trematoda Rudolphi, 1808, Digenea Carus, 1863, Hemiuroidea Looss, 1899, Plagiorchiida La Rue, 1957, Hemiurata, Skrjabin & Guschanskaja, 1954, Hemiuroidea, 1899
Hemiuridae Looss, 1899
Lecithochiriinae Lühe, 1901
Lecithochirium Lühe, 1901
Lecithochirium monticellii (Linton, 1898) Skrjabin & Guschankaja, 1955
Lecitochirium monticellii collected from Paralichthys patagonicus. (A) Entire worm, ventrolateral view; (B) Detail of terminal genitalia. Abbreviations: ev = ejaculatory vesicle, hd = hermaphroditic duct, m = metraterm, os = oral sucker, pp = pars prostatica, ph = pharynx, sv = seminal vesicle, ss = sinus sac, and vs = ventral sucker. Bars A = 0.2 mm, B = 0.1 mm.
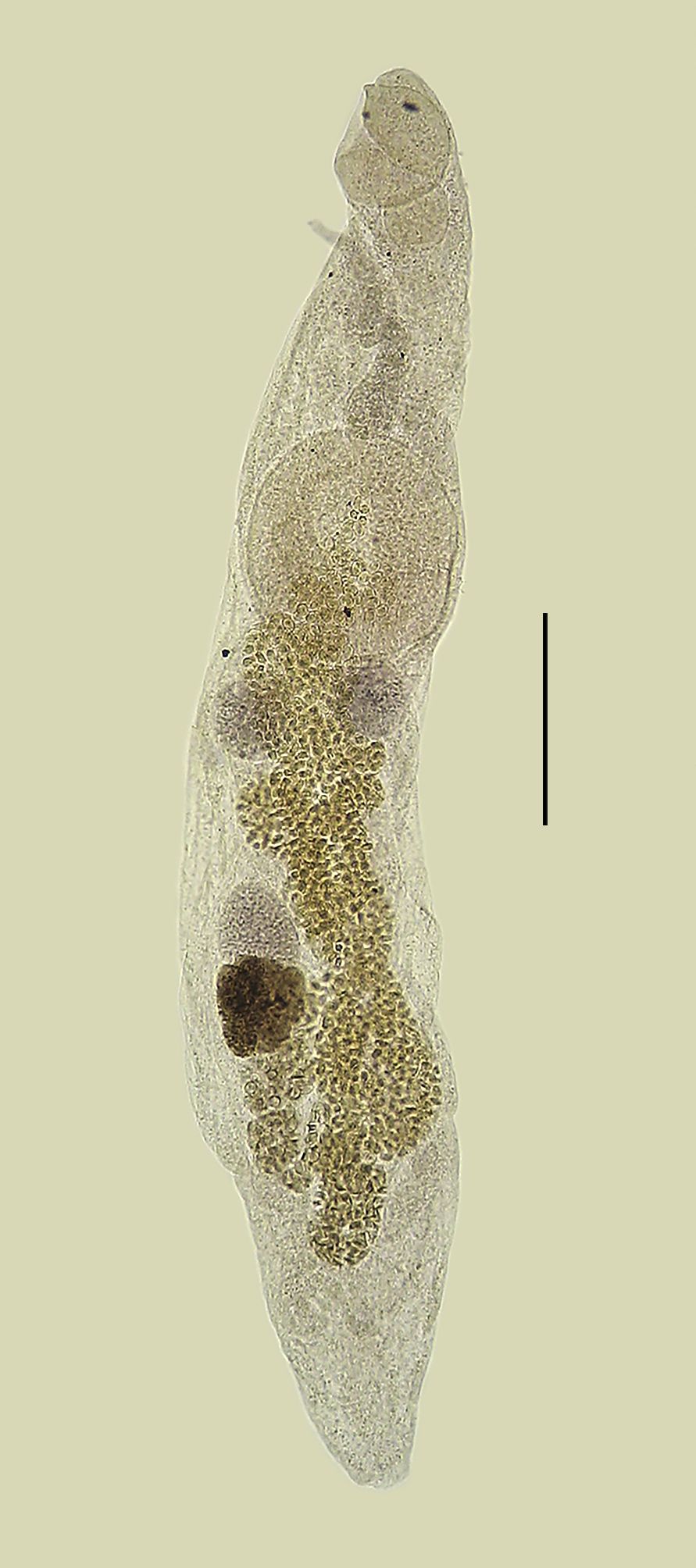
Lecitochirium monticellii collected from Paralichthys patagonicus. Entire worm, ventrolateral view. Bar = 0.2 mm.
Features observed in 35 specimens, 11 from P. isosceles, 13 from P. patagonicus and 11 from X. rasile. Body elongate, subcylindrical, with smooth tegument. Preoral lobe present. Ecsoma well developed, variable in length, retracted in some specimens. Oral sucker subglobular, subterminal. Ventrocervical groove present, with thickened walls. Pharynx subglobular, smaller than oral sucker, partly overlying oral sucker. Ventral sucker subglobular, developed, larger than oral sucker, pre-equatorial. Ceca lined with thick epithelium, entering ecsoma. Testes two, not lobed, rounded, smooth, variable in shape, tandem, lateral or diagonal, in larger specimens in distant zones, in smaller specimens the posterior portion of the anterior testis coincides with the anterior portion of the posterior testis, margins often obscured by uterine loops. Vas efferens uniting to vas deferens close to medial margin of ventral sucker. Vas deferens, small duct, uniting to seminal vesicle. Seminal vesicle tripartite, partially overlapping anterior margin of ventral sucker, with thick wall, posterior portion largest, anterior portion smallest, uniting to ejaculatory duct. Ejaculatory duct, small, slightly sinuous, sometimes swollen in its proximal, median or even distal portion, appearing as an external ejaculatory vesicle, surrounded by numerous prostatic cells, forming a well-developed pars prostatica. Ejaculatory duct uniting to sinus sac meeting the ejaculatory vesicle. Sinus sac ‘separogermiductus-type’, oval, well-developed, thick walled, containing well-developed ejacutatory vesicle (sometimes described as prostatic vesicle), and long, muscular, protrusible hermaphrodict duct opening in a genital pore. Genital pore medial, at or slightly posterior to pharyngeal level. Ovary rounded, smooth, post-testicular, intercecal, in mid-hindbody or more posterior, at some distance from posterior testis, in same field as testes but in separate zones, margins often obscured by uterine loops. Uterus descending into postvitelline space, reaching ecsoma and filling most of hindbody, metraterm entering sinus sac ventrally, joining male duct slightly anterior to ejaculatory vesicle. Vitellaria paired, contiguous, overlapping posterior portion of ovary, one trilobed and one quadrilobed with lobes somewhat longer than wide. Eggs small, operculate, numerous. Excretory pore terminal, excretory duct bifurcating posterior to ventral sucker, arms unite dorsal to oral sucker.
Morphometrics shown in Table 1.
Morphometric data of Lecithochirium monticellii collected from Paralichthys isosceles, P. patagonicus and Xystreurys rasile off the coast of the state of Rio de Janeiro, Brazil.
Hosts: P. isosceles, P. patagonicus and X. rasile
Parasitic indices: P = 33.33%, MI = 4.20 (± 4.93), MA = 1.40 (± 13.43), RI = 1-32 (P. isosceles); P = 75.00%, MI = 10.85 (± 15.35), MA = 8.13 (± 18.38), RI = 1-83 (P. patagonicus); P = 11.11%, MI = 5.25 (± 1.90), MA = 0.58 (± 2.12), RI = 2-9 (X. rasile).
Infection sites: stomach (P. isosceles, P. patagonicus and X. rasile).
Collected specimens: 84 in P. isosceles, 293 in P. patagonicus and 21 in X. rasile.
Deposited specimens: CHIOC 39206, 39207, 39208, 39209 (P. isosceles), CHIOC 39201a-b, 39202, 39203 (P. patagonicus) and CHIOC 39204a-b, 39205a-b (X. rasile).
Lecithasteridae Odhner, 1905
Lecithasterinae Odhner, 1905
Aponurus Looss, 1907
Aponurus laguncula Looss, 1907
Aponurus laguncula collected from Paralichthys isosceles. (A) Entire worm, ventrolateral view; (B) Detail of terminal genitalia. Abbreviations: m = metraterm, os = oral sucker, pp = pars prostatica, ph = pharynx, sv = seminal vesicle, ss = sinus sac, and vs = ventral sucker. Bars A = 0.2 mm, B = 0.1 mm.
