ABSTRACT
Queen palm is a tropical species of ecological importance and economic potential in various uses, including food. However, the knowledge about the nutritional value of fruits and the estimation of seed viability are still scarce. The aims of this work were to study the biometrics and chemical composition of fruits and seeds; describe the anatomy of embryos and assess the viability of the tetrazolium test. Mature fruits were collected, processed and submitted to biometry, water content, pulp and seeds chemical composition, germination and emergence in the laboratory evaluations. For the tetrazolium test, whole embryos were immersed in solution at 40 °C for one, two, four and six hours and compared with germination. The fruit pulp is rich in carbohydrates. The tetrazolium test performed at 40 ° C and 0.2% solution for two hours is adequate to estimate seed viability.
Index terms
Queen palm; Embryo; Germination; Palm; Pyrene
RESUMO
O coqueiro-jerivá é uma espécie tropical de importância ecológica e potencial econômico em diversos usos, inclusive alimentício. Contudo, o conhecimento sobre o valor nutricional dos frutos e a estimativa da viabilidade de sementes ainda são escassos na literatura. Os objetivos deste trabalho foram estudar a biometria e a composição centesimal de frutos e sementes; descrever a anatomia dos embriões, e avaliar o uso do teste de tetrazólio. Frutos maduros e recém-colhidos foram processados e submetidos à avaliação de dados biométricos, teor de água e composição química da polpa e das sementes, avaliação da germinação e emergência de plântulas em laboratório. Para o tetrazólio, embriões inteiros foram imersos na solução, a 40 °C, por uma, duas, quatro e seis horas, e comparados com teste de germinação e da emergência. A polpa dos frutos é rica em carboidratos.O teste de tetrazólio conduzido a 40°C, em solução de concentração a 0,2%, durante duas horas, é adequado para estimar a viabilidade das sementes.
Termos para indexação
Coqueiro-jerivá; Embrião; Germinação; Palmeira; Pirênio
INTRODUCTION
Queen palm is a palm native to Brazil of great ornamental value that produces palm and fruit for consumption, being also planted in other tropical and subtropical countries.
The pulp and almond are sources of oils for the food industry. Its fruits of sweet pulp are edible, and when dehydrated are consumed as dates. The palm, of slightly bitter flavor, is consumed in regional communities and has great economic potential (CARVALHO, 2006 CARVALHO, P.E.R. Espécies arbóreas brasileiras. Brasília, DF: Embrapa Informação Tecnológica, 2006. v.2, 627 p. ; LORENZI et al., 2010 LORENZI, H.; SOUZA, H.M.; MEDEIROS COSTA, J.T.;CERQUEIRA, L.S.C.; FERREIRA, E. Flora brasileira Arecaceae (Palmeiras). Nova Odessa: Plantarum, 2010. 368 p. ).
Its wood is used in rustic constructions and handicrafts. During drought, its leaves are consumed by equines as fodder. Flowers are melliform and of great apicultural potential, producing pollen and nectar. Its planting is recommended in the replacement of degraded areas in several Brazilian biomes. Its flowers and fruits are sources of food for insects, lizards, bats and other mammals like monkeys and raccoons. For several species of birds, in addition to providing food, the plant also serves as shelter. It grows well on the banks of rivers and its fruits feed fish. Leaves are used to produce lampshades and arrangements, while fibers are used to produce nets and other handmade articles. Syagrus romanzoffiana (Cham.) Glassman is a heliophilous species, selective hygrophilous and pioneer and has reaches 10-20 meters in height, and stipe with 20-30 cm in diameter and drupe type globular and ovalshape fruits with yellowish-orange pulp when ripe, fleshy and sweet (REIS, 2006 REIS, R.C.C. Palmeiras (Arecaceae) das Restingas do Estado do Rio de Janeiro, Brasil. Acta Botânica Brasilica, Belo Horizonte, v.20, n.3, p.501-512, 2006. ; Genini et al., 2009 GENINI, J.; GALETTI, M.; MORELLATO, L.P.C. Fruiting phenology of palms and trees in an Atlantic rianforeste land-bridge island. Flora, Freiberg, n.204, p.131-145, 2009. ). It has a wide dispersion in South America, being native to Brazil, Uruguay and Argentina (LORENZI, 2014 LORENZI, H. Árvores brasileiras, manual de identificação e cultivo de plantas arbóreas nativas do Brasil. 6.ed. Nova Odessa: Plantarum, 2014. v.1, 384p. ; REIS, 2006 REIS, R.C.C. Palmeiras (Arecaceae) das Restingas do Estado do Rio de Janeiro, Brasil. Acta Botânica Brasilica, Belo Horizonte, v.20, n.3, p.501-512, 2006. ).
Due to its ornamental and ecological importance as a native food species of the Brazilian Cerrado, Atlantic Forest and Pampas, the use of quality seeds becomes essential to subsidize regeneration of disturbed areas, commercial plantations or conservation and utilization of genetic resources (CRUZ; CARVALHO, 2003 CRUZ, E.D.; CARVALHO, J.E.U. Biometria de frutos e germinação de sementes de Couratari stellata A.C. Smith (Lecythidaceae). Acta Amazonica, Manaus, v.33, n.3, p.381-387, 2003. ).
