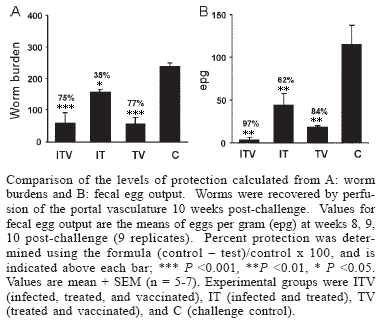
The high level of protection elicited in rodents and primates by the radiation-attenuated schistosome vaccine gives hope that a human vaccine relying on equivalent mechanisms is feasible. In humans, a vaccine would be undoubtedly administered to previously or currently infected individuals. We have therefore used the olive baboon to investigate whether vaccine-induced immunity is compromised by a schistosome infection. We showed that neither a preceding infection, terminated by chemotherapy, nor an ongoing chronic infection affected the level of protection. Whilst IgM responses to vaccination or infection were short-lived, IgG responses rose with each successive exposure to the vaccine. Such a rise was obscured by responses to egg deposition in already-infected animals. In human trials it would be necessary to use indirect estimates of infection intensity to determine vaccine efficacy. Using worm burden as the definitive criterion, we demonstrated that the surrogate measures, fecal eggs, and circulating antigens, consistently overestimated protection. Regression analysis of the surrogate parameters on worm burden revealed that the principal reason for overestimation was the threshold sensitivity of the assays. If we extrapolate our findings to human schistosomiasis mansoni, it is clear that more sensitive indirect measures of infection intensity are required for future vaccine trials.
Schistosoma mansoni; radiation-attenuated vaccine; infection; circulating antigens