Aponurus laguncula collected from Paralichthys patagonicus. Entire worm, ventral view. Bar = 0.2 mm.
Features observed in 31 specimens, 10 from P. isosceles, 13 from P. patagonicus and 8 from X. rasile: Body small, narrow, widest near posterior extremity. Tegument smooth. Pre-oral lobe small or not evident. Oral sucker subglobular, subterminal. Ventral sucker subglobular; immediately anterior to mid-body. Pharynx oval. Excretory system generally obscured by eggs, pore terminal, arms unite dorsal to oral sucker. Testes oval, oblique, in anterior hindbody. Seminal vesicle subglobular, in posterior forebody, near anterior margin of ventral sucker. Pars prostatica narrow, curved, with gland cells surrounding external ejaculatory duct. Sinus-sac oval, thin-walled. Genital pore medial. Ovary oval to sub-triangular, posterolateral to posterior testis and posterior to anterior testis. Seminal receptacle inconspicuous. Uterus in mature worms fills most of hindbody, also in median region dorsal to ventral sucker region and up to about middle of forebody, metraterm entering sinus sac ventrally joining male duct immediately at the beginning of sinus sac at the internal ejaculatory duct. Vitellaria paired, seven irregular follicles, one trilobed and one quadrilobed with follicles somewhat longer than wide, overlapping posterior portion of ovary. Eggs numerous, thin-shelled and operculate.
Morphometrics shown in Table 2.
Morphometric data of Aponurus laguncula collected from Paralichthys isosceles, P. patagonicus and Xystreurys rasile off the coast of the state of Rio de Janeiro, Brazil.
Hosts: P. isosceles, P. patagonicus and X. rasile
Parasitic indices: P = 36.66%, MI = 6.22 (± 5.11), MA = 2.28 (± 14.84), RI = 2-31 (P. isosceles); P = 52.77%, MI = 9.78 (± 7.94), MA = 5.16 (± 5.65), RI = 1-25 (P. patagonicus); P = 11.11%, MI = 15.50 (± 7.18), MA = 1.72 (± 2.12), RI = 1-39 (X. rasile).
Infection sites: stomach (P. isosceles, P. patagonicus and X. rasile).
Collected specimens: 137 in P. isosceles, 186 in P. patagonicus and 62 in X. rasile.
Deposited specimens: CHIOC 39214, 39215, 39216, 39217 (P. isosceles), CHIOC 39210, 39211a-c (P. patagonicus), and CHIOC 39212a-c, 39213 (X. rasile).
Nematoda Pottis, 1932, Chromadorea Inglis, 1893, Chromadoria Pearse, 1942, Rhabditida Chitwood, 1933, Spirurina Railliet & Henry, 1915
Spiruromorpha De Ley & Blaxter, 2002
Camallanoidea Railliet & Henry, 1915
Camallanidae Railliet & Henry, 1915
Procamallanus Baylis, 1923
Procamallanus (Spirocamallanus) Olsen, 1952
Procamallanus (Spirocamallanus) halitrophus (Fusco & Overstreet, 1978Fusco AC, Overstreet RM. Spirocamallanus cricotus sp. n. and S. halitrophus sp. n. (Nematoda: Camallanidae) from fishes in the northern Gulf of Mexico. J Parasitol 1978; 64(2): 239-244. http://dx.doi.org/10.2307/3279663. PMid:641665.
http://dx.doi.org/10.2307/3279663...
) Cárdenas & Lanfredi, 2005
Male of Procamallanus (Spirocamallanus) halithrophus collected from Paralichthys isosceles, DIC. (A) Anterior end, lateral view; (B) Detail of buccal capsule; (C) Posterior end, lateral view. Abbreviations: bc = buccal capsule, ge = glandular esophagus, me = muscular esophagus, nr = nerve ring, and s = spicules. Bars A and C = 0.2 mm, B = 0.05 mm.
Female of Procamallanus (Spirocamallanus) halithrophus collected from Paralichthys isosceles, ventral view, DIC. (A) genital portion of female body; (B) Posterior end; (C) Detail of tail tip showing a terminal digit-like projection with two minute, cuticular spike-like structures. Abreviations: va = vagina, v = vulva, and u = uteri. Bars A = 0.1 mm, B = 0.2 mm, C = 0.05 mm.
Features observed in 20 specimens, 8 from P. isosceles, and 12 from X. rasile: Large, reddish and slender nematodes. Both sexes characterized by orange-brown buccal capsule, longer than wide, thick-walled, with basal ring. Inner surface of buccal capsule with cuticular ridges. Muscular region of esophagus claviform, shorter than glandular region. Glandular region ending in paired bilobed valves. Lumen of esophagus triradiate. Oral aperture circular, sometimes rectangular, surrounded by three circlets of four papillae and by two lateral amphids. Outer papillae more prominent than middle papillae. Small deirids situated on lateral line near anterior end, between buccal capsule and nerve ring. Excretory pore ventrally located at anterior portion of body, between muscular and glandular portions of esophagus. Cuticle with deep transversal striations and modified laterally along the body forming the lateral line; striations lacking in cephalic region. Males and females with two terminal cuticular spike-like structures at end of tail, one dorsal and one ventral.
Male: Posterior region with wide caudal alae, ventrally bent and sometimes spiraled. Caudal alae with 11 pairs of transversally elongated papillae, three precloacal, six postcloacal, and two adcloacal, laterally located to cloacal opening. Three pairs of precloacal papillae located ventrally. First and second pairs of postcloacal papillae ventral, third pair lateral, fourth, fifth and sixth pairs dorsal. Cloacal opening longitudinal. Two spicules of unequal length, one covered by a loose sheath, with a pointed distal end.
Female: Body longer than that of males. Muscular vagina opens in vulva, a transversal ventral opening at mid-body. Anus at posterior end with transversal opening. Tail rounded. Pair of phasmids located laterally near tip of tail and anus.
Morphometrics shown in Table 3.
Morphometric data of Procamallanus (Spirocamallanus) halitrophus collected from Paralichthys isosceles and Xystreurys rasile off the coast of the state of Rio de Janeiro, Brazil.
Hosts: P. isosceles and X. rasile.
Parasitic indices: P = 25.00%, MI = 1.80 (± 1.03), MA = 0.45 (± 1.41), RI = 1-5 (P. isosceles); P = 30.55%, MI = 1.72 (± 0.90), MA = 0.52 (± 7.07), RI = 1-3 (X. rasile).
Infection sites: intestine (P. isosceles and X. rasile).
Collected specimens: 27 in P. isosceles and 19 X. rasile.
Deposited specimens: CHIOC 38732, 38733, 38734, 38735 (P. isosceles) and CHIOC 38724, 38725, 38726, 38727, 38728, 38729, 38730, 38731 (X. rasile).
Ascaridomorpha De Ley & Blaxter, 2002, Seuratoidea Hall, 1916
Cucullanidae Cobbold, 1864
Cucullaninae Cobbold, 1864
Cucullanus Müller, 1777
Cucullanus bonaerensis Lanfranchi, Timi & Sardella, 2004 (Figures 7-10)
Male of Cucullanus bonaerensis collected from Paralichthys patagonicus. (A) Anterior end, lateral view; (B) Posterior end, lateral view, visible caudal papillae, precloacal pairs (2, 3), medial adcloacal papilla (mad), adcloacal pairs (4, 5, 6, 7), postcloacal pairs (8, 9, 10, 11) and phasmid pair (ph). Abbreviations: e = esophagus, g = gubernaculum, m = mouth, nr = nerve ring, pc = pseudobuccal capsule, ps = pseudosucker, s = spicules. Bars: A and B = 0.2 mm.
Female of Cucullanus bonaerensis collected from Xystreurys rasile. (A) Anterior third, lateral view; (B) Eggs inside the uterus, lateral view; (C) Posterior end, lateral view. Abbreviations: a = anus, ph = phasmid, u = uteri, va = vagina, and v = vulva. Bars A and B = 0.2 mm, C = 0.05 mm.
Male of Cucullanus bonaerensis collected from Xystreurys rasile, latero-ventral view, SEM. (A) Anterior end; (B) Detail of cephalic end; (C) Detail of rectangle of Figure B showing triangular denticles. Abbreviations: am = amphid, ce = cephalic end, cd = collarette of triangular denticles, d = deirid, ip = inner papillae, m = mouth, and op = outer papillae. Bars A = 50 µm; B = 20 µm; C = 5 µm.