Thus, knowledge about a method of rapid and safe evaluation of the viability of seed lots becomes essential.
The propagation of S. romanzoffiana is done by seeds and according to Dransfield et al. (2008) DRANSFIELD, J.; UHL, N.W.; ASMUSSE, C.B.; BAKER, W.; HARLEY, M.; LEWIS, C. Genera palmarum: the evolution and classification of palms. Surrey: Royal Botanic Gardens, 2008. 732 p. , species of this genus present tubular remote or adjacent ligamentous germination. However, the germination of the Queen palm is considered slow and uneven, with great variation in the emergency process, which suggests the existence of some dormancy mechanism (CARVALHO, 2006 CARVALHO, P.E.R. Espécies arbóreas brasileiras. Brasília, DF: Embrapa Informação Tecnológica, 2006. v.2, 627 p. ).For the production of seedlings, this mechanism presents itself as an important disadvantage, as it induces great unevenness in the emergence of seedlings and loss of seeds due to deterioration, since they remain longer in the soil before germination (SMIDERLE et al, 2005 SMIDERLE, O.J.; MOURÃO JUNIOR, M.; SOUSA, R.C.P. Tratamentos pré-germinativos em sementes de acácia. Revista Brasileira de Sementes, Londrina, v.27, n.1, p.78-85, 2005. ).
In addition, other factors such as degree of maturation, presence or absence of pericarp, the time between harvest and sowing, environment and substrate temperature, and the water content of seeds may affect germination and emergence of seedlings (BROSCHAT, 1994 BROSCHAT, T.K. Palm seed propagation. Acta Horticulturae, The Hague, v.360, p.141-147, 1994. ).
Although some factors related to seed deterioration are known, the evaluation of seed viability by fast and efficient method, such as tetrazolium, is essential for the monitoring of seed quality (FRANÇA NETO et al., 1998 FRANÇA NETO, J.B.; KRZYZANOWSKI, F.C.; COSTA, N.P. O teste de tetrazólio em sementes de soja. Londrina: EMBRAPA-CNPSo, 1998. 72 p. (Documentos, 116). ). The test is based on the activity of dehydrogenase enzymes involved in cellular respiration. Thus, the reduction reaction of the tetrazolium salt solution occurs only in living cells and causes changes in the staining of embryo tissues, whereas dead tissues retain their original color (FRANÇA NETO et al., 2009 FRANÇA NETO, J.B.; KRZYZANOWSKI, F.C.; COSTA, N.P. Teste de tetrazólio em semente. In: BAALBAKI, R.; ELIAS, S.; MARCOS-FILHO, J.; MCDONALD, M.B. Seed vigor testing handbook. Ithaca: AOSA, 2009. (Contribuiton, 32). ).
To obtain accurate and reliable data using the tetrazolium test, in addition to the knowledge of the embryonic structure, the test requires standardization and improvement of specific techniques such as: procedures of preparation of seeds for staining, definition and correct interpretation of the resulting coloration, which are aspects that depend on the species under study and / or evaluation, as observed by several (BITTENCOURT et al., 1997 BITTENCOURT, S.R.M.; VIEIRA, R.D.; RODRIGUES, T.J.D. Criteria for peanut seed pre-conditioning for the tetrazolium test. Seed Science and Technology, Bassersdorf, v.25, n.3, p.337-342, 1997. ; FLORES et al., 2011 FLORES, P.C.; POGGI, D.; GARCÍA, S.M.; GARIGLIO, N.F. Topographic tetrazolium testing of black walnut (Juglans nigra L.) seeds. Seed Science and Technology, Zurich, v.39, n.1, p.230-235, 2011. ;SANTOS et al., 2012 SANTOS, J.F.; SANCHES, M.F.G.; BARBOSA, R.M.; LEÃO, E.F.; VIEIRA, R.D.Optimising tetrazolium test procedures to evaluate the physiological potential of peanut seeds. Seed Science and Technology, Bassersdorf, v.40, n.2, p.215-228, 2012. ; SILVA et al., 2012 SILVA, C.B.; BARBOSA, R.M.; VIEIRA, R.D. Evaluating sunnhemp (Crotalaria juncea) seed viability using the tetrazolium test. Seed Technology, Washington, v.34, n.2, p.263-272, 2012. ).
Due to the importance of the species and the lack of information, the aims of this work were to study the biometry and chemical composition of fruits and pyrenes; to describe the anatomy of embryos and to evaluate the use of the tetrazolium test in S. romanzoffiana seeds.
MATERIAL AND METHODS
Seeds: S. romanzoffiana (Cham.) Glassman fruits from 20 matrixes distant at least 100 m from each other were used. After collection, fruits were packed in polystyrene boxes and sent for analysis.
Freshly harvested ripe fruits were pulped (epicarp and mesocarp removal) using a knife and the remainder of the pulp was removed by manually rubbing them on the screen of a sieve (0.6 mm mesh) under constant water flow (Figure 1).Pyrenes, i.e., the structure represented by endocarp and seed, called here as seeds, were immersed in 0.05% sodium hypochlorite solution (NaOCl) for five minutes, washed in running water and submitted to shade drying on absorbent paper for four hours (LIMA JUNIOR, 2011 LIMA JUNIOR, M.J. (Ed.). Manual de procedimentos para análise de sementes florestais. Londrina: ABRATES, 2011. 83 p. ).
Biometry: Biometric data were obtained from 1000 fruits and 1000 seeds using a digital caliper graduated in millimeters with accuracy of 0.05 mm, calculating the mean and standard deviation for length and diameter of fruits and seeds, according to their respective water content (BRASIL, 2009 BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. Brasília, DF: Secretaria de Defesa Agropecuária, 2009. 399 p. ).
Centesimal composition: For the determination of the centesimal composition of pulp and seeds, samples were previously pre-dried in a forced air oven at 55 ° C for 72 h, being then cooled in glass desiccators, followed by grinding and sent for analysis at the laboratory of the Institute of Food Technology - ITAL, Campinas, SP.
The moisture and ash values of fruit pulp were determined (HORWITZ; LATIMER, 2010 HORWITZ, W.; LATIMER, G.W. (Ed.). Methods 920.151 and 940.26. In: AOAC. Official methods of analysis of the Association of Official Analytical Chemists International. 18th ed. Gaithersburg: AOAC, 2010. charp. 37, p.6-7. (Current Through Revision, 3). ), (ZENEBON; PASCUET, 2005 ZENEBON, O.; PASCUET, N.S. Métodos físico-químicos para análise de alimentos. 4.ed. Brasília: Ministério da Saúde/ANVISA; São Paulo: Instituto Adolfo Lutz, 2005. cap.4, p.98-124. ), as well as total lipids (ZENEBON; PASCUET, 2005 ZENEBON, O.; PASCUET, N.S. Métodos físico-químicos para análise de alimentos. 4.ed. Brasília: Ministério da Saúde/ANVISA; São Paulo: Instituto Adolfo Lutz, 2005. cap.4, p.98-124. ); caloric content (KALIL, 1975 KALIL, A. Manual básico de nutrição. São Paulo: Instituto de Saúde, 1975. ; PASSMORE et al., 1975 PASSMORE, R.; NICOL, B.M.; RAO, M.N. Manual sobre necesidades nutricionales del hombre. Ginebre: OMS, 1975. (Série de Monografia, 61). ; USDA, 1963 USDA - United States Department of Agriculture. Composition of foods. Washington: Agriculture Handbook, 1963. ) and starch (DIEMAIR, 1963 DIEMAIR, W. Laboratoriumsbuch fur Lebensmittelchemiker. 8th ed. Drisden: Verlag Von Theodor Steinkopff, 1963. ). Total carbohydrates were calculated by difference: 100 - (moisture + ash + protein + total lipids).
Tetrazolium test: The test was performed with whole and intact embryos. Seeds (pyrenes) were stored in plastic trays lined with paper for germination in greenhouse with forced air circulation at room temperature for two days. After this period, the operation of embryo extraction became easier, which was performed with pliers to break the endocarp and a metal clamp for the removal of the embryo in the endosperm. The extracted embryos were placed on filter paper moistened with buffered distilled water within Petri dish and held for two hours for soaking until treatments were assembled.
To perform the tetrazolium test, embryos were immersed in 50 mL plastic containers containing 0.2% 2,3,5-triphenyl chloride tetrazolium chloride solution kept in the dark at 40 ° C per periods of one, two, four and six hours. Five replicates of 10 embryos were used. After each period of exposure to tetrazolium, embryos were washed in running water and kept in distilled water for viability evaluation using stereoscope with 15X magnification. The staining results were compared with seed germination and seedling emergence tests according to FRANÇA NETO et al. (1999) FRANÇA NETO, J.B.; KRZYZANOWSKI, F.C.; VIEIRA, R.D. Vigor de sementes: conceitos e testes. Londrina: ABRATES, 1999. 218 p. .
Germination and Emergency Tests: The chemical treatment of seeds consisted of the application of fungicide based on 150 g-1L carbendazim and 350 g-1L thiram at dose of 100 mL of commercial product per kilogram of seeds. After treatment, the germination test was installed with four replicates of 25 Sphagnum sp. seeds moistened with distilled water + nystatin (0.2%) up to 60% of their retention capacity in plastic boxes (28.5 × 18.5 × 10 cm) wrapped in semi-permeable plastic packaging (0.06 µm low density polyethylene) in a germination chamber regulated to constant temperature of 30°C, white fluorescent light and photoperiod of eight hours. Seeds were considered to be germinated when primary root reached a length equal to or greater than the highest seed size, Figure 2 (i).