Male and female of Cucullanus bonaerensis collected from Xystreurys rasile, SEM. (A) Male, posterior end, lateral view showing caudal pseudosucker, caudal papilla pairs 1, 2, 3 (precloacal), 4, 5, 6, 7 (adcloacal), 8, 9, 10, 11 (postcloacal), and medial adcloacal papilla; (B) Female, posterior end, ventrolateral view. Abbreviations: a = anus, mad = medial adcloacal papilla, ph = phasmid and ps = caudal pseudosucker. Bars A = 100 µm, B = 50 µm.
Features observed in 40 specimens, 10 from P. isosceles, 20 from P. patagonicus and 10 from X. rasile: Body slender. Cuticle finely striated throughout. Lateral alae absent. Anterior end rounded, dorsoventrally expanded. Cephalic extremity with usual features of Cucullanus, with two pairs of prominent outer papillae, a pair of amphids, and three pairs of small labial inner papillae. Mouth slit-like dorsoventrally, surrounded by collarette armed with numerous triangular denticles. Pseudobuccal cavity well developed with internal cuticular lining, esophagus long and narrow, expanded at both extremities, opening into intestine through small valve; anterior end wider than posterior end. Nerve ring surrounding esophagus at its second third. Deirids and excretory pore situated between the second half and the distal end of esophagus. Right deirid preequatorial, left postequatorial. Tail conical.
Male: Precloacal sucker at posterior body region, between pairs 1 and 2 caudal papillae. Cloaca prominent. Caudal papillae consisting of one medial adcloacal papilla and 11 pairs of papillae, three precloacal pairs (pair 1 anterior and pairs 2 and 3 posterior to ventral sucker; pair 3 closer to cloaca), four adcloacal pairs (pairs 5, 6 and 7 subventral, pair 4 lateral situated slightly anterior to pair 7), and four postcloacal pairs (pairs 9 and 10 subventral, pairs 8 and 11 lateral) and one pair of lateral phasmids, at level of papillae pair 9. Spicules subequal with pointed distal ends. Gubernaculum Y- shaped.
Female: Ovijector directed anteriorly from vulva. Uteri amphidelphic. Eggs fully-developed, oval, thin-walled. Tail with pair of caudal papillae (phasmids) situated at posterior extremity.
Morphometrics shown in Table 4.
Morphometric data of Cucullanus bonaerensis collected from Paralichthys isosceles, P. patagonicus and Xystreurys rasile off the coast of the state of Rio de Janeiro, Brazil.
Hosts: P. isosceles, P. patagonicus and X. rasile.
Parasitic indices: P = 31.66%, MI = 8.05 (± 8.13), MA = 2.55 (± 12.72), RI = 1-58 (P. isosceles); P = 41.66%, MI = 7.00 (± 7.69), MA = 2.91 (± 9.89), RI = 1-42 (P. patagonicus); P = 27.77%, MI = 4.00 (± 3.39), MA = 1.11 (± 6.36), RI = 1-20 (X. rasile).
Infection site: intestine (P. isosceles, P. patagonicus and X. rasile).
Collected specimens: 153 in P. isosceles, 105 in P. patagonicus and 40 in X. rasile.
Deposited specimens: CHIOC 38745, 38746, 38747, 38748 (P. isosceles); CHIOC 38736, 38737, 38738, 38739, 38740 (P. patagonicus) and CHIOC 38741, 38742, 38743, 38744 (X. rasile).
This study allowed to evidence the first occurrence of parasitism of P. patagonicus by L. monticellii; X. rasile by A. laguncula and P. (S.) halitrophus; and P. isosceles and P. patagonicus by C. bonaerensis in the Western South Atlantic Ocean.
Discussion
The morphology and morphometry of the specimens of L. monticellii collected in the present study were in accordance with the original description (Linton, 1940Linton E. Trematodes from fishes mainly from the Woods Hole region, Massachusetts. Proc USNM 1940; 88(3078): 1-172. http://dx.doi.org/10.5479/si.00963801.88-3078.1.
http://dx.doi.org/10.5479/si.00963801.88...
) and redescriptions (Skrjabin & Guschanskaja, 1955Skrjabin KI, Guschanskaja LK. Suborder Hemiurata (Markevitsch, 1951) Skrjabin et Guschanskaja, 1954. Second part. In: Skrjabin KI, editor. Trematodes of animals and man. Moscow: Akademii Nauk SSSR; 1955. p. 337-643. (vol. 10).; Nasir & Díaz, 1971Nasir P, Díaz M. A revision of genus Lecithochirium Luehe, 1901, and redescription of L. monticellii (Linton, 1898) Skrjabin and Guschanskaja, 1955. Riv Parassitol 1971; 32(1): 27-36. PMid:5560150.; Fernandes et al., 1985Fernandes BMM, Kohn A, Pinto RM. Aspidogastrid and digenetic trematodes parasites of marine fishes of the coast of Rio de Janeiro State, Brazil. Rev Bras Biol 1985; 45(1-2): 109-116. PMid:4059605.; França et al., 2020França LF, Knoff M, Fonseca MCG, Gomes DC, Ferreira MS, Felizardo NN, et al. Lecithochirium monticellii digenetic trematode parasites of Trichiurus lepturus (Actinopterygii) from the state of Rio de Janeiro, Brazil, with notes on its taxonomy. An Acad Bras Cienc 2020; 92(2): e20190161. http://dx.doi.org/10.1590/0001-3765202020190161. PMid:32578665.
http://dx.doi.org/10.1590/0001-376520202...
) of the species. Morphometrically, the present specimens were smaller than those collected from Trichiurus lepturus L. from off the coast of Cumaná, State of Sucre, Venezuela (Nasir & Díaz 1971Nasir P, Díaz M. A revision of genus Lecithochirium Luehe, 1901, and redescription of L. monticellii (Linton, 1898) Skrjabin and Guschanskaja, 1955. Riv Parassitol 1971; 32(1): 27-36. PMid:5560150.), and from Trachurus lathami Nichols, 1920 and T. lepturus from off the coast of the state of Rio de Janeiro, Brazil (Fernandes et al., 1985Fernandes BMM, Kohn A, Pinto RM. Aspidogastrid and digenetic trematodes parasites of marine fishes of the coast of Rio de Janeiro State, Brazil. Rev Bras Biol 1985; 45(1-2): 109-116. PMid:4059605.; França et al., 2020França LF, Knoff M, Fonseca MCG, Gomes DC, Ferreira MS, Felizardo NN, et al. Lecithochirium monticellii digenetic trematode parasites of Trichiurus lepturus (Actinopterygii) from the state of Rio de Janeiro, Brazil, with notes on its taxonomy. An Acad Bras Cienc 2020; 92(2): e20190161. http://dx.doi.org/10.1590/0001-3765202020190161. PMid:32578665.
http://dx.doi.org/10.1590/0001-376520202...
), which can be considered intraspecific variation.
Alarcos & Timi (2012)Alarcos AJ, Timi JT. Parasite communities in three sympatric flounder species (Pleuronectiformes: Paralichthyidae): similar ecological filters driving toward repeatable assemblages. Parasitol Res 2012; 110(6): 2155-2166. http://dx.doi.org/10.1007/s00436-011-2741-5. PMid:22167375.
http://dx.doi.org/10.1007/s00436-011-274...
studied the same three species of flounders as the present study in Argentina and found L. microstomum [=L. monticellii, as suggested by França et al. (2020)França LF, Knoff M, Fonseca MCG, Gomes DC, Ferreira MS, Felizardo NN, et al. Lecithochirium monticellii digenetic trematode parasites of Trichiurus lepturus (Actinopterygii) from the state of Rio de Janeiro, Brazil, with notes on its taxonomy. An Acad Bras Cienc 2020; 92(2): e20190161. http://dx.doi.org/10.1590/0001-3765202020190161. PMid:32578665.
http://dx.doi.org/10.1590/0001-376520202...
] only parasitizing X. rasile. Alarcos et al. (2016)Alarcos AJ, Pereira NA, Taborda NL, Luque JL, Timi JT. Parasitogical evidence of stocks of Paralichthys isosceles (Pleuronectiformes: Paralichthyidae) at small end geographical scales in South American Atlantic coasts. Fish Res 2016; 173(3): 221-228. http://dx.doi.org/10.1016/j.fishres.2015.07.018.
http://dx.doi.org/10.1016/j.fishres.2015...
reported this digenean species parasitizing P. isosceles from off the state of Rio de Janeiro. The finding of L. monticellii parasitizing the flounders of the present study expands the knowledge of parasitism of marine teleostean hosts. This digenean has been previously reported occurring off the coast of the Western Atlantic Ocean from northern USA to Nuevo Gulf, Argentine Patagonia (Szidat & Nani, 1951Szidat L, Nani A. Las remoras del Atlántico Austral con un estudio de su nutricion natural y de sus parásitos (Pisc. Echeneidae). Rev Inst Nac Invest Cienc Nat, Mus Argent Cienc Nat “Bernardino Rivadavia”. Cien Zool 1951; 2(6): 385-417.; Nasir & Díaz, 1971Nasir P, Díaz M. A revision of genus Lecithochirium Luehe, 1901, and redescription of L. monticellii (Linton, 1898) Skrjabin and Guschanskaja, 1955. Riv Parassitol 1971; 32(1): 27-36. PMid:5560150.; Yamaguti, 1971Yamaguti S. Synopsis of the digenetic trematodes of vertebrates. Tokyo: Keigaku Publishing Co; 1971. (vol. 1-2).; Fernandes et al., 1985Fernandes BMM, Kohn A, Pinto RM. Aspidogastrid and digenetic trematodes parasites of marine fishes of the coast of Rio de Janeiro State, Brazil. Rev Bras Biol 1985; 45(1-2): 109-116. PMid:4059605., 2009Fernandes BMM, Arci ADN, Cohen SC. New data on some species of Monogenea and Digenea parasites of marine fish from the coast of the State of Rio de Janeiro, Brazil. Rev Bras Parasitol Vet 2009; 18(2): 13-18. http://dx.doi.org/10.4322/rbpv.01802003. PMid:19602310.
http://dx.doi.org/10.4322/rbpv.01802003...
; Pereira et al., 2000Pereira J Jr, Fernandes BMM, Robaldo RB. Digenea (Trematoda) of Micropogonias furnieri (Desmarest) (Perciformes, Sciaenidae) from Rio Grande do Sul, Brazil. Rev Bras Zool 2000; 17(3): 681-686. http://dx.doi.org/10.1590/S0101-81752000000300013.
http://dx.doi.org/10.1590/S0101-81752000...
; Luque et al., 2003Luque JL, Alves DR, Ribeiro RS. Community ecology of the metazoan parasites of Banded Croaker, Paralonchurus brasiliensis (Osteichthyes: Sciaenidae), from the coastal zone of the State of Rio de Janeiro, Brazil. Acta Sci Biol Sci 2003; 25(2): 273-278. http://dx.doi.org/10.4025/actascibiolsci.v25i2.2009.
http://dx.doi.org/10.4025/actascibiolsci...
, 2008Luque JL, Felizardo NN, Tavares LER. Community ecology of the metazoan parasites of namorado sandperches, Pseudopercis numida Miranda-Ribeiro, 1903 and P. semifasciata Cuvier, 1829 (Perciformes: Pinguipedidae), from the coastal zone of the State of Rio de Janeiro, Brazil. Braz J Biol 2008; 68(2): 269-278. http://dx.doi.org/10.1590/S1519-69842008000200007. PMid:18660954.
http://dx.doi.org/10.1590/S1519-69842008...
; Cordeiro & Luque, 2005Cordeiro AS, Luque JL. Metazoários parasitos do coió Dactylopterus volitans (Linnaeus, 1758) (Osteichthyes: Dactylopteridae) do litoral do Estado do Rio de Janeiro, Brasil. Acta Sci Biol Sci 2005; 27(2): 119-123. http://dx.doi.org/10.4025/actascibiolsci.v27i2.1320.
http://dx.doi.org/10.4025/actascibiolsci...
; Kohn et al., 2007Kohn A, Fernandes BMM, Cohen SC. South American Trematodes parasites of fishes. Rio de Janeiro: Imprinta Express; 2007.; Timi et al., 2010Timi JT, Lanfranchi AL, Luque JL. Similarity in parasite communities of the teleost fish Pinguipes brasilianus in the southwestern Atlantic: infracommunities as a tool to detect geographical patterns. Int J Parasitol 2010; 40(2): 243-254. http://dx.doi.org/10.1016/j.ijpara.2009.07.006. PMid:19682450.
http://dx.doi.org/10.1016/j.ijpara.2009....
; Carvalho & Luque, 2011Carvalho AR, Luque JL. Seasonal variation in metazoan parasites of Trichiurus lepturus (Perciformes: Trichiuridae) of Rio de Janeiro, Brazil. Braz J Biol 2011; 71(3): 771-782. http://dx.doi.org/10.1590/S1519-69842011000400024. PMid:21881803.
http://dx.doi.org/10.1590/S1519-69842011...
; Bueno et al., 2014Bueno GBF, Aguiar JCC, Santos SMC. Community structure of metazoan parasites of Trichiurus lepturus (Perciformes, Trichiuridae) from Ubatuba, Southwestern Atlantic Ocean, Brazil. Acta Sci Biol Sci 2014; 36(3): 357-364. http://dx.doi.org/10.4025/actascibiolsci.v36i3.21908.
http://dx.doi.org/10.4025/actascibiolsci...
; Justo & Kohn, 2014Justo MCN, Kohn A. Monogenoidea and Digenea parasites of Thunnus atlanticus (Perciformes, Scombridae) from Rio de Janeiro coast, Brazil. Neotrop Helminthol 2014; 8(2): 339-348., 2015Justo MCN, Kohn A. Thunnus obesus (Perciformes, Scombridae), a new host record for three species of Digenea from the coastal zone of the state of Rio de Janeiro, Brazil: research note. Bioikos 2015; 29(1): 35-38.; Pereira et al., 2014Pereira AN, Pantoja C, Luque JL, Timi JT. Parasites of Urophycis brasiliensis (Gadiformes: Phycidae) as indicators of marine ecoregions in coastal areas of the South American Atlantic. Parasitol Res 2014; 113(11): 4281-4292. http://dx.doi.org/10.1007/s00436-014-4106-3. PMid:25245108.
http://dx.doi.org/10.1007/s00436-014-410...
; Eiras et al., 2016Eiras JC, Velloso AL, Pereira J Jr. Parasitos de peixes marinhos da América do Sul. Rio Grande: Ed. FURG; 2016.; França et al., 2020França LF, Knoff M, Fonseca MCG, Gomes DC, Ferreira MS, Felizardo NN, et al. Lecithochirium monticellii digenetic trematode parasites of Trichiurus lepturus (Actinopterygii) from the state of Rio de Janeiro, Brazil, with notes on its taxonomy. An Acad Bras Cienc 2020; 92(2): e20190161. http://dx.doi.org/10.1590/0001-3765202020190161. PMid:32578665.
http://dx.doi.org/10.1590/0001-376520202...
). This is the first report of L. monticellii parasitizing P. isosceles and P. patagonicus.
The morphology and morphometry of the specimens of A. laguncula collected in the present study were in accordance with the redescriptions of Manter (1947)Manter HW. The digenetic trematodes of marine fishes of Tortugas, Florida. Am Midl Nat 1947; 38(2): 257-416. http://dx.doi.org/10.2307/2421571.
http://dx.doi.org/10.2307/2421571...
, Szidat (1961)Szidat L. Versuch einer Zoogeographie des Sud-Atlantik mit Hilfe von Leitparasiten der Meeresfische. Parasit Schrift 1961; 13: 1-98., Nahhas & Short (1965)Nahhas FM, Short RB. Digenetic trematodes of marine fishes from Apalachee Bay, Gulf of Mexico. Tulane Stud Zool 1965; 12: 39-50. http://dx.doi.org/10.5962/bhl.part.20210.
http://dx.doi.org/10.5962/bhl.part.20210...
and Fernandes et al. (1985)Fernandes BMM, Kohn A, Pinto RM. Aspidogastrid and digenetic trematodes parasites of marine fishes of the coast of Rio de Janeiro State, Brazil. Rev Bras Biol 1985; 45(1-2): 109-116. PMid:4059605.. Although Bray & MacKenzie (1990)Bray RA, MacKenzie K. Aponurus laguncula Looss, 1907 (Digenea: Lecithasteridae): a report from herring, Clupea harengus L., in the eastern English Channel and a review of its biology. Syst Parasitol 1990; 17(2): 115-124. http://dx.doi.org/10.1007/BF00009799.
http://dx.doi.org/10.1007/BF00009799...
demonstrated the entrance of the metraterm at the base of the sinus sac in an illustration in their redescription of this species, they did not describe this in the text, which is provided by the present study as “metraterm entering sinus sac ventrally joining male duct immediately to the beginning of sinus sac to the internal ejaculatory duct”.