Seedling emergence was performed under uncontrolled conditions in a laboratory environment, with four replicates of 25 seeds sown at a depth of one centimeter in moistened washed sand, with distilled water containing nystatin (0.2%), up to 60% of its capacity in plastic boxes (28.5 × 18.5 × 10 cm).
Containers were wrapped in semi-permeable plastic packaging (0.06 µm low density polyethylene).
Maximum and minimum temperatures were daily recorded, and the mean temperature in the evaluation period reached 28 and 24.4° C, respectively.
Seeds that have originated normal seedlings were considered germinated, Figure 2 (ii).
Embryo anatomy: For the anatomical description and to facilitate the observation of tissue differentiation, permanent slides with longitudinal and transverse cuts of embryos were made, being submitted to progressive alcoholic series for dehydration. After inclusion in paraplast, embryos were longitudinally and transversely sectioned with 10 µm thickness using a rotary microtome, deparafinized, and stained with 0.05% toluidine blue (O’BRIEN et al., 1964 O'BRIEN, T.P.; FEDER, N.; McCULLY, M.E. Polychromatic staining of plant cell walls by toluidine blue. Protoplasma, Viena, v.59, n.2, p.368-373, 1964. ). Then, the permanent slides were assembled.
Water content: determined in a greenhouse at 105 ± 3 ° C for 24 h, with two replicates of ten seeds, and the results were expressed in wet basis (BRASIL, 2009 BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. Brasília, DF: Secretaria de Defesa Agropecuária, 2009. 399 p. ).
RESULTS AND DISCUSSION
S. romanzoffiana (Cham.) Glassman fruits presented average length of 2.44 cm and 2.20 cm in diameter and seeds (pyrenes), 2.34 cm in length and 1.42 cm in diameter. The standard deviation and variation coefficient values of these parameters were relatively low, indicating high sample homogeneity (Table 1). According to Carvalho (2006) CARVALHO, P.E.R. Espécies arbóreas brasileiras. Brasília, DF: Embrapa Informação Tecnológica, 2006. v.2, 627 p. , fruits measure 3 to 5 cm in length and 2 to 3 cm in diameter and seeds 1 to 2 cm in length.
Although results are close to those of literature, some factors may influence the size of S. romanzoffiana (Cham.) Glassman fruits and seeds, such as genetic variability of parents, climatic conditions, availability of water and nutrients during the maturation process and the water content of fruits and seeds (Batista et al., 2011 BATISTA, G.S.; COSTA, R.S.; GIMENES, R.; PIVETTA, K.F.L.; MÔRO, F.V. Aspectos morfológicos dos diásporos e das plântulas de Syagrus oleracea (Mart.) Becc - Arecaceae. Comunicata Scientiae, Bom Jesus, v.2, n.3, p.170-176, 2011. ). In addition, the biometric characterization of fruits can provide important information that allows differentiating species of the same genus in the field (Cruz, Carvalho 2003 CRUZ, E.D.; CARVALHO, J.E.U. Biometria de frutos e germinação de sementes de Couratari stellata A.C. Smith (Lecythidaceae). Acta Amazonica, Manaus, v.33, n.3, p.381-387, 2003. ).
The mass of one kilogram of S. romanzoffiana (Cham.) Glassman seeds presented 160 units, which number is lower than that observed by Brasil (2009), with 448 units, or in works by Leite (2001) LEITE, J.A.C. Divergência genética, morfométrica e potencial germinativo de Syagrus romanzoffiana. 2001. 86f. Dissertação (Mestrado em Agronomia) - Universidade Federal de Lavras, Lavras, 2001. , with average of 497 diaspores and Lopes (2007) LOPES, V.S. Morfologia e fenologia reprodutiva do ariri (Syagrus vagans (Bondar) Hawkes) - ARECACEAE - numa área de caatinga do município de Senhor do Bonfim-BA. 2007. 87f. Dissertação (Mestrado em Agronomia) - Universidade Federal da Paraíba, João Pessoa, 2007. with Syagrus vagans (Bondar) A.D.Hawkes, with 691 dipores. As can be observed, these values are extremely variable. This occurrence is attributed to the wild features of the species (LORENZI et al., 2010 LORENZI, H.; SOUZA, H.M.; MEDEIROS COSTA, J.T.;CERQUEIRA, L.S.C.; FERREIRA, E. Flora brasileira Arecaceae (Palmeiras). Nova Odessa: Plantarum, 2010. 368 p. ) and because it develops in environments with very different climate and soil characteristics. The size and mass of the seeds of a given species are extremely variable characteristics since they change among different sites, times and within the same individual (VALADARES et al., 2009 VALADARES, J.; PAULA, R.C.; MÔRO, F.V. Germinação, desenvolvimento de plântulas e teste de tetrazólio em Poecilanthe parviflora Bentham (Fabaceae - Faboideae). Científica, Jaboticabal, v.37, n.1, p.39-47, 2009. ).