The species A. laguncula was found parasitizing P. patagonicus in Argentinean waters by Szidat (1961)Szidat L. Versuch einer Zoogeographie des Sud-Atlantik mit Hilfe von Leitparasiten der Meeresfische. Parasit Schrift 1961; 13: 1-98. and in waters off Necochea, Argentina, by Alarcos & Timi (2012)Alarcos AJ, Timi JT. Parasite communities in three sympatric flounder species (Pleuronectiformes: Paralichthyidae): similar ecological filters driving toward repeatable assemblages. Parasitol Res 2012; 110(6): 2155-2166. http://dx.doi.org/10.1007/s00436-011-2741-5. PMid:22167375.
http://dx.doi.org/10.1007/s00436-011-274...
, which differs from the present study, which also found it parasitizing P. isosceles and X. rasile. The species A. laguncula was found in all three of the flounder species studied, which differs from Alarcos et al. (2016)Alarcos AJ, Pereira NA, Taborda NL, Luque JL, Timi JT. Parasitogical evidence of stocks of Paralichthys isosceles (Pleuronectiformes: Paralichthyidae) at small end geographical scales in South American Atlantic coasts. Fish Res 2016; 173(3): 221-228. http://dx.doi.org/10.1016/j.fishres.2015.07.018.
http://dx.doi.org/10.1016/j.fishres.2015...
, who reported it only in P. isosceles from off the state of Rio de Janeiro, Brazil. Furthermore, the species has been previously reported parasitizing a variety of marine teleostean fish species off Brazil, namely Balistes capriscus Gmelin, 1789, B. vetula L., Chaetodipterus faber (Broussonet, 1782), Ctenosciaena gracilicirrhus (Metzelaar, 1919), Dactylopterus volitans L., Micropogonias furnieri (Desmarest, 1823), Mullus argentinae Hubbs & Marini, 1933, Paralonchurus brasiliensis (Steindachner, 1875), Peprilus paru (L.), Pseudopercis numida Miranda Ribeiro, 1903, Rhomboplites aurorubens (Cuvier, 1829), Scomber japonicas Houttuyn, 1782, Trachurus lathami Nichols, 1920, Umbrina coroides Cuvier, 1830, and Urophycis brasiliensis (Kaup, 1858) (Kohn et al., 2007Kohn A, Fernandes BMM, Cohen SC. South American Trematodes parasites of fishes. Rio de Janeiro: Imprinta Express; 2007.; Fernandes et al., 2009Fernandes BMM, Arci ADN, Cohen SC. New data on some species of Monogenea and Digenea parasites of marine fish from the coast of the State of Rio de Janeiro, Brazil. Rev Bras Parasitol Vet 2009; 18(2): 13-18. http://dx.doi.org/10.4322/rbpv.01802003. PMid:19602310.
http://dx.doi.org/10.4322/rbpv.01802003...
; Cárdenas et al., 2012aCárdenas MQ, Fernandes BMM, Justo MCN, Santos AL, Cohen SC. Helminth parasites of Ctenosciaena gracilicirrhus (Perciformes: Sciaenidae) from the coast of Angra dos Reis, Rio de Janeiro State, Brazil. Rev Mex Biodivers 2012a; 83(1): 31-35. http://dx.doi.org/10.22201/ib.20078706e.2012.1.1242.
http://dx.doi.org/10.22201/ib.20078706e....
, bCárdenas MQ, Lanfredi RM, Oliveira-Menezes A. Cytochemical analysis of the body wall of the flounder parasite Procamallanus (Spirocamallanus) halitrophus (Nematoda: camallanidae). Comp Parasitol 2012b; 79(2): 173-181. http://dx.doi.org/10.1654/4565.1.
http://dx.doi.org/10.1654/4565.1...
; Eiras et al., 2016Eiras JC, Velloso AL, Pereira J Jr. Parasitos de peixes marinhos da América do Sul. Rio Grande: Ed. FURG; 2016.). Therefore, the list of hosts for A. laguncula is expanded to include the flounders studied in the present study. This is the first report of A. laguncula parasitizing P. isosceles and X. rasile.
The morphology and morphometry of the specimens of P. (S.) halitrophus collected in the present study were in accordance with redescription of the species using specimens collected from Syacium papillosum L. and Citharichthys macrops Dresel, 1889, from off the coast of the state of Rio de Janeiro, Brazil (Cárdenas & Lanfredi, 2005Cárdenas MQ, Lanfredi RM. Further description of Procamallanus (Spirocamallanus) halitrophus comb. n. (Nematoda: Camallanidae) from flounder off the Brazilian coast by light and scanning electron microscopy. J Parasitol 2005; 91(3): 606-613. http://dx.doi.org/10.1645/GE-3422. PMid:16108554.
http://dx.doi.org/10.1645/GE-3422...
).
In South America, P. (S.) halitrophus has been registered parasitizing some marine teleostean fish off the coast of Brazil, namely P. isosceles, C. macrops, M. argentinae, S. papillosum and U. brasiliensis off the states of Rio de Janeiro, Rio Grande do Sul and Santa Catarina (Alarcos et al., 2016Alarcos AJ, Pereira NA, Taborda NL, Luque JL, Timi JT. Parasitogical evidence of stocks of Paralichthys isosceles (Pleuronectiformes: Paralichthyidae) at small end geographical scales in South American Atlantic coasts. Fish Res 2016; 173(3): 221-228. http://dx.doi.org/10.1016/j.fishres.2015.07.018.
http://dx.doi.org/10.1016/j.fishres.2015...
; Eiras et al., 2016Eiras JC, Velloso AL, Pereira J Jr. Parasitos de peixes marinhos da América do Sul. Rio Grande: Ed. FURG; 2016.; Di Azevedo & Iñiguez, 2018Di Azevedo MIN, Iñiguez AM. Nematode parasites of commercially important fish from the southeast coast of Brazil: morphological and genetic insight. Int J Food Microbiol 2018; 267: 29-41. http://dx.doi.org/10.1016/j.ijfoodmicro.2017.12.014. PMid:29278825.
http://dx.doi.org/10.1016/j.ijfoodmicro....
). Therefore, this is the first report of P. (S.) halitrophus parasitizing P. patagonicus and X. rasile.
The morphology and morphometry of the specimens of C. bonaerensis collected in the present study were in accordance with the description of the species using specimens collected from Urophycis brasiliensis (Kaup, 1858) from off the coast of Mar del Plata, Argentina by Lanfranchi et al. (2004)Lanfranchi AL, Timi JT, Sardella NH. Cucullanus bonaerensis n. sp. (Nematoda: Cucullanidae) parasitizing Urophycis brasiliensis (Pisces: Phycidae) from Argentinean Waters. J Parasitol 2004; 90(4): 808-812. http://dx.doi.org/10.1645/GE-3276. PMid:15357074.
http://dx.doi.org/10.1645/GE-3276...
.
Alarcos & Timi (2012)Alarcos AJ, Timi JT. Parasite communities in three sympatric flounder species (Pleuronectiformes: Paralichthyidae): similar ecological filters driving toward repeatable assemblages. Parasitol Res 2012; 110(6): 2155-2166. http://dx.doi.org/10.1007/s00436-011-2741-5. PMid:22167375.
http://dx.doi.org/10.1007/s00436-011-274...
found Cucullanus sp. and C. bonaerensis parasitizing X. rasile off the Argentine Coast, while the present study found only C. bonaerensis in the three paralichthyid hosts studied. Alarcos et al. (2016)Alarcos AJ, Pereira NA, Taborda NL, Luque JL, Timi JT. Parasitogical evidence of stocks of Paralichthys isosceles (Pleuronectiformes: Paralichthyidae) at small end geographical scales in South American Atlantic coasts. Fish Res 2016; 173(3): 221-228. http://dx.doi.org/10.1016/j.fishres.2015.07.018.
http://dx.doi.org/10.1016/j.fishres.2015...
reported Cucullanus sp. in P. isosceles from off the coast of the state of Rio de Janeiro, Brazil, which differs from the present study, which found only C. bonaerensis. Therefore, the present study expands the occurrence of this species in South America. This is the first report of C. bonaerensis parasitizing P. isosceles and P. patagonicus.