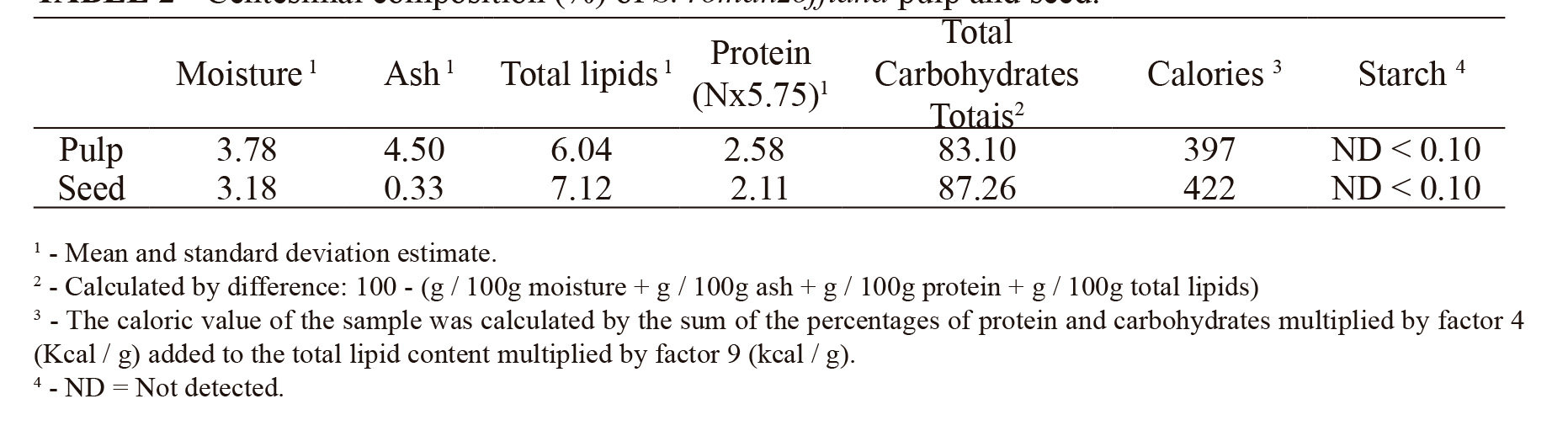
Regarding the centesimal composition, the amount of ash in the pulp (4.5%) was almost 14 times higher than in the seed (0.33%). Both S. romanzoffiana pulp and seeds have low and similar percentages of total lipids (6.04 and 7.12%) and proteins (2.58 and 2.11%), respectively. The percentages of total carbohydrates are high in the pulp (83.10%) and in the seed (87.26%), both containing about 400 calories, with 397 Kcal-1.g in the pulp and 422 Kcal-1.g in the seed. The starch percentage was not detectable in any of the materials analyzed (Table 2).
In this study, the centesimal composition results are in agreement with those found by Goudel et al. (2013) GOUDEL, F.; SHIBATA, M.; COELHO, C.M.M.; MILLER, P.R.M. Fruit biometry and seed germination of Syagrus romanzoffiana (Cham.) Glassm. Acta Botanica Brasilica, Belo Horizonte, v.27, n.1, p.147-154, 2013. , but for Coimbra and Jorge (2011) COIMBRA, M.C.; JORGE, N. Proximate composition of guariroba (Syagrus oleracea), jerivá (Syagrus romanzoffiana) and macaúba (Acrocomia aculeata) palm fruits. Food Research International, Toronto, v.44, p.2139–2142, 2011. , different values were observed for the species.
Variations in the contents of chemical components in palms of the same species can be caused by genetic factors, differences in edaphoclimatic conditions or cultivation regions (NEIDA; ELBA, 2007 NEIDA, S.; ELBA, S. Caracterizacíon del acai o manacá (Euterpe oleracea Mart): un fruto del Amazonas. Archivos Latinoamericanos de Nutricion, Caracas, v.57, p.94-99, 2007. ; BORGES et al., 2011 BORGES, G.S.C.; VIEIRA, F.G.K.; COPETTI, C.; GONZAGA, L.V.; ZAMBIAZI, R.C.; MANCINI-FILHO, J.; FETT, R. Chemical characterization, bioactive compounds, and antioxidant capacity of jussara (Euterpe edulis) fruit from the Atlantic Forest in southern Brazil. Food Research International, Toronto, v.44, n.7, p.2128–2133, 2011. ; MHANHMAD et al., 2011 MHANHMAD, S.; LEEWANISH, P.; PUNSUVON, V.; SRINIVES, P. Seasonal effects on bunch components and fatty acid composition in Dura oil palm (Elaeis guineensis). African Journal of Agricultural Research, Victoria Island, v.6, n.7, p.1835-1843, 2011. ).
For the tetrazolium test conducted at 40°C, temperature and TZ concentration of 0.2%, during the different periods, embryos with minimum of 90% of uniformity and the pink tone of the coloration were considered as viable (Figure 3).