Alarcos & Timi (2012)Alarcos AJ, Timi JT. Parasite communities in three sympatric flounder species (Pleuronectiformes: Paralichthyidae): similar ecological filters driving toward repeatable assemblages. Parasitol Res 2012; 110(6): 2155-2166. http://dx.doi.org/10.1007/s00436-011-2741-5. PMid:22167375.
http://dx.doi.org/10.1007/s00436-011-274...
found gravid females of C. bonaerensis in X. rasile from off the Argentine Coast and suggested this species as their definitive host. In the present study, gravid females of this nematode were only found in P. patagonicus. Lanfranchi et al. (2004)Lanfranchi AL, Timi JT, Sardella NH. Cucullanus bonaerensis n. sp. (Nematoda: Cucullanidae) parasitizing Urophycis brasiliensis (Pisces: Phycidae) from Argentinean Waters. J Parasitol 2004; 90(4): 808-812. http://dx.doi.org/10.1645/GE-3276. PMid:15357074.
http://dx.doi.org/10.1645/GE-3276...
did not find any gravid females of C. bonaerensis, which they attributed to seasonality in the life cycle of the parasites and/or host suitability.
The sites of infection found here were the same as reported by Alarcos & Timi (2012)Alarcos AJ, Timi JT. Parasite communities in three sympatric flounder species (Pleuronectiformes: Paralichthyidae): similar ecological filters driving toward repeatable assemblages. Parasitol Res 2012; 110(6): 2155-2166. http://dx.doi.org/10.1007/s00436-011-2741-5. PMid:22167375.
http://dx.doi.org/10.1007/s00436-011-274...
, stomach for hemiurid and lecithasterid digenean trematodes and intestine for camallanid and cucullanid nematodes.
Comparisons of the parasitic indices of the present study with those for the hemiurid and lecithasterid digeneans and camalanid and cucullanid nematodes collected from flounders in Argentine waters by Alarcos & Timi (2012)Alarcos AJ, Timi JT. Parasite communities in three sympatric flounder species (Pleuronectiformes: Paralichthyidae): similar ecological filters driving toward repeatable assemblages. Parasitol Res 2012; 110(6): 2155-2166. http://dx.doi.org/10.1007/s00436-011-2741-5. PMid:22167375.
http://dx.doi.org/10.1007/s00436-011-274...
found that the latter had a lower prevalence (10.42%) and mean abundance (0.17) for L. microstomum in X. rasile; a lower prevalence (23.53%) and mean abundance (0.94) for A. laguncula in P. patagonicus; and a higher prevalence (39.58%) and lower mean abundance (0.73) for C. bonaerensis in X. rasile.
Comparisons with the parasitic indices of helminths parasitizing P. isosceles off of the state of Rio de Janeiro, Brazil, reported by Alarcos et al. (2016)Alarcos AJ, Pereira NA, Taborda NL, Luque JL, Timi JT. Parasitogical evidence of stocks of Paralichthys isosceles (Pleuronectiformes: Paralichthyidae) at small end geographical scales in South American Atlantic coasts. Fish Res 2016; 173(3): 221-228. http://dx.doi.org/10.1016/j.fishres.2015.07.018.
http://dx.doi.org/10.1016/j.fishres.2015...
, revealed that these authors found lower prevalences (32.80%, 5.30%,11.10%) and mean abundances (0.95, 0.10, 0.17) for L. microstomum, A. laguncula and P. halitrophus (respectively). These differences in parasitic indices could be related to the greater number of collection locations of the present study since, beyond the municipalities of Cabo Frio and Niterói, the present study also collected in the municipalities of Rio de Janeiro and Angra dos Reis. The differences could also be due to various environmental and seasonal factors intrinsic to the collections themselves.
Differences observed in helminthofauna composition and parasitic indices for the same hosts collected in Necochea, Argentina, and Rio de Janeiro, Brazil, could be correlated with the different ecoregions of these areas. According to Spalding et al. (2007)Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson M, et al. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience 2007; 57(7): 573-583. http://dx.doi.org/10.1641/B570707.
http://dx.doi.org/10.1641/B570707...
, marine ecoregions comprise relatively homogeneous sets of species that are clearly distinct from adjacent systems. The dominant biogeographic agents that define ecoregions vary from location to location but may include physico-chemical and biological factors. Thereby, features observed within ecoregions can influence their fish parasite communities and, thus, explain the parasite compositions found for flounder species from waters off Argentina by Alarcos & Timi (2012)Alarcos AJ, Timi JT. Parasite communities in three sympatric flounder species (Pleuronectiformes: Paralichthyidae): similar ecological filters driving toward repeatable assemblages. Parasitol Res 2012; 110(6): 2155-2166. http://dx.doi.org/10.1007/s00436-011-2741-5. PMid:22167375.
http://dx.doi.org/10.1007/s00436-011-274...
and those found for the same flounder species from waters off of the state of Rio de Janeiro, Brazil, by the present study and for that found for P. isosceles by Alarcos et al. (2016)Alarcos AJ, Pereira NA, Taborda NL, Luque JL, Timi JT. Parasitogical evidence of stocks of Paralichthys isosceles (Pleuronectiformes: Paralichthyidae) at small end geographical scales in South American Atlantic coasts. Fish Res 2016; 173(3): 221-228. http://dx.doi.org/10.1016/j.fishres.2015.07.018.
http://dx.doi.org/10.1016/j.fishres.2015...
.
Acknowledgments
The authors thank Ricardo Baptista Schmidt (Serviço de Produção e Tratamento de Imagens do Instituto Oswaldo Cruz/FIOCRUZ) for processing the figures, and Isaac Albert Mallet (Laboratório de Microscopia Eletrônica de Varredura da Universidade Estadual do Rio de Janeiro) for assistance in obtaining SEM photographs. This work was supported by Conselho Nacional de Desenvolvimento Cientifico e Tecnológico - CNPq fellowship (MCGF: 150140/2018-5) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES grant (NNF: EXPPD 000020).
-
How to cite: Fonseca MCG, Felizardo NN, Torres EJL, Gomes DC, Knoff M. Hemiurid and lecithasterid digenean trematodes and camallanid and cucullanid nematodes parasitizing flounders collected off the coast of Rio de Janeiro State, Brazil. Braz J Vet Parasitol 2022; 31(1): e019921. https://doi.org/10.1590/S1984-29612022011
References
- Acha PN, Szyfres B. Zoonoses and communicable diseases common to man and animals. Vol. III. Parasitoses 3rd ed. Washington, DC: PAHO; 2003. Scientific and Technical Publication; no. 580.
- Alarcos AJ, Pereira NA, Taborda NL, Luque JL, Timi JT. Parasitogical evidence of stocks of Paralichthys isosceles (Pleuronectiformes: Paralichthyidae) at small end geographical scales in South American Atlantic coasts. Fish Res 2016; 173(3): 221-228. http://dx.doi.org/10.1016/j.fishres.2015.07.018
» http://dx.doi.org/10.1016/j.fishres.2015.07.018 - Alarcos AJ, Timi JT. Parasite communities in three sympatric flounder species (Pleuronectiformes: Paralichthyidae): similar ecological filters driving toward repeatable assemblages. Parasitol Res 2012; 110(6): 2155-2166. http://dx.doi.org/10.1007/s00436-011-2741-5 PMid:22167375.
» http://dx.doi.org/10.1007/s00436-011-2741-5 - Bernardes RA, Figueiredo JL, Rodrigues AR, Fischer LG, Vooren CM, Haimovici M, et al. Peixes da zona econômica exclusiva da região sudeste-sul do Brasil: Levantamento com armadilhas, pargueiras e rede de arrasto de fundo São Paulo: Editora da Universidade de São Paulo; 2005.
- Bray RA, MacKenzie K. Aponurus laguncula Looss, 1907 (Digenea: Lecithasteridae): a report from herring, Clupea harengus L., in the eastern English Channel and a review of its biology. Syst Parasitol 1990; 17(2): 115-124. http://dx.doi.org/10.1007/BF00009799
» http://dx.doi.org/10.1007/BF00009799 - Bueno GBF, Aguiar JCC, Santos SMC. Community structure of metazoan parasites of Trichiurus lepturus (Perciformes, Trichiuridae) from Ubatuba, Southwestern Atlantic Ocean, Brazil. Acta Sci Biol Sci 2014; 36(3): 357-364. http://dx.doi.org/10.4025/actascibiolsci.v36i3.21908
» http://dx.doi.org/10.4025/actascibiolsci.v36i3.21908 - Bush AO, Lafferty KD, Lotz JM, Shostak AW. Revisited. J Parasitol 1997; 83(4): 575-583. http://dx.doi.org/10.2307/3284227 PMid:9267395.