As for the percentage of colored embryos during the different periods, 45% were obtained in the period of one, 61% in two, 64% in four and 96% in six hours. S. romanzoffiana embryos were partially or totally stained pink in periods of one and two hours (Figure 3). In the germination test carried out in parallel with the tetrazolium test, seeds presented 59% germination and 57% seedling emergence, while the average viability by the tetrazolium test was 61% when submitted to two hours of imbibition. In this case, agreement among evaluations carried out between tests was observed. Therefore, in view of the adequate staining pattern, the two-hour period may be recommended for the safe evaluation of S. romanzoffiana seeds by the tetrazolium test.
This variation between germination and viability by the tetrazolium test should be less than 5% (BITTENCOURT; VIEIRA, 1997 BITTENCOURT, S.R.M.; VIEIRA, R.D. Use of reduced concentration of tetrazolium solutions for the evaluation of the viability of peanut seed lots. Seed Science and Technology, Bassersdorf, v.25, n.1, p.75-82, 1997. ; FRANÇA NETO et al., 1999 FRANÇA NETO, J.B.; KRZYZANOWSKI, F.C.; VIEIRA, R.D. Vigor de sementes: conceitos e testes. Londrina: ABRATES, 1999. 218 p. ).
When the vigorous seeds stain slowly, they present shining pink surface coloration.
This demonstrates that the solution had difficulty penetrating tissues due to the higher integrity of the cell membrane system. Older, deteriorated or damaged seeds usually color faster and deeper and show reddish and more intense staining (SILVA et al., 2012 SILVA, C.B.; BARBOSA, R.M.; VIEIRA, R.D. Evaluating sunnhemp (Crotalaria juncea) seed viability using the tetrazolium test. Seed Technology, Washington, v.34, n.2, p.263-272, 2012. ). However, intense red staining may indicate excessive time of contact with tetrazolium or inadequate solution concentration, as well as region of high physiological activity such as the end of the primary root.
By observing the seed germination type of Queen palm, it could be classified as a tubular remote type, in which, according to Dransfield et al. (2008) DRANSFIELD, J.; UHL, N.W.; ASMUSSE, C.B.; BAKER, W.; HARLEY, M.; LEWIS, C. Genera palmarum: the evolution and classification of palms. Surrey: Royal Botanic Gardens, 2008. 732 p. , the cotyledonary petiole is striking and there is no presence of ligule.
To determine the embryo viability, the uniformity of the structure staining is considered essential, comparing them with control embryos submitted to immersion in buffered distilled water in the same periods and temperatures applied in the different treatments. Embryos from hollow seeds and with signs of pest attacks were considered unviable.
It was observed that the control embryo has a milky and uniform coloration.
As the tetrazolium test is mainly based on the distribution of living and dead tissues among the various embryo organs, for the test evaluation, it is necessary to know the structure of seeds with certainty. In this sense, the absence of considerations about the anatomy of the palm embryo can cause numerous errors in the interpretation of the tetrazolium test.
For the description and detailing of the anatomy of the S. romanzoffiana embryo, permanent slides were made with longitudinal and transverse cuts of the embryo, according to the method described by Johansen (1940) JOHANSEN, D.A. Plant microtechnique. New York: McGraw – Hill Book, 1940. 523 p. (Figure 4).
The embryo of species of the Arecaceae family is formed by a single cotyledon, generally little differentiated, and the embryonic axis is in its proximal region. During the germinative process, this proximal region will give rise to the cotyledonary petiole, while the distal region develops as haustorium.
The Syagrus romanzofian embryo is elongated, poorly differentiated, and does not present constriction or morpho-anatomical difference that distinguishes where the proximal region ends and the distal region begins, as observed in Figure 4 (i), except for the longitudinal invaginations observed in whole embryos in the distal region (Figure 3). Some embryos have a slight curvature in the distal region, and the transverse median extension of both regions has similar dimensions (Figure 3). It was observed that some embryos may not present this curvature, be less elongated, and have a slightly conical shape.
The plumule of the embryonic axis is differentiated in two leaf primordia, constituted by the first and second leaf sheath (Figure 4 - vi and vii). The cotyledonary cleft is conspicuous and the protoderm is unstratified, formed by flattened cells of easily identifiable (Figure 4 - viii).
The protoderm, the fundamental meristem, and the procambium can be identified throughout the embryonic structure by the shapes of their cells, sizes, and positions. The procambium cells are elongated, narrow and intensively colored by toluidine blue (Figure 4 - ix).
In Figure 4 (i to vi), procambium, which will give rise to vascular bundles, is observed above the embryonic axis, passing through its lateral, distributing in an ordered way, as it distances from the embryonic axis, it follows branching, becoming peripheral near the protoderm, and remaining thus in all its distal region. The fundamental meristem cells are isodiametric, larger in the medulla than in the cortical region of the cotyledon (Figure 4 - ix).
Criteria for germination of S. romanzoffiana seeds. (I) Primary root of length equal to or greater than the largest seed measure. (ii) Normal seedling; pr) primary root.
S. romanzoffiana embryos submitted to the tetrazolium test in 0.2% concentration under 40 ° C, during the period of one (i), two (ii), four (iii) and six (iv) hours compared to the control embryo on the right.