» http://dx.doi.org/10.2307/3284227 - Cárdenas MQ, Fernandes BMM, Justo MCN, Santos AL, Cohen SC. Helminth parasites of Ctenosciaena gracilicirrhus (Perciformes: Sciaenidae) from the coast of Angra dos Reis, Rio de Janeiro State, Brazil. Rev Mex Biodivers 2012a; 83(1): 31-35. http://dx.doi.org/10.22201/ib.20078706e.2012.1.1242
» http://dx.doi.org/10.22201/ib.20078706e.2012.1.1242 - Cárdenas MQ, Lanfredi RM, Oliveira-Menezes A. Cytochemical analysis of the body wall of the flounder parasite Procamallanus (Spirocamallanus) halitrophus (Nematoda: camallanidae). Comp Parasitol 2012b; 79(2): 173-181. http://dx.doi.org/10.1654/4565.1
» http://dx.doi.org/10.1654/4565.1 - Cárdenas MQ, Lanfredi RM. Further description of Procamallanus (Spirocamallanus) halitrophus comb. n. (Nematoda: Camallanidae) from flounder off the Brazilian coast by light and scanning electron microscopy. J Parasitol 2005; 91(3): 606-613. http://dx.doi.org/10.1645/GE-3422 PMid:16108554.
» http://dx.doi.org/10.1645/GE-3422 - Carvalho AR, Luque JL. Seasonal variation in metazoan parasites of Trichiurus lepturus (Perciformes: Trichiuridae) of Rio de Janeiro, Brazil. Braz J Biol 2011; 71(3): 771-782. http://dx.doi.org/10.1590/S1519-69842011000400024 PMid:21881803.
» http://dx.doi.org/10.1590/S1519-69842011000400024 - Cerqueira VR, Mioso R, Macchiavello JAG, Brügger AM. Ensaios de indução de desova do linguado (Paralichthys orbignyanus Valenciennes, 1839). Bol Inst Pesca 1997; 24(special): 247-254.
- Cordeiro AS, Luque JL. Metazoários parasitos do coió Dactylopterus volitans (Linnaeus, 1758) (Osteichthyes: Dactylopteridae) do litoral do Estado do Rio de Janeiro, Brasil. Acta Sci Biol Sci 2005; 27(2): 119-123. http://dx.doi.org/10.4025/actascibiolsci.v27i2.1320
» http://dx.doi.org/10.4025/actascibiolsci.v27i2.1320 - De Ley P, Blaxter M. A new system for Nematoda: combining morphological characters with molecular trees, and translating clades into ranks and taxa. In: Cook R, Hunt DJ, editors. Nematology monographs and perspectives Leiden: EJ Brill; 2004. p. 633-653.
- Di Azevedo MIN, Iñiguez AM. Nematode parasites of commercially important fish from the southeast coast of Brazil: morphological and genetic insight. Int J Food Microbiol 2018; 267: 29-41. http://dx.doi.org/10.1016/j.ijfoodmicro.2017.12.014 PMid:29278825.
» http://dx.doi.org/10.1016/j.ijfoodmicro.2017.12.014 - Eiras JC, Takemoto RM, Pavanelli GC. Métodos de estudo e técnicas laboratoriais em parasitologia de peixes 2ª ed. Maringá: Eduem; 2006.
- Eiras JC, Velloso AL, Pereira J Jr. Parasitos de peixes marinhos da América do Sul. Rio Grande: Ed. FURG; 2016.
- Felizardo NN, Justo MC, Knoff M, Fonseca MCG, Pinto RM, Gomes DC. Juvenile didymozoids of the types, Torticaecum and Neotorticaecum (Didymozoidae: Digenea), from new marine fish hosts (Pisces: Teleostei) in the neotropical region of Brazil. J Helminthol 2011; 85(3): 270-275. http://dx.doi.org/10.1017/S0022149X10000507 PMid:20854704.
» http://dx.doi.org/10.1017/S0022149X10000507 - Felizardo NN, Knoff M, Diniz JB, Torres EJL, Calixto FAA, São Clemente SC. Pterobothrium crassicolle parasitizing Paralichthys orbignyanus (Osteichthyes, Paralichthyidae) in Brazil. An Acad Bras Cienc 2018; 90(2): 1605-1610. http://dx.doi.org/10.1590/0001-3765201820170323 PMid:29768571.
» http://dx.doi.org/10.1590/0001-3765201820170323 - Felizardo NN, Knoff M, Pinto RM, Gomes DC. Larval anisakid nematodes of the flounder, Paralichthys isosceles Jordan, 1890 (Pisces: Teleostei) from Brazil. Neotrop Helminthol 2009a; 3(2): 57-64.
- Felizardo NN, Menezes RC, Tortelly R, Knoff M, Pinto RM, Gomes DC. Larvae of Hysterothylacium sp. (Nematoda: Anisakidae) in the sole fish Paralichthys isosceles Jordan, 1890 (Pisces: Teleostei) from the littoral of the state of Rio de Janeiro, Brazil. Vet Parasitol 2009b; 166(1-2): 175-177. http://dx.doi.org/10.1016/j.vetpar.2009.08.004 PMid:19713041.
» http://dx.doi.org/10.1016/j.vetpar.2009.08.004 - Felizardo NN, Torres EJL, Fonseca MCG, Pinto RM, Gomes DC, Knoff M. Cestodes of the flounder Paralichthys isosceles Jordan, 1890 (Osteichthyes - Paralichthyidae) from the State of Rio de Janeiro, Brazil. Neotrop Helminthol 2010; 4(2): 113-125.
- Fernandes BMM, Arci ADN, Cohen SC. New data on some species of Monogenea and Digenea parasites of marine fish from the coast of the State of Rio de Janeiro, Brazil. Rev Bras Parasitol Vet 2009; 18(2): 13-18. http://dx.doi.org/10.4322/rbpv.01802003 PMid:19602310.
» http://dx.doi.org/10.4322/rbpv.01802003 - Fernandes BMM, Kohn A, Pinto RM. Aspidogastrid and digenetic trematodes parasites of marine fishes of the coast of Rio de Janeiro State, Brazil. Rev Bras Biol 1985; 45(1-2): 109-116. PMid:4059605.
- Figueiredo JL, Menezes NA. Manual de peixes marinhos do sudeste do Brasil. VI. Teleostei (5). São Paulo: Museu de Zoologia, USP; 2000.
- Fonseca MCG, Knoff M, Felizardo NN, Di Azevedo MIN, Torres EJL, Gomes DC, et al. Integrative taxonomy of Anisakidae and Raphidascarididae (Nematoda) in Paralichthys patagonicus and Xystreurys rasile (Pisces: teleostei). Int J Food Microbiol 2016; 235: 113-124. http://dx.doi.org/10.1016/j.ijfoodmicro.2016.07.026 PMid:27491056.
» http://dx.doi.org/10.1016/j.ijfoodmicro.2016.07.026 - Fonseca MCG, Knoff M, Felizardo NN, Torres EJL, Di Azevedo MIN, Gomes DC, et al. Acanthocephalan parasites of the flounder species Paralichthys isosceles, Paralichthys patagonicus and Xystreurys rasile from Brazil. Rev Bras Parasitol Vet 2019; 28(3): 346-359. http://dx.doi.org/10.1590/s1984-29612019031 PMid:31215609.
» http://dx.doi.org/10.1590/s1984-29612019031 - Fonseca MCG, São Clemente SC, Felizardo NN, Gomes DC, Knoff M. Trypanorhyncha cestodes of hygienic-sanitary importance infecting flounders Paralichthys patagonicus Jordan, 1889 and Xystreurys rasile (Jordan, 1891) of the Neotropical region, Brazil. Parasitol Res 2012; 111: 865-874. http://dx.doi.org/10.1007/s00436-012-2912-z PMid:22488201.
» http://dx.doi.org/10.1007/s00436-012-2912-z - França LF, Knoff M, Fonseca MCG, Gomes DC, Ferreira MS, Felizardo NN, et al. Lecithochirium monticellii digenetic trematode parasites of Trichiurus lepturus (Actinopterygii) from the state of Rio de Janeiro, Brazil, with notes on its taxonomy. An Acad Bras Cienc 2020; 92(2): e20190161. http://dx.doi.org/10.1590/0001-3765202020190161 PMid:32578665.
» http://dx.doi.org/10.1590/0001-3765202020190161 - Fusco AC, Overstreet RM. Spirocamallanus cricotus sp. n. and S. halitrophus sp. n. (Nematoda: Camallanidae) from fishes in the northern Gulf of Mexico. J Parasitol 1978; 64(2): 239-244. http://dx.doi.org/10.2307/3279663 PMid:641665.
» http://dx.doi.org/10.2307/3279663 - Gibson DI, Jones A, Bray RA. Keys to the Trematoda Wallingford: CABI Publishing; 2002. (vol. 1). http://dx.doi.org/10.1079/9780851995472.0000
» http://dx.doi.org/10.1079/9780851995472.0000 - Justo MCN, Fernandes BMM, Knoff M, Cárdenas MQ, Cohen SC. Checklist of Brazilian Cestoda. Neotrop Helminthol 2017; 11(1): 187-282.
- Justo MCN, Kohn A. Monogenoidea and Digenea parasites of Thunnus atlanticus (Perciformes, Scombridae) from Rio de Janeiro coast, Brazil. Neotrop Helminthol 2014; 8(2): 339-348.
- Justo MCN, Kohn A. Thunnus obesus (Perciformes, Scombridae), a new host record for three species of Digenea from the coastal zone of the state of Rio de Janeiro, Brazil: research note. Bioikos 2015; 29(1): 35-38.
- Klimpel S, Palm HW. Anisakid Nematode (Ascaridoidea) life cycles and distribution: increasing zoonotic potential in the time of climate change? In: Mehlhorn H, editor. Progress in parasitology Berlin: Springer; 2011. p. 201-221. (Parasitology Research Monographs, vol. 2). http://dx.doi.org/10.1007/978-3-642-21396-0_11
» http://dx.doi.org/10.1007/978-3-642-21396-0_11 - Knoff M, Felizardo NN, Iniguez AM, Maldonado JRA Jr, Torres EJL, Pinto RM, et al. Genetic and morphological characterisation of a new species of the genus Hysterothylacium (Nematoda) from Paralichthys isosceles Jordan, 1890 (Pisces: Teleostei) of the Neotropical region, State of Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 2012; 107(2): 186-193. http://dx.doi.org/10.1590/S0074-02762012000200006 PMid:22415256.
» http://dx.doi.org/10.1590/S0074-02762012000200006 - Knoff M, Gomes DC. Metodologia básica para coleta e o processamento de helmintos parasitos. In: Molinaro EM, Caputo LFG, Amendoeira MRR, editors. Conceitos e métodos para a formação de profissionais em laboratórios de saúde. Rio de Janeiro: EPSJV, IOC; 2012. (vol. 5).
- Kohn A, Fernandes BMM, Cohen SC. South American Trematodes parasites of fishes Rio de Janeiro: Imprinta Express; 2007.
- Lanfranchi AL, Timi JT, Sardella NH. Cucullanus bonaerensis n. sp. (Nematoda: Cucullanidae) parasitizing Urophycis brasiliensis (Pisces: Phycidae) from Argentinean Waters. J Parasitol 2004; 90(4): 808-812. http://dx.doi.org/10.1645/GE-3276 PMid:15357074.
» http://dx.doi.org/10.1645/GE-3276 - Linton E. Trematodes from fishes mainly from the Woods Hole region, Massachusetts. Proc USNM 1940; 88(3078): 1-172. http://dx.doi.org/10.5479/si.00963801.88-3078.1
» http://dx.doi.org/10.5479/si.00963801.88-3078.1 - Looss A. Beiträge zur Systematik der Distomen. Zur Kenntnis der Familie Hemiuridae. Zool Jahrb (Syst) 1908; 26: 63-180.
- Lopes Torres EJ, Souza W, Miranda K. Comparative analysis of Trichuris muris surface using conventional, low vacuum, environmental and field emission scanning electron microscopy. Vet Parasitol 2013; 196(3-4): 409-416. http://dx.doi.org/10.1016/j.vetpar.2013.02.026 PMid:23537947.
» http://dx.doi.org/10.1016/j.vetpar.2013.02.026 - Luque JL, Alves DR, Ribeiro RS. Community ecology of the metazoan parasites of Banded Croaker, Paralonchurus brasiliensis (Osteichthyes: Sciaenidae), from the coastal zone of the State of Rio de Janeiro, Brazil. Acta Sci Biol Sci 2003; 25(2): 273-278. http://dx.doi.org/10.4025/actascibiolsci.v25i2.2009
» http://dx.doi.org/10.4025/actascibiolsci.v25i2.2009 - Luque JL, Felizardo NN, Tavares LER. Community ecology of the metazoan parasites of namorado sandperches, Pseudopercis numida Miranda-Ribeiro, 1903 and P. semifasciata Cuvier, 1829 (Perciformes: Pinguipedidae), from the coastal zone of the State of Rio de Janeiro, Brazil. Braz J Biol 2008; 68(2): 269-278. http://dx.doi.org/10.1590/S1519-69842008000200007 PMid:18660954.
» http://dx.doi.org/10.1590/S1519-69842008000200007 - Manter HW. The digenetic trematodes of marine fishes of Tortugas, Florida. Am Midl Nat 1947; 38(2): 257-416. http://dx.doi.org/10.2307/2421571
» http://dx.doi.org/10.2307/2421571 - Massa AE, Palacios DL, Paredi ME, Crupkin M. Postmortem changes in quality indices of ice-stored flounder (Paralichthys patagonicus). J Food Biochem 2005; 29(5): 570-590. http://dx.doi.org/10.1111/j.1745-4514.2005.00050.x
» http://dx.doi.org/10.1111/j.1745-4514.2005.00050.x - Nahhas FM, Short RB. Digenetic trematodes of marine fishes from Apalachee Bay, Gulf of Mexico. Tulane Stud Zool 1965; 12: 39-50. http://dx.doi.org/10.5962/bhl.part.20210
» http://dx.doi.org/10.5962/bhl.part.20210 - Nakamura I, Inada T, Takeda M, Hatanaka H. Important fishes trawled off Patagonia Tokyo: JAMARC; 1986.
- Nasir P, Díaz M. A revision of genus Lecithochirium Luehe, 1901, and redescription of L. monticellii (Linton, 1898) Skrjabin and Guschanskaja, 1955. Riv Parassitol 1971; 32(1): 27-36. PMid:5560150.
- Pereira AN, Pantoja C, Luque JL, Timi JT. Parasites of Urophycis brasiliensis (Gadiformes: Phycidae) as indicators of marine ecoregions in coastal areas of the South American Atlantic. Parasitol Res 2014; 113(11): 4281-4292. http://dx.doi.org/10.1007/s00436-014-4106-3 PMid:25245108.
» http://dx.doi.org/10.1007/s00436-014-4106-3 - Pereira J Jr, Fernandes BMM, Robaldo RB. Digenea (Trematoda) of Micropogonias furnieri (Desmarest) (Perciformes, Sciaenidae) from Rio Grande do Sul, Brazil. Rev Bras Zool 2000; 17(3): 681-686. http://dx.doi.org/10.1590/S0101-81752000000300013
» http://dx.doi.org/10.1590/S0101-81752000000300013 - Skrjabin KI, Guschanskaja LK. Suborder Hemiurata (Markevitsch, 1951) Skrjabin et Guschanskaja, 1954. Second part. In: Skrjabin KI, editor. Trematodes of animals and man. Moscow: Akademii Nauk SSSR; 1955. p. 337-643. (vol. 10).
- Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson M, et al. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience 2007; 57(7): 573-583. http://dx.doi.org/10.1641/B570707
» http://dx.doi.org/10.1641/B570707 - Szidat L, Nani A. Las remoras del Atlántico Austral con un estudio de su nutricion natural y de sus parásitos (Pisc. Echeneidae). Rev Inst Nac Invest Cienc Nat, Mus Argent Cienc Nat “Bernardino Rivadavia”. Cien Zool 1951; 2(6): 385-417.
- Szidat L. Versuch einer Zoogeographie des Sud-Atlantik mit Hilfe von Leitparasiten der Meeresfische. Parasit Schrift 1961; 13: 1-98.
- Timi JT, Lanfranchi AL, Luque JL. Similarity in parasite communities of the teleost fish Pinguipes brasilianus in the southwestern Atlantic: infracommunities as a tool to detect geographical patterns. Int J Parasitol 2010; 40(2): 243-254. http://dx.doi.org/10.1016/j.ijpara.2009.07.006 PMid:19682450.
» http://dx.doi.org/10.1016/j.ijpara.2009.07.006 - Williams H, Jones A. Parasitic worms of fish. London: Taylor & Francis; 1994. http://dx.doi.org/10.1201/b12595
» http://dx.doi.org/10.1201/b12595 - Yamaguti S. Synopsis of the digenetic trematodes of vertebrates Tokyo: Keigaku Publishing Co; 1971. (vol. 1-2).
Publication Dates
-
Publication in this collection
11 Mar 2022 -
Date of issue
2022
History
-
Received
05 Nov 2021 -
Accepted
28 Jan 2022