(i) Longitudinal section of S. romanzoffiana embryo. (vi) Detail of the embryonic axis of the image (i). Dotted lines indicate the positions of the cross-sections of figures (ii) to (v). Details of cross sections (vii to ix). The structure presents the following: b1: first leaf sheath; b2: second leaf sheath; cc: cotyledonary cavity; fc: cotyledonary slit; mf: fundamental meristem; pc: procambium; pd: protoderm; pf: leaf primordia; pm: pro-meristem.
CONCLUSION
The mean seed size (pyrene) is 2.34 cm in length and 1.42 cm in diameter. One kilogram of seeds has 160 units. The embryo is slightly differentiated, elongated and with longitudinal invaginations in the distal region.
Pulp and seed are rich in total carbohydrates, with contents higher than 80% and approximately 400 Kcal-1.g.
The tetrazolium test conducted with solution 0.2% concentration and at 40°C for two hours is adequate to estimate the viability of S. romanzoffiana seeds.
S. romanzoffiana seeds present germination of tubular remote type.
ACKNOWLEDGMENTS
To the Foundation for Research Support of the State of São Paulo (FAPESP, process 11 / 06907-2), to the Coordination of Improvement of Higher Education Personnel (CAPES, project PNPD process No. 02672 / 09-0) and to the Institute of Food Technology (ITAL).
- BATISTA, G.S.; COSTA, R.S.; GIMENES, R.; PIVETTA, K.F.L.; MÔRO, F.V. Aspectos morfológicos dos diásporos e das plântulas de Syagrus oleracea (Mart.) Becc - Arecaceae. Comunicata Scientiae, Bom Jesus, v.2, n.3, p.170-176, 2011.
- BITTENCOURT, S.R.M.; VIEIRA, R.D. Use of reduced concentration of tetrazolium solutions for the evaluation of the viability of peanut seed lots. Seed Science and Technology, Bassersdorf, v.25, n.1, p.75-82, 1997.
- BITTENCOURT, S.R.M.; VIEIRA, R.D.; RODRIGUES, T.J.D. Criteria for peanut seed pre-conditioning for the tetrazolium test. Seed Science and Technology, Bassersdorf, v.25, n.3, p.337-342, 1997.
- BORGES, G.S.C.; VIEIRA, F.G.K.; COPETTI, C.; GONZAGA, L.V.; ZAMBIAZI, R.C.; MANCINI-FILHO, J.; FETT, R. Chemical characterization, bioactive compounds, and antioxidant capacity of jussara (Euterpe edulis) fruit from the Atlantic Forest in southern Brazil. Food Research International, Toronto, v.44, n.7, p.2128–2133, 2011.
- BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. Brasília, DF: Secretaria de Defesa Agropecuária, 2009. 399 p.
- BROSCHAT, T.K. Palm seed propagation. Acta Horticulturae, The Hague, v.360, p.141-147, 1994.
- CARVALHO, P.E.R. Espécies arbóreas brasileiras. Brasília, DF: Embrapa Informação Tecnológica, 2006. v.2, 627 p.
- COIMBRA, M.C.; JORGE, N. Proximate composition of guariroba (Syagrus oleracea), jerivá (Syagrus romanzoffiana) and macaúba (Acrocomia aculeata) palm fruits. Food Research International, Toronto, v.44, p.2139–2142, 2011.
- CRUZ, E.D.; CARVALHO, J.E.U. Biometria de frutos e germinação de sementes de Couratari stellata A.C. Smith (Lecythidaceae). Acta Amazonica, Manaus, v.33, n.3, p.381-387, 2003.
- DIEMAIR, W. Laboratoriumsbuch fur Lebensmittelchemiker. 8th ed. Drisden: Verlag Von Theodor Steinkopff, 1963.
- DRANSFIELD, J.; UHL, N.W.; ASMUSSE, C.B.; BAKER, W.; HARLEY, M.; LEWIS, C. Genera palmarum: the evolution and classification of palms. Surrey: Royal Botanic Gardens, 2008. 732 p.
- FLORES, P.C.; POGGI, D.; GARCÍA, S.M.; GARIGLIO, N.F. Topographic tetrazolium testing of black walnut (Juglans nigra L.) seeds. Seed Science and Technology, Zurich, v.39, n.1, p.230-235, 2011.
- FRANÇA NETO, J.B.; KRZYZANOWSKI, F.C.; COSTA, N.P. O teste de tetrazólio em sementes de soja. Londrina: EMBRAPA-CNPSo, 1998. 72 p. (Documentos, 116).
- FRANÇA NETO, J.B.; KRZYZANOWSKI, F.C.; COSTA, N.P. Teste de tetrazólio em semente. In: BAALBAKI, R.; ELIAS, S.; MARCOS-FILHO, J.; MCDONALD, M.B. Seed vigor testing handbook. Ithaca: AOSA, 2009. (Contribuiton, 32).
- FRANÇA NETO, J.B.; KRZYZANOWSKI, F.C.; VIEIRA, R.D. Vigor de sementes: conceitos e testes. Londrina: ABRATES, 1999. 218 p.
- GENINI, J.; GALETTI, M.; MORELLATO, L.P.C. Fruiting phenology of palms and trees in an Atlantic rianforeste land-bridge island. Flora, Freiberg, n.204, p.131-145, 2009.
- GOUDEL, F.; SHIBATA, M.; COELHO, C.M.M.; MILLER, P.R.M. Fruit biometry and seed germination of Syagrus romanzoffiana (Cham.) Glassm. Acta Botanica Brasilica, Belo Horizonte, v.27, n.1, p.147-154, 2013.
- HORWITZ, W.; LATIMER, G.W. (Ed.). Methods 920.151 and 940.26. In: AOAC. Official methods of analysis of the Association of Official Analytical Chemists International. 18th ed. Gaithersburg: AOAC, 2010. charp. 37, p.6-7. (Current Through Revision, 3).
- JOHANSEN, D.A. Plant microtechnique. New York: McGraw – Hill Book, 1940. 523 p.
- KALIL, A. Manual básico de nutrição. São Paulo: Instituto de Saúde, 1975.
- LEITE, J.A.C. Divergência genética, morfométrica e potencial germinativo de Syagrus romanzoffiana. 2001. 86f. Dissertação (Mestrado em Agronomia) - Universidade Federal de Lavras, Lavras, 2001.
- LIMA JUNIOR, M.J. (Ed.). Manual de procedimentos para análise de sementes florestais. Londrina: ABRATES, 2011. 83 p.
- LOPES, V.S. Morfologia e fenologia reprodutiva do ariri (Syagrus vagans (Bondar) Hawkes) - ARECACEAE - numa área de caatinga do município de Senhor do Bonfim-BA. 2007. 87f. Dissertação (Mestrado em Agronomia) - Universidade Federal da Paraíba, João Pessoa, 2007.
- LORENZI, H. Árvores brasileiras, manual de identificação e cultivo de plantas arbóreas nativas do Brasil. 6.ed. Nova Odessa: Plantarum, 2014. v.1, 384p.
- LORENZI, H.; SOUZA, H.M.; MEDEIROS COSTA, J.T.;CERQUEIRA, L.S.C.; FERREIRA, E. Flora brasileira Arecaceae (Palmeiras). Nova Odessa: Plantarum, 2010. 368 p.
- MHANHMAD, S.; LEEWANISH, P.; PUNSUVON, V.; SRINIVES, P. Seasonal effects on bunch components and fatty acid composition in Dura oil palm (Elaeis guineensis). African Journal of Agricultural Research, Victoria Island, v.6, n.7, p.1835-1843, 2011.
- NEIDA, S.; ELBA, S. Caracterizacíon del acai o manacá (Euterpe oleracea Mart): un fruto del Amazonas. Archivos Latinoamericanos de Nutricion, Caracas, v.57, p.94-99, 2007.
- O'BRIEN, T.P.; FEDER, N.; McCULLY, M.E. Polychromatic staining of plant cell walls by toluidine blue. Protoplasma, Viena, v.59, n.2, p.368-373, 1964.
- PASSMORE, R.; NICOL, B.M.; RAO, M.N. Manual sobre necesidades nutricionales del hombre. Ginebre: OMS, 1975. (Série de Monografia, 61).
- REIS, R.C.C. Palmeiras (Arecaceae) das Restingas do Estado do Rio de Janeiro, Brasil. Acta Botânica Brasilica, Belo Horizonte, v.20, n.3, p.501-512, 2006.
- SANTOS, J.F.; SANCHES, M.F.G.; BARBOSA, R.M.; LEÃO, E.F.; VIEIRA, R.D.Optimising tetrazolium test procedures to evaluate the physiological potential of peanut seeds. Seed Science and Technology, Bassersdorf, v.40, n.2, p.215-228, 2012.
- SILVA, C.B.; BARBOSA, R.M.; VIEIRA, R.D. Evaluating sunnhemp (Crotalaria juncea) seed viability using the tetrazolium test. Seed Technology, Washington, v.34, n.2, p.263-272, 2012.
- SMIDERLE, O.J.; MOURÃO JUNIOR, M.; SOUSA, R.C.P. Tratamentos pré-germinativos em sementes de acácia. Revista Brasileira de Sementes, Londrina, v.27, n.1, p.78-85, 2005.
- USDA - United States Department of Agriculture. Composition of foods. Washington: Agriculture Handbook, 1963.
- VALADARES, J.; PAULA, R.C.; MÔRO, F.V. Germinação, desenvolvimento de plântulas e teste de tetrazólio em Poecilanthe parviflora Bentham (Fabaceae - Faboideae). Científica, Jaboticabal, v.37, n.1, p.39-47, 2009.
- ZENEBON, O.; PASCUET, N.S. Métodos físico-químicos para análise de alimentos. 4.ed. Brasília: Ministério da Saúde/ANVISA; São Paulo: Instituto Adolfo Lutz, 2005. cap.4, p.98-124.
Publication Dates
-
Publication in this collection
2016
History
-
Received
13 Apr 2015 -
Accepted
03 May 2016